Abstract
Mice with a targeted mutation of the gastric inhibitory polypeptide (GIP) receptor gene (GIPR) were generated to determine the role of GIP as a mediator of signals from the gut to pancreatic β cells. GIPR−/− mice have higher blood glucose levels with impaired initial insulin response after oral glucose load. Although blood glucose levels after meal ingestion are not increased by high-fat diet in GIPR+/+ mice because of compensatory higher insulin secretion, they are significantly increased in GIPR−/− mice because of the lack of such enhancement. Accordingly, early insulin secretion mediated by GIP determines glucose tolerance after oral glucose load in vivo, and because GIP plays an important role in the compensatory enhancement of insulin secretion produced by a high insulin demand, a defect in this entero-insular axis may contribute to the pathogenesis of diabetes.
A much greater insulin response and a smaller increase in blood glucose level are observed after oral glucose load than after i.v. injection of the same amount of glucose (1, 2). The hormonal factor(s) thus implicated as transmitters of signals from the gut to pancreatic β cells originally was referred to incretin and was thought to be crucial in glucose homeostasis by promoting insulin secretion immediately on meal ingestion (3, 4).
Two incretins have been identified: gastric inhibitory polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). GIP is a gastrointestinal peptide hormone of 42 aa that is released from duodenal endocrine K cells after absorption of glucose or fat (5). It was first isolated from porcine intestine on the basis of its ability to inhibit gastric acid secretion. Subsequent studies of the physiological role of GIP found that GIP potentiates glucose-induced insulin secretion from pancreatic β cells, so GIP also is referred to as glucose-dependent insulinotropic polypeptide. We have isolated a human cDNA encoding the GIP precursor and determined its exon-intron organization (6, 7), confirming that GIP belongs to the vasoactive intestinal peptide (VIP)/glucagon/secretin family. We also isolated the human GIP receptor gene (GIPR) and cDNA (8). The GIPR has seven potential membrane-spanning domains, a feature characteristic of the VIP/glucagon/secretin receptor family of G protein-coupled receptors (9, 10). Stimulation of the GIPR on pancreatic β cells activates adenylyl cyclase and mitogen-activated protein kinase, resulting in increased insulin secretion (11, 12).
GLP-1 is synthesized by specific posttranslational processing from proglucagon in L cells in the lower intestinal tract and secreted mainly as truncated GLP-1 [7–36 amide] (13). Recently, Scrocchi et al. (14) generated GLP-1 receptor-lacking mice with glucose intolerance accompanied by impaired insulin secretion even after i.p. glucose load. Various nutrients are known to directly stimulate the secretion of GLP-1 (15). However, because these nutrient-related GLP-1 responses begin early after ingestion (16), it seems unlikely that the nutrients could have reached the lower portion of small intestine by that time. Damholt et al. (17) have shown that GIP increases secretion of GLP-1 from canine L cells, but that glucose does not, suggesting that glucose stimulates GLP-1 release via GIP. Nutrients such as glucose and fat are known to be potent secretagogues of GIP after meal ingestion (5). Glucose together with GIP may synergically stimulate insulin secretion, and, whereas fat produces no insulin secretion directly, it elicits a large amount of GIP (15), so also contributes to early insulin release after meal. The insulinotropic effect of GIP is selectively impaired despite exaggerated GIP secretion in type 2 diabetic patients, while the effect of GLP-1 is preserved (18, 19). These results strongly suggest that, although GLP-1 is a potent therapeutic agent in type 2 diabetic patients, the modulation of pancreatic β cells via GIP is more involved in the pathogenesis of type 2 diabetes mellitus.
Materials and Methods
Targeting Construct.
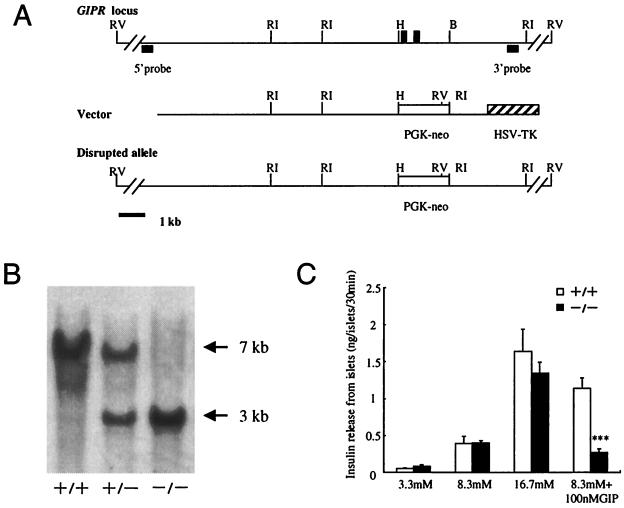
Four overlapping genomic clones containing the partial GIPR gene were isolated from a 129SVJ mouse genomic library (Strategene). The organization of the targeting vector is shown in Fig. 1A. Briefly, the 1.2-kb HindIII/BglII fragment containing exons 4 and 5 was replaced with a cassette containing a positively selectable marker, the neomycin resistance gene (neo), under the control of the phosphoglycerate kinase (PGK) promoter. External to the 3′ region of homology with the target locus was placed a negatively selectable marker gene, the herpes simplex virus thymidine kinase gene (HSV-TK) under the control of the MC1 promoter.
Figure 1.
Disruption of the GIPR gene by homologous recombination. (A) Schematic drawings of the wild-type GIPR locus, the targeting vector, and the mutant allele generated after homologous recombination. Exons 4 and 5 of GIPR are indicated by closed boxes. The 5′ and 3′ probes for DNA blotting are indicated. Restriction enzymes: B, BglII; H, HindIII; RI, EcoRI; RV, EcoRV. PGK-neo, phosphoglycerate kinase-neomycin; HSV-TK, herpes simplex virus thymidine kinase. (B) EcoRI-digested genomic DNA from a litter derived from a GIPR+/− intercross was hybridized with a 3′ probe. The sizes of wild-type (7 kb) and targeted (3 kb) alleles are indicated. (C) Insulin secretion from isolated pancreatic islets was examined in response to the indicated concentrations of glucose with or without 100 nM GIP. Data were obtained from 12-week-old mice. Values are expressed as mean ± SE. ***, P < 0.001 for GIPR−/− mice vs. GIPR+/+.
Generation of Mutant Animals.
CGR8 embryonic stem (ES) cells (20) and derivatives were cultured without feeders in Glasgow modification of Eagle medium (GMEM) supplemented with 10% FCS, 0.1 mM 2-mercaptoethanol, and 1,000 units/ml of leukemia inhibitory factor (21). For transfection, 1 × 107 ES cells were electroporated with 100 μg of the linearized targeting construct at 800 V and 3 μF in a 0.4-cm cuvette by using a Bio-Rad gene pulsar and then selected in the presence of 150 μg/ml G418 (Sigma) and 1 mM gancyclovir (Tanabe Seiyaku, Osaka, Japan). Colonies surviving positive/negative selection were isolated and screened by Southern blot of EcoRV- and EcoRI-digested genomic DNA by using 5′ and 3′ external probes, respectively, to confirm that genuine homologous recombination had occurred on both sides of the gene. The chimeric mice were produced by microinjection of a targeted ES clone into C57BL/6 blastocysts (22) and were bred with female C57BL/6 mice. Mice with the targeted allele were backcrossed to C57BL/6 mice before analysis of homozygous mice. Male mice were subjected to experiments.
Glucose Tolerance and Insulin Secretion.
Different groups of age-matched 8- to 12-week-old male mice were used for oral glucose tolerance test and i.p. glucose tolerance test. After a 16-hr fast (18:00 to 10:00), plasma insulin and glucose levels were measured and d-glucose (2 g/kg body weight) was loaded. Blood samples were taken from the tail vein at indicated times. Blood glucose levels were measured by enzyme-electrode method. Plasma insulin levels were measured by using an ELISA kit (Morinaga, Yokohama, Japan).
Measurement of Insulin Release from Intact Islets.
Pancreatic islets were isolated by collagenase digestion as described (23). Isolated islets were cultured for 18 hr in RPMI medium 1640 containing 11.1 mM glucose, 10% FCS, 100 units/ml of penicillin, and 100 μg/ml streptomycin. Insulin release from intact islets was monitored by using static incubation as described (24). Briefly, the islets were preincubated at 37°C for 30 min in Krebs–Ringer bicarbonate buffer (KRBB) supplemented with 3.3 mM glucose and 0.2% BSA. Groups of islets then were batch-incubated for 30 min in 0.4 ml of KRBB with test materials. At the end of the incubation period, islets were pelleted by centrifugation (10,000 g, 3 min) and aliquots of the buffer were sampled. The amount of immunoreactive insulin was determined by RIA, using rat insulin as standard.
High-Fat Diet Study.
At 7 weeks of age, mutant (n = 5) and wild-type (n = 5) mice were provided either a high-fat diet or normal control diet. The high-fat diet contained 45% kcal as fat, 35% kcal as carbohydrate, and 20% kcal as protein, with an energy density of 3.57 kcal/g. The control diet contained 13% kcal as fat, 60% kcal as carbohydrate, and 27% as protein, with an energy density of 3.57 kcal/g. Mice were exposed to the respective diets for 3 weeks until the experiments. The meal (13.3 kcal/kg body weight) containing 25% kcal as fat, 59% kcal as carbohydrate, and 16% as protein; an energy density of 1 kcal/g was orally administrated.
Statistical Analysis.
Data are expressed as means ± SE. Statistical analyses were performed by an unpaired t test.
Results
Development of GIPR-Deficient Mice.
We generated mice lacking the GIPR gene (GIPR), by replacing exons 4 and 5 of GIPR with the PGK-neo cassette (Fig. 1A). Exons 4 and 5 are 108 bp and 104 bp in size, respectively, and encode the N-terminal extracellular region (8). CGR8 embryonic stem cells (20) were transfected with this construct and cultured without feeders in a leukemia inhibitory factor-supplemented medium (21), then efficiently transmitted to germ lines with the expected Mendelian frequency (data not shown). Transmission of the mutant allele was confirmed by DNA blotting analysis using a 3′ probe, which was set in the external region of the targeting construct (Fig. 1B). The absence of GIPRs was confirmed by batch-incubation studies using isolated pancreatic islets (Fig. 1C). GIP stimulated insulin secretion 2.9-fold from the islets of GIPR+/+ mice, but had no insulinotropic effect in GIPR−/− mice. The islets isolated from heterozygous mice did respond to GIP (data not shown), indicating that the truncated GIPR does not have a dominant-negative effect, but has lost its function. Although various receptors of the vasoactive intestinal peptide/glucagon/secretin family are expressed in pancreatic islets (9, 10), GIP signals are transmitted only via the GIPR, so GIPR−/− mice are especially suitable to analyze the functional role of GIP in the entero-insular axis.
No gross abnormalities were seen in the mutant mice in general behavior or feeding (Table 1). No histological abnormalities were found in pancreas, antrum, duodenum, or adrenal gland (data not shown). No significant differences in plasma glucose, HbA1c, triglyceride, total cholesterol, free fatty acids levels, or body weight were observed in GIPR−/− mice (Table 1 and Fig. 2).
Table 1.
General characteristics of GIPR−/− and GIPR+/+ mice
| Group (n = 5) | Casual BS, mg/dl | HbA1c, % | Food intake, g/day | Water, g/day | TG, mg/dl | FFA, mEq/dl | TC, mg/dl | LDLC, mg/dl | HDLC, mg/dl |
|---|---|---|---|---|---|---|---|---|---|
| +/+ | 152 ± 4 | 10.2 ± 0.4 | 4.2 ± 0.3 | 6.9 ± 0.3 | 12.0 ± 0.6 | 579 ± 133 | 104 ± 8 | 11.0 ± 1.0 | 95.7 ± 7.2 |
| −/− | 160 ± 6 | 10.2 ± 0.3 | 4.0 ± 0.2 | 6.4 ± 0.4 | 12.4 ± 0.8 | 755 ± 53 | 109 ± 4 | 11.0 ± 1.6 | 102.1 ± 3.3 |
Values indicated as mean ± SE. BS, blood glucose; TG, triglyceride; FFA, free fatty acid; TC, total cholesterol; LDLC, low density lipoprotein cholesterol; HDLC, high density lipoprotein cholesterol.
Figure 2.
Body weight and fasting blood glucose levels in GIPR−/− and GIPR+/+ mice. (A) Growth curve. ○, GIPR+/+; ■, GIPR−/−. Body weight was measured in g at 1, 2, 4, 8, 12, and 20 weeks. (B) Fasting plasma glucose levels were measured after a 16-hr fast. Open bars, GIPR+/+; filled bars, GIPR−/−.
GIP as Incretin.
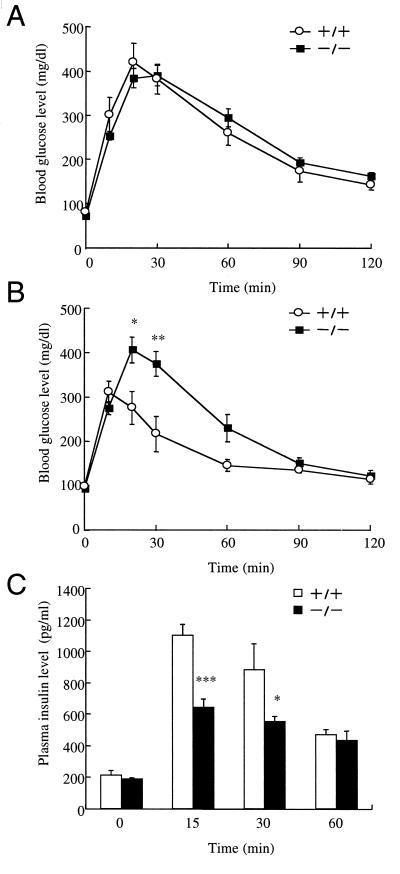
To determine the effect of GIP on glucose homeostasis, we carried out i.p. glucose tolerance test (IPGTT) and oral glucose tolerance test (OGTT) by administration of 2 g d-glucose per kg body weight. In IPGTT, blood glucose levels were not significantly different in GIPR−/− mice and GIPR+/+ mice (Fig. 3A). In OGTT, fasting glucose levels of GIPR−/− mice were not significantly different from GIPR+/+ mice (Fig. 3B). However, the peak levels of blood glucose were delayed in GIPR−/− mice, and the maximum blood glucose levels were significantly higher than in GIPR+/+ mice (406 ± 28 mg/dl at 20 min vs. 312 ± 24 mg/dl at 10 min, P < 0.01).
Figure 3.
Glucose tolerance test. (A) Intraperitoneal glucose tolerance test in age-matched GIPR+/+ mice (○, n = 4) and GIPR−/− (■, n = 6). (B) Oral glucose tolerance test in age-matched GIPR+/+ mice (○, n = 4) and GIPR−/− (■, n = 6). (C) Plasma insulin levels after oral glucose loading for age-matched GIPR+/+ mice (open bars, n = 4) and GIPR−/− (filled bars, n = 6). Statistical significance was assessed by using the unpaired t test. Values are indicated as mean ± SE. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for GIPR−/− mice vs. GIPR+/+.
To determine the cause of the glucose intolerance of GIPR−/− mice, we measured the plasma insulin levels. Fasting insulin levels in GIPR−/− mice were identical to those in GIPR+/+ mice. In contrast, insulin levels in GIPR−/− mice were significantly lower than in GIPR+/+ mice 15 min after glucose challenge (Fig. 3C, 641 ± 54 pg/ml vs. 1,101 ± 68 pg/ml, P < 0.001). Because the islets isolated from GIPR−/− mice preserve a normal response to glucose in a dose-dependent manner (Fig. 1C), the decrease is not caused by impaired glucose-induced insulin secretion, but by disruption of the GIP entero-insular axis.
GIP Is Required for Compensatory Insulin Secretion.
Nutritional composition of the diet, especially in the case of high fat, causes insulin resistance and is associated with the development of diabetes mellitus (25). Because fat is a potent GIP secretagogue and plasma GIP levels are elevated under high-fat feeding (26, 27), we investigated the pathophysiological role of GIP in these conditions.
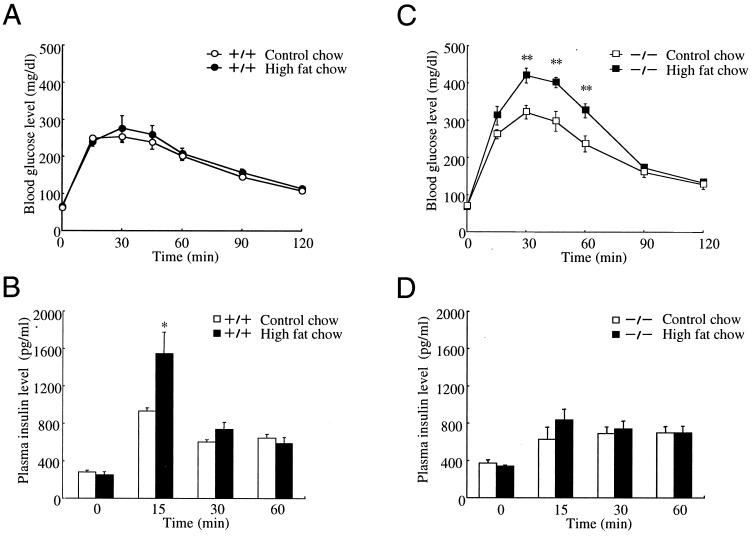
GIPR+/+ and GIPR−/− mice were fed high-fat chow for 3 weeks. Analysis of mice on control and high-fat diet showed no significant differences in body weight (high fat 22.8 ± 0.6 g vs. control 21.7 ± 0.8 g in GIPR+/+, high fat 21.1 ± 1.0 g vs. control 22.6 ± 0.3 g in GIPR−/−). In GIPR+/+ mice, plasma glucose levels (peak levels, 275 ± 34 mg/dl vs. 253 ± 16 mg/dl at 30 min) remained unchanged and plasma insulin levels (peak levels, 1564 ± 229 pg/ml vs. 931 ± 36 pg/ml at 15 min, P < 0.05) were increased, compared with normal chow-fed GIPR+/+ mice after meal ingestion (Fig. 4 A and B). On the other hand, the high-fat diet further increased plasma glucose levels (peak levels, 419 ± 20 mg/dl vs. 322 ± 18 mg/dl at 30 min, P < 0.01) without a significant enhancement of insulin secretion (peak levels, 831 ± 118 pg/ml vs. 627 ± 128 pg/ml at 15 min) after meal ingestion in GIPR−/− mice (Fig. 4 C and D).
Figure 4.
Effects of diet on blood glucose levels and plasma insulin levels after meal ingestion. GIPR+/+ mice (A and B) and GIPR−/− mice (C and D) were fed control diet (open symbols) or high fat diet (closed symbols). Blood glucose levels (A and C) and plasma insulin levels (B and D) were measured after meal ingestion. Values are indicated as mean ± SE. *, P < 0.05; **, P < 0.01.
Discussion
GIPR−/− mice show normal glucose tolerance after i.p. administration of glucose and glucose intolerance accompanied with impaired insulin secretion after oral administration of glucose. Glucose-induced insulin secretion from isolated pancreatic islets is preserved in GIPR−/− mice in vitro. These results show the important role of GIP as incretin, and that insulin secretion from the pancreatic β cells is regulated not only by glucose but also by GIP, especially in the early phase after glucose ingestion.
It is well known that the early insulin response after oral glucose challenge is decreased in type 2 diabetic patients (28), and that disorders in glucose metabolism, especially in the glycerol phosphate shuttle, can cause decreased glucose-induced insulin secretion from pancreatic β cells of type 2 diabetic animals (29). In the present study, we find that the early insulin secretion after oral glucose ingestion is impaired in GIPR−/− mice, whereas the insulin response to glucose is preserved, in a dose-dependent manner, in isolated pancreatic islets. Nauck et al. (19) have reported that GIP-induced insulin secretion is selectively impaired in type 2 diabetes. We examined mutations in the GIPR gene of type 2 diabetic patients and found a missense mutation (G198C) that causes a decreased response of cAMP to GIP (9), suggesting that an impaired GIP/insular axis is involved in the impaired early insulin response to oral glucose load that is characteristic of type 2 diabetes.
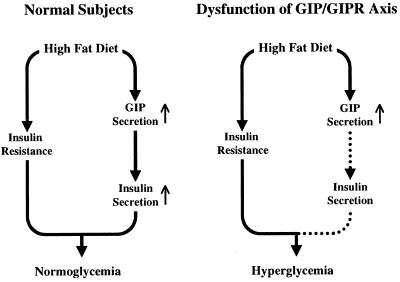
It generally is accepted that glucose homeostasis in the insulin-resistant state is maintained because of higher secretion of insulin (30) and that high-fat feeding leads to insulin resistance (25). In GIPR+/+ mice, high-fat feeding did not alter the glucose levels, because of compensatory insulin secretion. On the contrary, in GIPR−/− mice, high-fat feeding did not increase the insulin level, and the glucose level further deteriorated after meal ingestion, indicating the absence of such compensating insulin secretion. A model linking insulin resistance and hyperinsulinemia via the entero-insular axis is shown in Fig. 5. In normal subjects (Fig. 5 Left), fat and carbohydrate promote GIP secretion from K cells in the upper portion of the gastrointestinal tract, and this amount of GIP leads to sufficient additional secretion of insulin from the pancreatic β cells to compensate for the insulin resistance, maintaining glucose homeostasis, consistently with previous studies showing elevated GIP levels in human and laboratory animals on a high-fat diet (26, 27). It is important to emphasize that high-fat intake can produce both insulin resistance and exaggerated GIP and insulin secretion, synergically creating additional obesity. In the case of disruption of the GIP/GIPR axis (Fig. 5 Right), however, GIP cannot stimulate insulin secretion from the pancreatic β cells, and glucose homeostasis deteriorates. Such disruption of the GIP/GIPR axis, as is observed in type 2 diabetic patients (19), may contribute to pathogenesis of diabetes.
Figure 5.
Schematic models for insulin resistance and insulin secretion induced by high-fat feeding. (Left) The model in normal subjects. Both insulin resistance and compensatory insulin secretion are induced by high-fat feeding. Blood glucose levels are kept normal. (Right) The model if the GIP/GIPR axis is disturbed. The insulin secretion is insufficient and glucose intolerance develops.
GIP and GLP-1 belong to the vasoactive intestinal peptide/glucagon/secretin family; secreted from the small intestine, they directly accelerate insulin secretion of the pancreatic β cells in a glucose-dependent manner (5). The GIP producing K cells are distributed in the antrum and upper portion of the small intestine, while the GLP-1 producing L cells are distributed in the lower portion of the small intestine. Both GIPR-deficient and GLP-1 receptor-deficient mice (14) show glucose intolerance after oral glucose loading. On the other hand, high-fat feeding does not alter glucose tolerance in GLP-1 receptor-deficient mice (31), while glucose homeostasis in GIPR-deficient mice does deteriorate. In addition, it has been demonstrated that the plasma insulin response to GLP-1 is preserved while the plasma insulin response to GIP is impaired in type 2 diabetes patients (19). These results indicate that, although GIP and GLP-1 both act as incretin physiologically, the GIP/insular axis may have an important role in the pathogenesis of type 2 diabetes mellitus, especially in insulin-deficient and insulin-resistant conditions.
Acknowledgments
We thank Drs. S. Seino and T. Miki (Chiba University) for technical advice on meal ingestion. This study was supported in part by Grants-in-Aid for Creative Basic Research (10NP0201) and Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan, a grant for “Research for the Future” Program from the Japan Society for the Promotion of Science (JSPS-RFTF97I00201), and a grant for Diabetic Research from Tsumura & Co., Japan.
Abbreviations
- GIP
gastric inhibitory polypeptide
- GIPR
GIP receptor
- GLP-1
glucagon-like peptide 1
References
- 1.Elrick H, Stimmler L, Hlad C J, Arai Y. J Clin Endocrinol Metab. 1964;24:1076–1082. doi: 10.1210/jcem-24-10-1076. [DOI] [PubMed] [Google Scholar]
- 2.Perley M J, Kipnis D M. J Clin Invest. 1967;46:1954–1962. doi: 10.1172/JCI105685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unger R H, Eisentraut A M. Arch Intern Med. 1969;123:261–266. [PubMed] [Google Scholar]
- 4.Creutzfeldt W. Diabetologia. 1979;16:75–85. doi: 10.1007/BF01225454. [DOI] [PubMed] [Google Scholar]
- 5.Pederson R A. In: Gut Peptides: Biochemistry and Physiology. Walsh J H, Dockray G J, editors. New York: Raven; 1994. pp. 217–260. [Google Scholar]
- 6.Takeda J, Seino Y, Tanaka K, Fukumoto H, Kayano T, Takahashi H, Mitani T, Kurono M, Suzuki T, Tobe T, Imura H. Proc Natl Acad Sci USA. 1987;84:7005–7008. doi: 10.1073/pnas.84.20.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inagaki N, Seino Y, Takeda J, Yano H, Yamada Y, Bell G I, Eddy R L, Fukushima Y, Byers M G, Shows T B, Imura H. Mol Endocrinol. 1989;3:1014–1021. doi: 10.1210/mend-3-6-1014. [DOI] [PubMed] [Google Scholar]
- 8.Yamada Y, Hayami T, Nakamura K, Kaisaki P J, Someya Y, Wang C Z, Seino S, Seino Y. Genomics. 1995;29:773–776. doi: 10.1006/geno.1995.9937. [DOI] [PubMed] [Google Scholar]
- 9.Thorens B. Proc Natl Acad Sci USA. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuda K, Inagaki N, Yamada Y, Kubota A, Seino S, Seino Y. Biochem Biophys Res Commun. 1994;205:1556–1562. doi: 10.1006/bbrc.1994.2844. [DOI] [PubMed] [Google Scholar]
- 11.Kubota A, Yamada Y, Hayami T, Yasuda K, Someya Y, Ihara Y, Kagimoto S, Watanabe R, Taminato T, Tsuda K, Seino Y. Diabetes. 1996;45:1701–1705. doi: 10.2337/diab.45.12.1701. [DOI] [PubMed] [Google Scholar]
- 12.Kubota A, Yamada Y, Yasuda K, Someya Y, Ihara Y, Kagimoto S, Watanabe R, Kuroe A, Ishida H, Seino Y. Biochem Biophys Res Commun. 1997;235:171–175. doi: 10.1006/bbrc.1997.6743. [DOI] [PubMed] [Google Scholar]
- 13.Fehmann H C, Goke R, Goke B. Endocr Rev. 1995;16:390–410. doi: 10.1210/edrv-16-3-390. [DOI] [PubMed] [Google Scholar]
- 14.Scrocchi L A, Brown T J, MaClusky N, Brubaker P L, Auerbach A B, Joyner A L, Drucker D J. Nat Med. 1996;2:1254–1258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 15.Elliott R M, Morgan L M, Tredger J A, Deacon S, Wright J, Marks V. J Endocrinol. 1993;138:159–166. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- 16.Nauck M A. Diabetologia. 1999;42:373–379. doi: 10.1007/s001250051165. [DOI] [PubMed] [Google Scholar]
- 17.Damholt A B, Buchan A M, Kofod H. Endocrinology. 1998;139:2085–2091. doi: 10.1210/endo.139.4.5921. [DOI] [PubMed] [Google Scholar]
- 18.Takemura J, Seino Y, Tsuda K, Seino S, Ikeda M, Sakurai H, Imura H. Endocrinol Jpn. 1981;28:17–21. doi: 10.1507/endocrj1954.28.17. [DOI] [PubMed] [Google Scholar]
- 19.Nauck M A, Heimesaat M M, Orskov C, Holst J J, Ebert R, Creutzfeldt W. J Clin Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mountford P, Zevnik B, Duwel A, Nichols J, Li M, Dani C, Robertson M, Chambers I, Smith A. Proc Natl Acad Sci USA. 1994;91:4303–4307. doi: 10.1073/pnas.91.10.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith A G. J Tissue Cult Methods. 1991;13:89–94. [Google Scholar]
- 22.Schwartzberg P L, Goff S P, Robertson E J. Science. 1989;246:799–803. doi: 10.1126/science.2554496. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto S, Ishida H, Kato S, Okamoto Y, Tsuji K, Mizuno N, Ueda S, Mukai E, Seino Y. Endocrinology. 1998;139:1133–1140. doi: 10.1210/endo.139.3.5771. [DOI] [PubMed] [Google Scholar]
- 24.Kato S, Ishida H, Tsuura Y, Tsuji K, Nishimura M, Horie M, Taminato T, Ikehara S, Odaka H, Ikeda I, Okada Y, Seino Y. J Clin Invest. 1996;97:2417–2425. doi: 10.1172/JCI118688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Storlien L H, Pan D A, Kriketos A D, Baur L A. Ann NY Acad Sci. 1993;683:82–90. doi: 10.1111/j.1749-6632.1993.tb35694.x. [DOI] [PubMed] [Google Scholar]
- 26.Hampton S M, Kwasowski P, Tan K, Morgan L M, Marks V. Diabetologia. 1983;24:278–281. doi: 10.1007/BF00282713. [DOI] [PubMed] [Google Scholar]
- 27.Morgan L M, Hampton S M, Tredger J A, Cramb R, Marks V. Br J Nutr. 1988;59:373–380. doi: 10.1079/bjn19880046. [DOI] [PubMed] [Google Scholar]
- 28.Porte D., Jr Diabetes. 1991;40:166–180. doi: 10.2337/diab.40.2.166. [DOI] [PubMed] [Google Scholar]
- 29.Tsuura Y, Ishida H, Okamoto Y, Kato S, Horie M, Ikeda H, Seino Y. Diabetologia. 1994;37:1082–1087. doi: 10.1007/BF00418371. [DOI] [PubMed] [Google Scholar]
- 30.Reaven G M. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 31.Scrocchi L A, Drucker D J. Endocrinology. 1998;139:3127–3132. doi: 10.1210/endo.139.7.6092. [DOI] [PubMed] [Google Scholar]