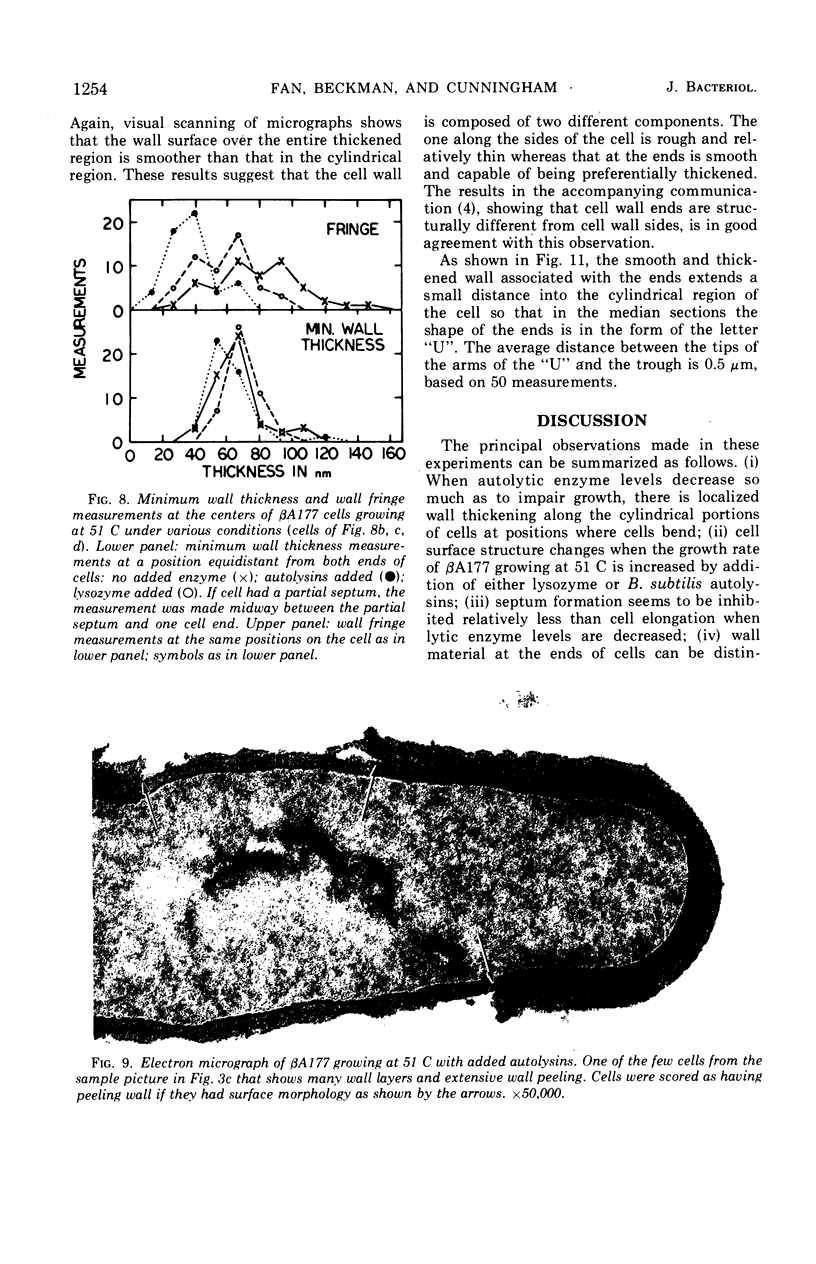
Abstract
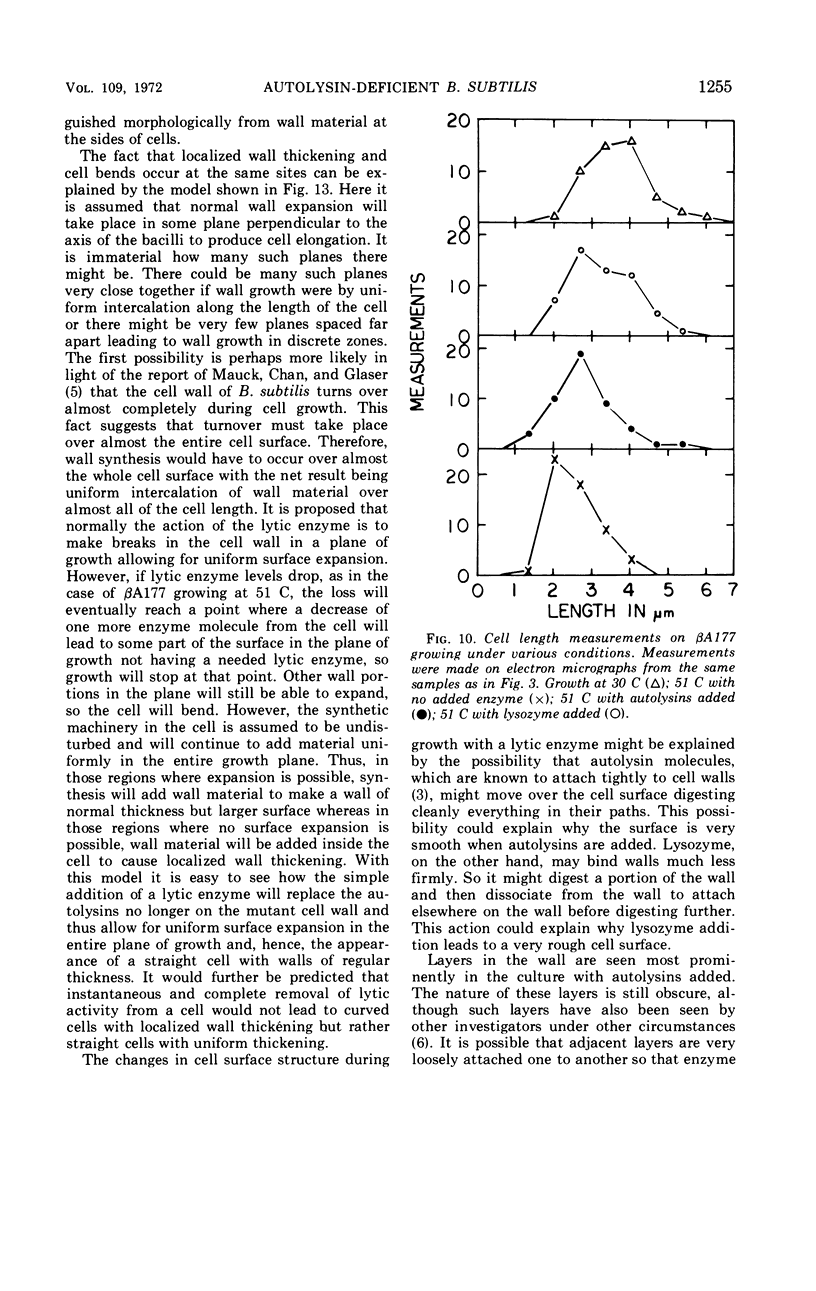
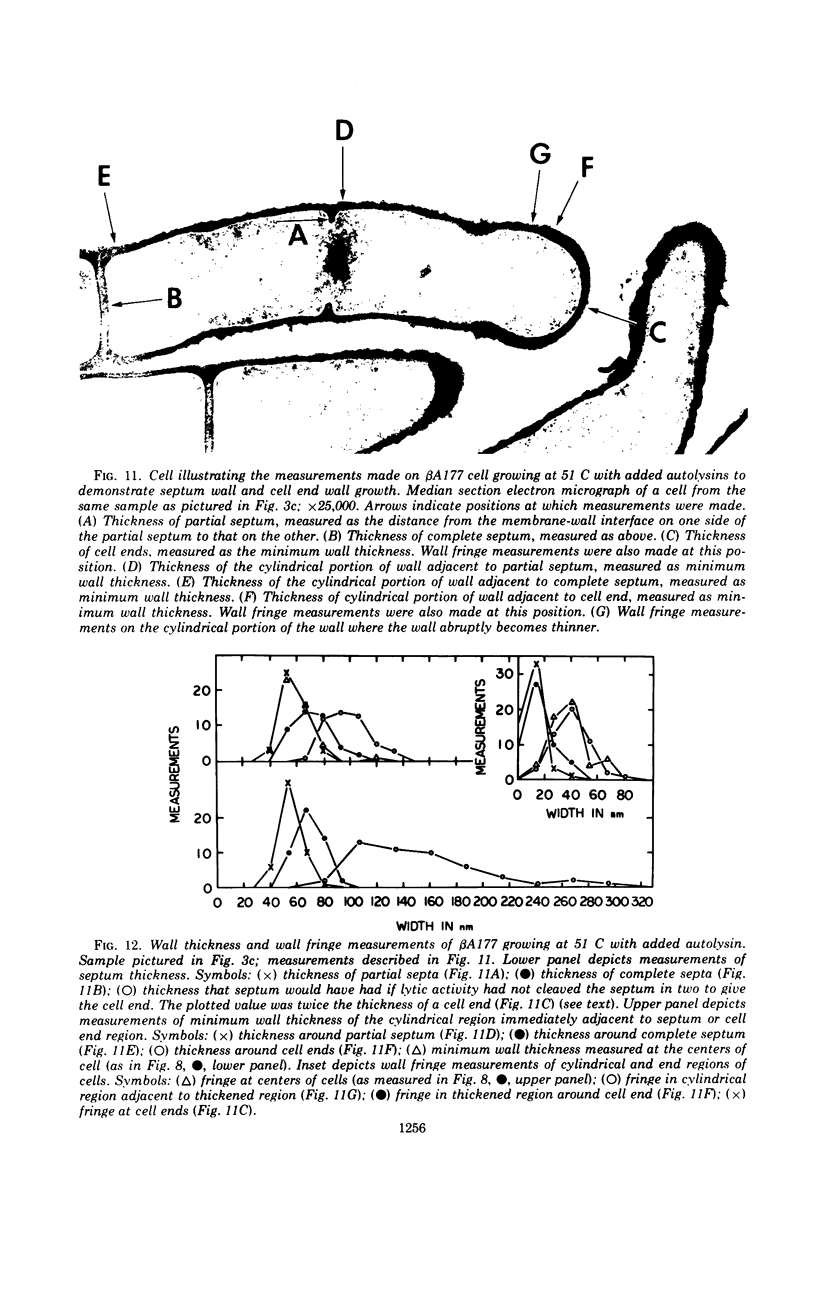
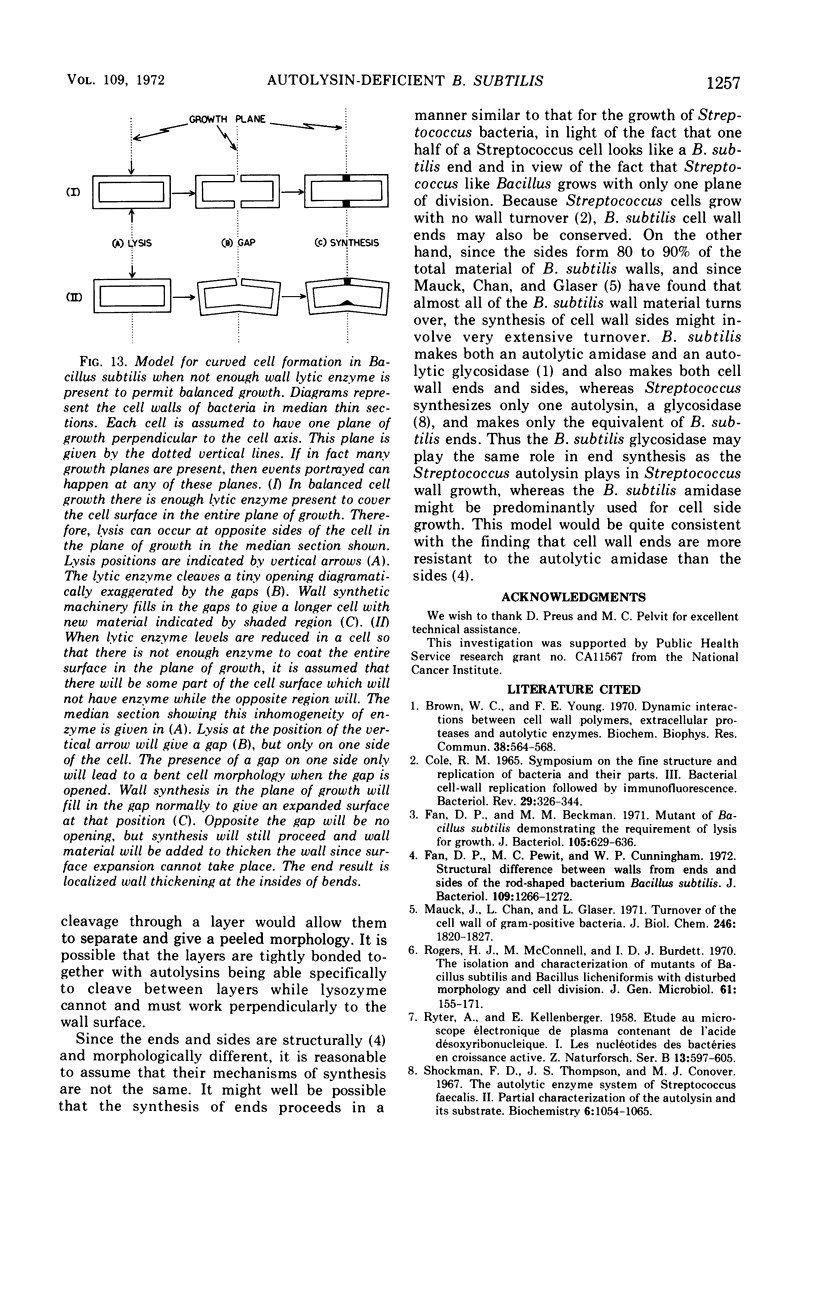
The growth of Bacillus subtilis mutant βA177 can be inhibited under special conditions in which not enough autolytic enzymes are produced for optimal growth. Electron microscopy studies show that during growth inhibition there is localized thickening of the cell wall at positions where cells bend. A model is proposed to explain this result. Rapid growth can be restored by adding lysozyme or a B. subtilis autolysin mixture to a growth-inhibited βA177 culture. Such addition reduces the localized wall thickening and causes other changes in surface morphology which are described and discussed. Septum formation seems to be relatively less inhibited than cell elongation when lytic enzyme levels are reduced. Measurements were made demonstrating that walls at ends of cells are morphologically different from walls at sides of cells in cultures of βA177 growing at 51 C.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown W. C., Young F. E. Dynamic interactions between cell wall polymers, extracellular proteases and autolytic enzymes. Biochem Biophys Res Commun. 1970 Feb 20;38(4):564–568. doi: 10.1016/0006-291x(70)90618-2. [DOI] [PubMed] [Google Scholar]
- Cole R. M. Symposium on the fine structure and replication of bacteria and their parts. 3. Bacterial cell-wall replication followed by immunofluorescence. Bacteriol Rev. 1965 Sep;29(3):326–344. doi: 10.1128/br.29.3.326-344.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M. Mutant of Bacillus subtilis demonstrating the requirement of lysis for growth. J Bacteriol. 1971 Feb;105(2):629–636. doi: 10.1128/jb.105.2.629-636.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Pelvit M. C., Cunningham W. P. Structural difference between walls from ends and sides of the rod-shaped bacterium Bacillus subtilis. J Bacteriol. 1972 Mar;109(3):1266–1272. doi: 10.1128/jb.109.3.1266-1272.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Rogers H. J., McConnell M., Burdett I. D. The isolation and characterization of mutants of Bacillus subtilis and Bacillus licheniformis with disturbed morphology and cell division. J Gen Microbiol. 1970 May;61(2):155–171. doi: 10.1099/00221287-61-2-155. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]