Interest in the interplay between mind and body is deep-rooted. Written accounts in Western society are found as far back as the second century, when the physician Galen remarked that cancer seemed to occur more frequently in melancholic (depressed) than sanguine (happy, spirited) women [1]. In traditional Eastern medicine, mind and body have been considered entwined for centuries, and this attitude continues to today (see [2] for an overview). Relative to these examples, it is only recently that scientific inquiry has taken an interest in the mind-body connection. The past three decades have witnessed an explosion of discoveries in this area, and we have seen psychological factors related to physical ailments from asthma to heart disease [3–9]. The present review focuses on just one aspect of mindbody interaction, but one with far-reaching effects. We review evidence for the relationship between psychological stress and immune function. Using a conceptual model, we review psychological, behavioral, and physiological responses to stressful events before entering into a more in-depth discussion of the potential role of subjective experiences of stress.
The Model
Stressors can produce profound health consequences. In one epidemiological study, for example, all-cause mortality increased in the month following a severe stressor – the death of a spouse [10]. Theorists propose that stressful events trigger cognitive and affective responses which, in turn, induce sympathetic nervous system and endocrine changes, and these ultimately impair immune function. [7, 11–14]. Potential health consequences are broad, but include rates of infection [15, 16], HIV progression [17, 18], and cancer incidence and progression [10, 19, 20]. Figure 1 depicts the major steps in this hypothesized chain of events, along with their mediators and moderators. Each step in this model (i.e. stressor, psychological stress response, physiological stress response, immune changes, and disease processes) is hypothesized to be causal. Individual difference factors (e.g. personality) moderate the effect of stressors on psychological stress response.
Figure 1.

Hypothesized pathway by which stress affects immune function and, ultimately, disease processes. NB: CNS = central nervous system; HPA = hypothalamic-pituitary-adrenal; SAM = sympathetic-adrenal-medullary.
This literature is rapidly developing. The purpose of this review is to highlight one aspect which promises to be of great interest in the coming years: the role of individual differences in the subjective experience of stress and their implications for immune function. As we will describe, subjective stress is often implicitly considered in this literature but rarely explicitly studied. In fact, data supporting the causal role of subjective stress in immune change are surprisingly weak.
Stressors and the Psychological Stress Response
Stressors and the stress response are distinct concepts. Stress is classically defined as a perturbation of the normal homeostasis of the body that results when environmental demands exceed a person’s perceived resources to meet those demands [21]. More recent theorists have introduced a broader view of stress involving allostasis, or “stability through change.” This view conceptualizes bodily systems as existing in a state of fluidity that is responsive to environmental demands. Repeated demands, however, tax the body’s ability to respond and return to normal, producing “wear and tear” or “allostatic load [22]. In both theories, an environmental demand (the stressor) precedes one’s reaction to the demand (the stress response).
Theories suggest that the principal aspect of the psychological stress response is cognitive - the event must be perceived as stressful. Lazarus and Folkman’s [21] model of stress and coping proposes that, in response to a potentially stressful stimulus, an individual appraises the threat value of the stimulus (primary appraisal), as well as his or her capacity to respond to the stimulus (secondary appraisal) [21], and this appraisal process determines the type, direction, and intensity of the stress-related emotions (i.e. anxiety, anger, guilt, sadness, shame, envy, and disgust; [23]). Appraisals vary based on individual differences such as personality traits, past experience with the stimulus, and perceived self-efficacy [21, 23, 24]. Thus, stress responses can vary, even when the precipitating events are similar.
Although responses vary, most investigations examine stress by studying stressors, which are circularly defined as events which most people would find stressful. Indeed, data confirm that, on average, people undergoing divorce, bereavement, family caregiving, academic examinations, daily hassles, and financial hardship report greater distress than those not experiencing these stressors [25–32]. Consistent with theory, data also show that individuals vary in their subjective reactions to stress, and this variation is often based on individual differences in personality, coping, self efficacy, and social characteristics [21, 23, 24, 33–42].
The Behavioral Stress Response
In addition to cognitive and emotional effects, stressors can promote behavioral change. Although there are some exceptions, people generally pursue less healthy behaviors when under stress, and these effects are seen whether “stress” is defined as the presence of stressful events or perceptions of stress. For example, people reporting greater perceived stress are likely to exercise for less time on fewer days, report lower self-efficacy for meeting exercise goals, and report feeling less satisfied with their exercise [43, 44], and these effects are particularly strong among older adults [45]. Stress is also related to sleep difficulties. It is not only the leading cause of temporary insomnia, but lack of sleep may also be a source of stress [46]. Stressors such as job demands and lack of control at work correlate with insomnia, sleep deprivation, and daytime fatigue [47].
Stress is not only associated with diminished health-promoting behaviors, but also with increased health-damaging behaviors. Alcohol intake is likely to increase when individuals are under stress [48–51]. Among students, controlling for daily coping, affect, and weekly drinking trends, participants drink more alcohol on days when perceived stress is high [52]. People under stress also smoke more cigarettes [53–55] and report that smoking cessation is more difficult [56, 57]. Finally, diet is also affected, as people typically eat more “fast food,” higher calorie foods, and more fat and sugar when under stress [58–61].
Like psychological responses to stress, behavioral responses are moderated by individual differences. For example, use of alcohol in response to stress is moderated by gender (men are more likely to use alcohol to cope than women; [62]), coping style (individuals who cope through avoidance are likely drink in response to stress; [58]), and social support (people with high social support reduce alcohol intake during stress, while those with low support increase alcohol intake; [44]). Dietary choices are also moderated by individual differences. While most people increase caloric intake during periods of high perceived stress, this effect is strongly pronounced in those who typically restrain their intake (e.g. dieters; [63]), and others reduce caloric intake during stressful periods [64]. These behavioral changes could combine with the cognitive and emotional effects of stressors to affect immune function.
The Physiological Stress Response
Cognitive and emotional events cannot influence immune function directly. Instead, stress is thought to affect immune function through central nervous system control of the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic-adrenalmedullary (SAM) axis (for reviews, see [12, 65–67]). These axes are illustrated in Figure 2. Briefly, the HPA axis is activated when stress-related sensory signals are processed in the paraventricular nucleus of the hypothalamus, triggering release of corticotropin-releasing hormone (CRH). This hormone stimulates the release of peptides from the pituitary, most notably adrenocorticotropin hormone (ACTH) and ß-endorphin. ACTH, in turn, induces the release of glucocorticoids (cortisol in humans) from the adrenal cortex.
Figure 2.
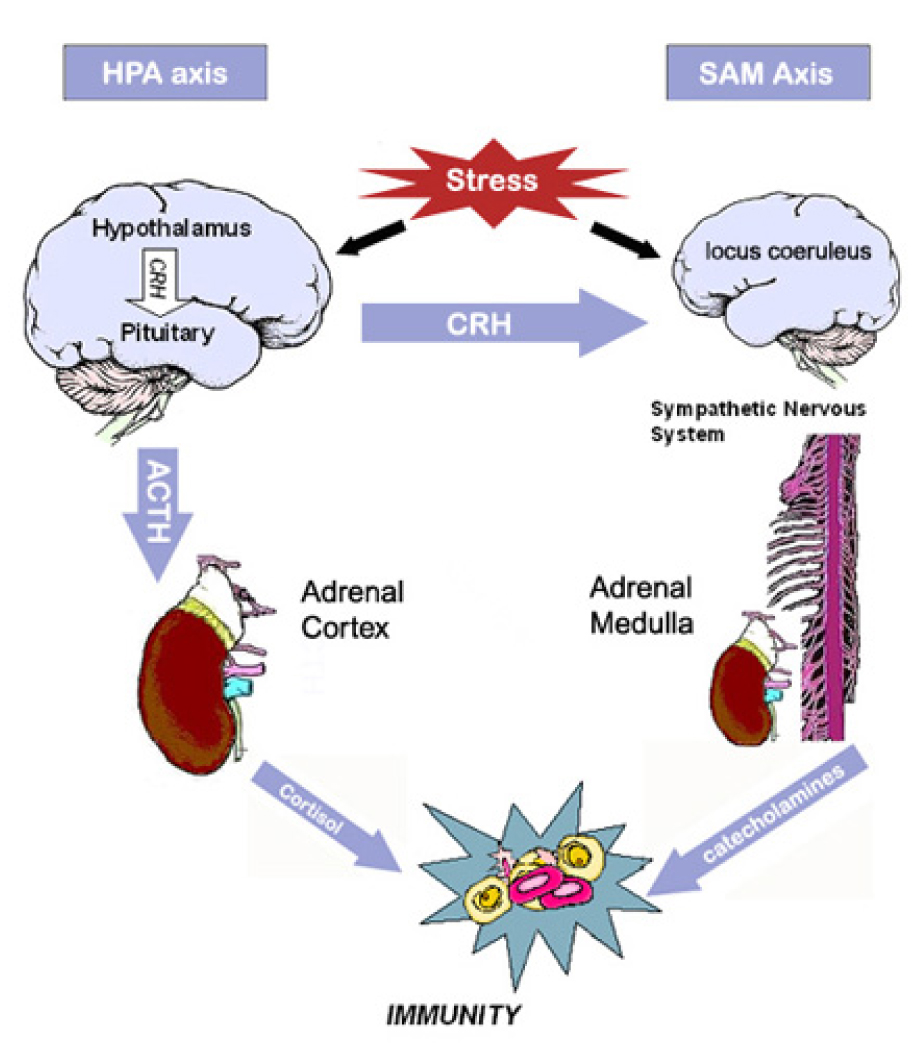
Main components of the hypothalamic-pituitary-adrenal (HPA) and sympatheticadrenal-medullary (SAM) axes. NB: CRH=corticotropin releasing hormone; ACTH= adrenocorticotropin hormone.
Activation of the SAM axis begins with the processing of stress-related sensory signals in the locus coeruleus of the pons. Release of CRH from the hypothalamus further contributes to activation of the SAM axis. Sympathetic nerve fibers trigger release of catecholamines (norepinephrine and epinephrine) into the bloodstream by the adrenal medulla, and peripheral sympathetic nerve fibers release additional norepinephrine. This axis produces the classic “fight or flight” response, characterized by increased heart rate and respiration and a redirection of blood flow from the digestive organs to the skeletal muscles.
Hormones released from the HPA and SAM axes can affect immune function. Glucocorticoids are believed to have strong effects on the immune system, but catecholamines, CRH, and opioids also play a role [68]. Although the specific modes of action are not fully understood, some immune cells, most notably lymphocytes and macrophages, express receptors that are responsive to high levels of circulating glucocorticoids [67]. Glucocorticoids can directly suppress the actions of T lymphocytes and macrophages and may affect cell trafficking and circulation [65, 66]. Further, glucocorticoids affect the production and release of cytokines. When circulating glucocorticoids levels are elevated, T-cell production of pro-inflammatory cytokines, such as interleukin-2 and interferon-γ, is suppressed. These altered cytokines, in turn, exert influence on multiple aspects of immune function, including cell cytotoxicity, proliferation, and secretion of additional regulatory cytokines [65].
Lymphocytes and macrophages also express catecholamine receptors. Norepinephrine and epinephrine can affect the production of cytokines in vitro, suppressing interleukin-12 and enhancing interleukin-10. Such an imbalance could increase an individual’s risk for viral infection and tumor growth. Further, these alterations in cytokine secretions combine with CRH to impair the functioning of NK cells [69].
The Behavioral Stress Response and Immunity
In addition to CNS and endocrine effects, stress-related behavioral changes can have immune consequences. As noted above, stressed individuals have poorer sleep, exercise, and dietary habits, and they may increase drug and alcohol use. While relatively little is known about the immune effects of such behaviors, some evidence suggests that better health behaviors yield better immune functioning. First, exercise is thought to have protective effects on immune function, especially among older adults [70, 71]. In fact, a recent randomized study shows exercise to improve healing times in experimentally-produced wounds [72]. Second, dissatisfaction with sleep correlates with lower numbers and poorer functioning of immune cells [73– 75]. Third, although moderate amounts of alcohol intake appear to have beneficial effects on immune function, high alcohol intake has been shown to be detrimental to immune function [76]. Interestingly, health behaviors may even moderate the physiological consequences of stress. Exercise has been shown to buffer the effects of stress [45, 77], while smoking intensifies them [78].
Stressors and Immunity
The model presented in Figure 1 depicts the psychological stress response (perceived stress, distress) as the mediator in the relationship between stressors and immunity. Although most researchers would characterize subjective stress as an important element, the majority of studies examining the physiological effects of stress have studied stressors rather than the stress response. That is, rather than assessing whether individuals perceive that their circumstances are stressful, researchers identify an event that most people would find stressful and assess immune function in relation to it. Research designs include repeated measures designs, which compare a group’s immune function during “stressed” and “nonstressed” time periods (e.g. during academic examinations vs. during summer break), and case-control designs, which compare groups experiencing a stressor (e. g. caregiving) to those not experiencing the stressor. Among studies comparing humans within “stress” and “non-stress” conditions, both laboratory and naturalistic stressors have been examined. The former permit greater experimental control, whereas the latter are associated with greater stimulus impact. Acute laboratory stressors (e.g. speech task, problem-solving task) are usually short (less than 30 minutes), and an experimental design is used. Naturalistic stressors include brief (e. g. academic examinations, natural disasters, space flight) and chronic stressors (e.g. bereavement, caregiving, job-related stress).
Immune outcomes are typically enumerative or functional analysis of immune cells (such as B and T cells) and immune substances (such as antibody and cytokines). Natural killer (NK) and T lymphocytes have received the most study, as these measures have been in use for decades and have clinical relevance. T lymphocytes are considered part of the specific (or acquired) immune system, because they demonstrate “memory,” showing an enhanced response upon second presentation of an antigen [79]. They are divided into three classes: (a) T-helper cells, which are identified by their expression of the CD4 marker, are involved in the initial recognition of a foreign protein (an antigen); (b) T-cytotoxic cells, which express the CD8 marker, have the ability to lyse cells to which they bind; and (c) T suppressor cells primarily inhibit the action of other T-cells. Natural Killer cells are a distinct class of lymphocytes that are considered part of the non-specific (or natural) immune system. They can lyse both allogenic (non-self) and autologous (self) cells without prior sensitization [80], producing a rapid response to initial infection. Additional immune outcomes are chosen for their clinical relevance. These outcomes include antibody response to in vivo vaccine, susceptibility to cold viruses, and wound healing [15, 81, 82].
Stressors produce reliable immune changes. Meta-analytic reviews have summarized results from the large number of studies using the "stress/non-stress" paradigm [83–85], revealing consistent immune changes in the presence of psychological stressors. In the most recent review, Segerstrom and Miller [85] analyzed different types of stressors separately and found that the immunological effect of stressors depends on their duration (as has been noted by others, see [83, 88]). Distinct data patterns were found with acute (<30 minutes), brief naturalistic (days or weeks), and chronic stressors (years).
First, Segerstrom and Miller [85] identified 85 studies which employed acute, laboratory stressors (e.g. public speaking). These stressors appear to elicit redistribution of immune cells [89]. The most prominent effect observed is an increase in numbers of natural killer cells in peripheral blood with accompanying increases in NK cell cytotoxicity assays (although per cell NK cytotoxicity is not seen to increase). Coincident increases were also seen in numbers of T-cytotoxic cells, neutrophils, and large granular lymphocytes in peripheral blood. As the time frame in these studies is too short for production of new immune cells, increases of circulating cells are likely due to their movement from other areas. In addition to cell redistribution, impaired cellular immunity was observed. T cell function (proliferative response to mitogens) was diminished with acute stressors. Researchers have speculated that changes in response to acute stress are an aspect of the adaptive “fight or flight” response, and thus generally beneficial [88]. These changes have been interpreted as a shift away from specific immunity (e.g. T cell function) toward natural immunity (e.g. NK cells, neutrophils; [85]), and this shift toward the faster-acting natural immunity mechanisms might yield improved response to physical injury.
The second pattern, seen in response to brief naturalistic stressors (e.g. academic examinations), contrasts with acute stressors [85]. Specifically, meta-analysis of 63 studies of brief stressors showed no reliable changes in numbers of circulating white blood cells. Instead, consistent reductions in T cell proliferation and NK cell cytotoxicity were seen. Additionally, the cytokine profile changed, with increases in interleukin-6, interleukin-10, and decreases in interferon-γ. These effects are consistent with a shift away from cellular (Th1) immunity toward humoral (Th2) immunity [85, 90]. The third pattern, seen in chronic stressors (e.g. caregiving), implies broad immune impairment. Meta-analysis of 23 studies suggested that both natural and specific immunity are impaired. Reductions are seen in NK cell cytotoxicity, T cell proliferation, and antibody response to influenza vaccination.
Despite differences in the immune results with acute, brief, and chronic stressors, the mechanisms by which stressors affect immune function are believed to be similar. Specifically, perception of stress triggers the activation of the HPA and SAM axes, initiating hormonal influence on immune systems. The detrimental effects of chronic stress have been explained as the overuse of systems whose function is to address short term threats (c.f., “allostatic load”; [88]). It has been observed that chronic stress reduces the flexibility of the immune system to adjust to new challenges (see [91], for a discussion). For example, animals that have been chronically stressed showed decreased HPA responsiveness to novel stressors [92]. This inflexibility could potentially lead to poorer immune response to challenge across a variety of situations (e.g. T cell proliferation, response to immunization).
Interpretation of the literature comparing “stress” to “non-stress” conditions involves the implicit consideration of subjective stress. As noted above, events are chosen for study based on the researcher’s judgment that most people would find them stressful. Indeed, some studies have measured perceived stress or distress and confirmed that, on average, participants report greater subjective stress when in the “stress” condition (e.g., [93, 94]). This literature is therefore naturally interpreted to show that subjective stress (a feature which all the events have in common) produces immune change.
Although all the events under study share the property of being stress-producing, all the participants do not necessarily share the property of experiencing stress. There may be some participants included in the studies who would not consider the event stressful (and for them the event would not be a “stressor”). Despite this possibility, the data show that, on average, people do consider the events stressful and, on average, they show immune change. What these studies do not tell us, however, is whether perception that an event is stressful is necessary for immune change.
Psychological Moderators of the Stress Response
Additional studies that implicitly consider subjective stress are those investigating individual differences (e.g. personality, coping) and the stress response. As described above, ample research shows that individual differences moderate people’s psychological reactions to stressors. In addition, some evidence shows that individual differences moderate people’s physiological reactions to stressors, including their immune responses.
Personality factors can moderate immune response to stress. For example, men who are high in hostility show greater change in blood pressure, cortisol, and NK cell cytotoxicity when discussing a marital problem with their wives as compared to nonhostile men [95]. In an investigation of naturally-occurring acute and chronic stressors, optimistic women showed little immune change (NK cell cytotoxicity) after acute stressors, while showing large immune changes after chronic stressors [96]. Additional personality traits have shown cross-sectional correlations with immunity (i.e. repression, defensiveness, introversion, pessimistic attributional style, and trait anxiety; [97–102].
Coping can also moderate the immune response to stress. For example Stowell and colleagues [103] showed that active coping was associated with better T cell function when stress was high, while avoidance coping appeared beneficial when stress was low. Other studies confirm the relationship between coping efforts and immune outcomes [41, 104–106].
Researchers speculate that personality and coping moderate immune function by affecting subjective stress (as depicted in Figure 1). For example, hostile individuals may perceive more threat (i.e., primary appraisal [21]) and experience greater anger in response to conflict than non-hostile people. Coping could also affect the appraisal process. Effective copers might have high expectations of their ability to respond to the event (i.e. secondary appraisal), and this might reduce anxiety. One study explicitly tested the hypothesis that personality could affect immunity via subjective stress. In a study in which all participants were undergoing a stressor (entering law school), Segerstrom and colleagues [107] measured optimism, perceived stress, mood, and immune outcomes on two occasions. Optimism was, indeed, related to immunity (T-helper cell counts and NK cell cytotoxicity). Further, the hypothesized pathway was supported. Mood partially accounted for the relationship between optimism and T-helper cell counts, while perceived stress partially accounted for the relationship between optimism and NK cell cytotoxicity. Thus, as depicted in Figure 1, it is plausible that personal characteristics ultimately affect the physiological stress response through their effects on subjective stress.
The Psychological Stress Response and Immunity
Examination of the psychological stress response, per se, can be accomplished through participants’ self-reports. Questionnaires of subjective stress (perceived stress, emotional distress) can be correlated with immune measures, and this can be done in samples in which all participants are undergoing a stressor or in the general population. Subjective stress is also incorporated into “checklist” methods of assessing stress. Participants are asked to recall stressful events they have experienced. Typically, they are presented with a list of events and asked to identify which events they have experienced within the past year and how upsetting they found them to be. In contrast to studies in which the researcher identifies the stressor, this design has the advantage that the events under study are identified as stressors by the participants. Experimental methods of investigating subjective stress are less common, but have been employed. Perceptions of stress may be manipulated through stress protocols or through clinical interventions designed to alleviate stress.
The model outlined in Figure 1 implies that subjective stress (perceived stress and distress) will be more closely related to immune outcomes than are the events themselves. However, the observed relationships between subjective stress and immunity are surprisingly weak. Experimental manipulations of perceived stress, self-reports of subjective stress, participant-identified stressors, and clinical interventions have all yielded mixed results in determining the role of subjective stress in affecting immunity.
No experimental study has attempted to manipulate perceptions of stress (independent of the presence of a stressor) to affect immune response; however, one experimental study showed that manipulation of perceptions can affect cardiovascular response to stress. Tomaka and colleagues presented participants with one of two sets of instructions for a mental arithmetic stress task. One instructional set was intended to emphasize threat, while the other portrayed the task as a challenge [108]. As predicted, stronger cardiovascular reactions (heart rate, cardiac output, pre-ejection period, and total peripheral resistance) were seen in the threat condition. Because cardiovascular and immunologic responses to acute stress tend to coincide [109, 110], these results may be relevant for immune function. Other studies of acute laboratory stressors measured participants’ perceptions of the stressor, and these support the findings of Tomaka and colleagues. Although they did not manipulate stress perceptions, researchers observed larger physiological responses among participants who appraised the task as more overwhelming and less controllable [111, 112].
Correlational studies have investigated subjective stress and immunity in naturalistic stressors. Many significant relationships between perceptions of stress and immune outcomes have been reported, yet overall, these results are not robust. Perceived stress correlates with reduced neutrophil activity [113], impaired response to vaccines [114–116], increased susceptibility to the common cold [15], and lower pro-inflammatory cytokine production in wounds [117], [118]. Emotional distress has also been correlated with immune function. With greater distress, NK and T cell function are lower [119–121], and interleukin-6 (a predictor of future disability among older adults) is higher among spousal caregivers of Alzheimer’s patients [122]. The clinical relevance of distress is implied by the work of Jabaaij and colleagues [123], who showed that distress predicted response to a hepatitis vaccine.
Despite the promising results of correlational studies such as these, the true relationships between subjective stress and immune function are yet unknown. Metaanalysis of 21 studies in which global perceived stress was correlated with immunity in the general population showed no significant relationships [85]. Meta-analysis of 9 studies in which participants were experiencing a common traumatic stressor (e.g. cancer diagnosis [124]) showed only NK cell cytotoxicity to relate to subjective stress, and the effect was modest (r=−.15, p=.02). The reviewers cited limited numbers of studies and methodological issues as potential explanations for the null results.
The “checklist” method is a more common way to assess stress in relation to immunity. Like subjective reports of stress, checklist methods should theoretically show stronger relationships with immunity than do researcher-identified stressors, because the events are identified as stressful by the participants. However, like correlational studies of subjective stress, results are surprisingly weak. In fact, meta-analysis of 53 studies using this approach found no consistent relationship between life event checklists and immunity [85].
Finally, psychological interventions have sought to improve immune function by reducing subjective experiences of stress [125]. In fact, some psychological interventions have successfully improved both subjective stress and immune function in stressed populations [126–130]. Predictably, other interventions have not affected immune function [131–135], and overall, only relaxation interventions appear to have reliable effect on immune function [125]. Most importantly, psychological improvements may not coincide with immune improvements. While some interventions showed immunological benefits to correspond to psychological benefits (e.g. [136]), others have not (e.g. [127]). Further, some psychological interventions have demonstrated psychological improvements without affecting immunity (e.g. [137]), or immune improvement without affecting subjective stress (e.g. [138]).
The Ambivalent Role of Subjective Stress
How can we explain the fact that stressors are consistently related to immunity, but the hypothesized causal factor, perceived stress, is not? How do we explain the capacity of individual differences such as personality to modulate the physiological stress response, if the subjective experience of stress is irrelevant? Circumstantial evidence implies that subjective stress is an important factor in determining the physiological stress response, but studies that directly test the relationship do not confirm this.
While it may be true that there simply have not been enough studies examining this question [85], it is nevertheless surprising that results are not stronger among published studies. One consideration is the nature of self-reported data. These data can be influenced by individual differences in the ways people interpret and report their subjective experience. For example, some individuals may be prone to chronically under-report their subjective stress, others to over-report it. In addition, there may be persistent individual differences in tonic levels of immune measures [139]. Such individual differences could obscure the true relationships between subjective stress and immunity.
A longitudinal study offers the possibility of evaluating these confounding factors while also testing the effects of a stressor. Because each subject serves as his or her own control, differences between subjects can be differentiated from change within subjects over time. Three studies offer examination of subjective stress and immunity in a longitudinal design. Their results highlight the importance of change in subjective stress relative to absolute levels of stress. In the first study, Maes and colleagues measured students’ perceived stress and immune function at two time points, mid-semester and during the examination period [89]. They found that the difference in perceived stress across the two time-points correlated with the difference in immune cell counts and in the T-helper/suppressor ratio. Students whose perceived stress increased the most also showed the greatest change in immunity. The second study, by Stone and colleagues, employed multiple measurement time-points and found that students showed lower antibody titers to a harmless protein on days when their negative mood was high relative to days with low negative mood [140]. In the third, a Japanese investigation of the immune effects of confinement, change in mood over the confinement period predicted immune change, although overall level of mood did not. Two groups of 5 subjects each were confined for 10 days, and participants rated their mood daily. Participants were classified as “sensitive” or “non-sensitive” based on their mood change. Sensitive participants showed greater change in mood over the confinement period as well as greater immune change across all measures (percentages of granulocytes, natural killer cells, and CD69 positive cells) as compared with non-sensitive participants [141].
A second consideration with self-report data is its dependence on the participants’ ability to accurately rate their psychological state. Theorists have suggested that the cognitive processing required to produce a physiological stress response need not be conscious [14]. Participants might subconsciously appraise an event as threatening, yet be unable to report this appraisal. In particular, participants who use denial and repression to cope may be less aware of their subjective state. In fact, people who use denial or repressive coping report little psychological distress, yet their physiological response is consistent with participants reporting high stress [14, 142].
Conclusions
Conclusive evidence demonstrates that stressful events are related to immune change. For acute stressors, this relationship is causal, and it is reasonable to interpret causality from chronic stressors as well. The present review explores an aspect of the stress response with significant theoretical and clinical implications: subjective experiences of stress. Perceptions of stress and emotional distress are proposed to be the mechanisms by which events elicit immune change. In addition, subjective stress has been used to explain between-subject variability in the immunological stress response, and clinical researchers seek to mitigate the immunological consequences of stress by improving subjective stress. These interpretations persist despite modest support for an association between subjective stress and immune function.
Two potential solutions to this dilemma are explored. First, longitudinal data may better illuminate the role of subjective stress in immune function than do cross-sectional studies. A few data suggest that change in subjective stress is more relevant for immune function than are absolute levels [89, 140, 141], but more research is needed to establish this. Second, it is possible that the appraisal of events as stressful is not always accessible to the conscious mind [14]. Participants using repression or denial may not be aware of feelings of stress, yet may show stress-related physiological reactions. Consistent evaluation of such traits is needed to evaluate this possibility.
Clarification of the role of subjective stress in immune change has both theoretical and clinical implications. It will further our understanding of how stressful events modulate immune activity as well as identifying targets for psychological intervention. With these tools in hand, we become better equipped to improve the health and quality of life for those enduring chronic stress.
References
- 1.Dunn AJ. In: Psychoneuroimmunology: Introduction and general perspectives, in Stress, the Immune System, and Psychiatry. Leonard BE, Miller K, editors. New York: John Wiley & Sons Ltd.; 1996. [Google Scholar]
- 2.Daruna JH. Introduction to Psychoneuroimmunology. San Diego: Elsevier; 2004. [Google Scholar]
- 3.DeLongis A, Folkman S, Lazarus RS. The impact of daily stress on health and mood - Psychological and social resources as mediators. Journal of Personality and Social Psychology. 1988;54(3):486–495. doi: 10.1037//0022-3514.54.3.486. [DOI] [PubMed] [Google Scholar]
- 4.Frasure-Smith N. In-hospital symptoms of psychological stress as predictors of long-term outcome after acute myocardial infarction in men. American Journal of Cardiology. 1991;67(2):121–127. doi: 10.1016/0002-9149(91)90432-k. [DOI] [PubMed] [Google Scholar]
- 5.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 6.Adler NE, Matthews KA. Health psychology - Why do some people get sick and some stay well. Annual Review of Psychology. 1994;45:229–259. doi: 10.1146/annurev.ps.45.020194.001305. [DOI] [PubMed] [Google Scholar]
- 7.Cohen S, Herbert TB. Health Psychology: Psychological factors and physical disease from the perspective of human psychoneuroimmunology. Annual Review of Psychology. 1996;47:113–142. doi: 10.1146/annurev.psych.47.1.113. [DOI] [PubMed] [Google Scholar]
- 8.Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology. Annual Review of Psychology. 2002;53(1):83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- 9.Young CR, Welsh CJ. Stress, health, and disease. Cellscience. 2005;2(2):132–158. [Google Scholar]
- 10.Kaprio J, Koskenvuo M, Rita H. Mortality after bereavement: a prospective study of 95,647 widowed persons. American Journal of Public Health. 1987;77(3):283–287. doi: 10.2105/ajph.77.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992 Mar 4;267:1244–1252. [PubMed] [Google Scholar]
- 12.Glaser R, Kiecolt-Glaser JK. Handbook of Human Stress and Immunity. San Diego: Academic Press; 1994. [Google Scholar]
- 13.McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Research Reviews. 1997;23(1–2):79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 14.Olff M. Stress, depression and immunity: the role of defense and coping styles. Psychiatry Research. 1999 Jan 18;85:7–15. doi: 10.1016/s0165-1781(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 15.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. The New England Journal of Medicine. 1991;325(9):606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 16.Cohen S, Williamson GM. Stress and infectious disease in humans. Psychological Bulletin. 1991;109(1):5–24. doi: 10.1037/0033-2909.109.1.5. [DOI] [PubMed] [Google Scholar]
- 17.Leserman J, Jackson ED, Petitto JM, Golden RN, Silva SG, Perkins DO, Cai J, Folds JD, Evans DL. Progression to AIDS: the effects of stress, depressive symptoms, and social support. Psychosomatic Medicine. 1999;61(3):397–406. doi: 10.1097/00006842-199905000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Leserman J, Petitto JM, Golden RN, Gaynes BN, Gu H, Perkins DO, Silva SG, Folds JD, Evans DL. Impact of stressful life events, depression, social support, coping, and cortisol on progression to AIDS. The American Journal of Psychiatry. 2000;157(8):1221–1228. doi: 10.1176/appi.ajp.157.8.1221. [DOI] [PubMed] [Google Scholar]
- 19.Andersen BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. American Psychologist. 1994;49(5):389–404. doi: 10.1037//0003-066x.49.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiecolt-Glaser JK, Glaser R. Psychoneuroimmunology and cancer: fact or fiction? European Journal of Cancer. 1999;35:1603–1607. doi: 10.1016/s0959-8049(99)00197-5. [DOI] [PubMed] [Google Scholar]
- 21.Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984. [Google Scholar]
- 22.McEwen BS. Protective and damaging effects of stress mediators. The New England Journal of Medicine. 1998 Jan 15;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 23.Lazarus RS. Coping theory and research: Past, present, and future. Psychosomatic Medicine. 1993;55(3):234–247. doi: 10.1097/00006842-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Bandura A. Self-efficacy: The exercise of control. New York: W.H. Freeman; 1997. [Google Scholar]
- 25.Holmes TH, Rahe RH. The social readjustment rating scale. Journal of Psychosomatic Research. 1967;11(2):213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 26.Miller MA, Rahe RH. Life changes scaling for the 1990s. Journal of Psychosomatic Research. 1997;43(3):279–292. doi: 10.1016/s0022-3999(97)00118-9. [DOI] [PubMed] [Google Scholar]
- 27.Folkman S. Positive psychological states and coping with severe stress. Social Science and Medicine. 1997;45(8):1207–1221. doi: 10.1016/s0277-9536(97)00040-3. [DOI] [PubMed] [Google Scholar]
- 28.Martin JL, Dean L. Effects of AIDS-related bereavement and HIV-related illness on psychological distress among gay men - A 7-year longitudinal study 1985–1991. Journal of Consulting and Clinical Psychology. 1993;661(1):94–103. doi: 10.1037//0022-006x.61.1.94. [DOI] [PubMed] [Google Scholar]
- 29.Horwitz AV, White HR, Howell-White S. The use of multiple outcomes in stress research: A case study of gender differences in responses to marital dissolution. Journal of Health and Social Behavior. 37(3):278–291. [PubMed] [Google Scholar]
- 30.Hope S, Power C, Rodgers B. Does financial hardship account for elevated psychological distress in lone mothers? Social Science and Medicine. 1999;49(12):1637–1649. doi: 10.1016/s0277-9536(99)00251-8. [DOI] [PubMed] [Google Scholar]
- 31.Kinney JM, Stephens MAP. Caregiving hassles scale - assessing the daily hassles of caring for a family member with dementia. Gerontologist. 1989;29(3):328–332. doi: 10.1093/geront/29.3.328. [DOI] [PubMed] [Google Scholar]
- 32.Anderson CS, Linto J, Stewart-Wynne EG. A population-based assessment of the impact and burden of caregiving for long-term stroke survivors. Stroke. 1995;26(5):843–849. doi: 10.1161/01.str.26.5.843. [DOI] [PubMed] [Google Scholar]
- 33.Gerits P, De Brabander B. Psychosocial predictors of psychological, neurochemical and immunological symptoms of acute stress among breast cancer patients. Psychiatry Research. 1999;85(1):95–103. doi: 10.1016/s0165-1781(98)00136-x. [DOI] [PubMed] [Google Scholar]
- 34.Cvengros JA, Christensen AJ, Lawton WJ. Health Locus of Control and Depression in Chronic Kidney Disease: A Dynamic Perspective. Journal of Health Psychology. 2005;10(5):677–686. doi: 10.1177/1359105305055320. [DOI] [PubMed] [Google Scholar]
- 35.Shelley M, Pakenham KI. External health locus of control and general self-efficacy: Moderators of emotional distress among university students. Australian Journal of Psychology. 2004;56(3):191–199. [Google Scholar]
- 36.Chan DW. Stress, self-efficacy, social support, and psychological distress among prospective Chinese teachers in Hong Kong. Educational Psychology. 2002;22(5):557–569. [Google Scholar]
- 37.Benight CC, Harper ML. Coping self-efficacy perceptions as a mediator between acute stress response and long-term distress following natural disasters. Journal of Traumatic Stress. 2002;15(3):177–186. doi: 10.1023/A:1015295025950. [DOI] [PubMed] [Google Scholar]
- 38.Benight CC, Freyaldenhoven RW, Hughes J, Ruiz JM, Zoschke TA, Lovallo WR. Coping self-efficacy and psychological distress following the Oklahoma City bombing. Journal of Applied Social Psychology. 2000;30(7):1331–1344. [Google Scholar]
- 39.Sharts-Hopco NC, Regan-Kubinski MJ, Lincoln PS, Heverly MA. Problem focused coping in HIV-infected mothers in relation to self-efficacy, uncertainty, social support, and psychological distress. IMAGE: Journal of Nursing Scholarship. 1996;28(2):107–111. doi: 10.1111/j.1547-5069.1996.tb01201.x. [DOI] [PubMed] [Google Scholar]
- 40.Clarke D. Neuroticism: Moderator or mediator in the relation between locus of control and depression. Personality and Individual Differences. 2004;37(2):245–258. [Google Scholar]
- 41.Molassiotis A, Van Den Akker OBA, Milligan DW, Goldman JM. Symptom distress, coping style and biological variables as predictors of survival after bone marrow transplantation. Journal of Psychosomatic Research. 1997;42(3):275–285. doi: 10.1016/s0022-3999(96)00298-x. [DOI] [PubMed] [Google Scholar]
- 42.Lackner JB, Joseph JG, Ostrow DG, Kessler RC, Eshleman S, Wortman CB, O'Brien K, Phair JP, Chmiel J. A longitudinal study of psychological distress in a cohort of gay men: Effects of social support and coping strategies. Journal of Nervous and Mental Disease. 1993;181(1):4–12. doi: 10.1097/00005053-199301000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Stetson BA, Rahn JM, Dubbert PM, Wilner BI, Mercury MG. Prospective evaluation of the effects of stress on exercise adherence in community-residing women. Health Psychology. 1997;16(6):515–520. doi: 10.1037//0278-6133.16.6.515. [DOI] [PubMed] [Google Scholar]
- 44.Steptoe A, Wardle J, Pollard TM, Canaan L. Stress, social support and health-related behavior: A study of smoking, alcohol consumption and physical exercise. Journal of Psychosomatic Research. 1996;41(2):171–180. doi: 10.1016/0022-3999(96)00095-5. [DOI] [PubMed] [Google Scholar]
- 45.Zuzanek J, Robinson JP, Iwasaki Y. The relationships between stress, health, and physically active leisure as a function of life-cycle. Leisure Sciences. 1998;20(4):253–275. [Google Scholar]
- 46.Rosch PJ. Stress and sleep: Some startling and sobering statistics. Stress Medicine. 1996;12(4):207–210. [Google Scholar]
- 47.Kalimo R, Tenkanen L, Harma M, Poppius E, Heinsalmi P. Job stress and sleep disorders: Findings from the Helsinki Heart Study. Stress Medicine. 2000;16(2):65–75. [Google Scholar]
- 48.Johnstone BM, Garrity TF, Straus R. In: The relationship between alcohol and life stress, in Clinical Disorders and Stressful Life Events. Miller TW, editor. Madison, CT: International Universities Press, Inc; 1997. pp. 247–279. [Google Scholar]
- 49.Russell M, Cooper L, Frone MR, Peirce RS. A longitudinal study of stress, alcohol, and blood pressure in community-based samples of blacks and non-blacks. Alcohol Research and Health. 1999;23(4):299–306. [PMC free article] [PubMed] [Google Scholar]
- 50.Frone MR. Work stress and alcohol use. Alcohol Research and Health. 1999;23(4):284–291. [PMC free article] [PubMed] [Google Scholar]
- 51.Armeli S, Todd M, Mohr C. A daily process approach to individual differences in stress-related alcohol use. Journal of Personality. 2005;73(6):1–30. doi: 10.1111/j.0022-3506.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- 52.Park CL, Armeli S, Tennen H. The daily stress and coping process and alcohol use among college students. Journal of Studies on Alcohol. 2004;65(1):126–135. doi: 10.15288/jsa.2004.65.126. [DOI] [PubMed] [Google Scholar]
- 53.Posner I, Leitner LA, Lester D. Diet, cigarette smoking, stressful life events, and subjective feelings of stress. Psychological Reports. 1994;74(3):841–842. doi: 10.2466/pr0.1994.74.3.841. [DOI] [PubMed] [Google Scholar]
- 54.Niaura R, Shadel WG, Britt DM, Abrams DB. Response to social stress, urge to smoke, and smoking cessation. Addictive Behaviors. 2002;27(2):241–250. doi: 10.1016/s0306-4603(00)00180-5. [DOI] [PubMed] [Google Scholar]
- 55.Kouvonen A, Kivimaki M, Virtanen M, Pentti J, Vahtera J. Work stress, smoking status, and smoking intensity: An observational study of 46,190 employees. Journal of Epidemiology and Community Health. 2005;59(1):63–69. doi: 10.1136/jech.2004.019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westman M, Eden D, Shirom A. Job stress, cigarette smoking and cessation: The conditioning effects of peer support. Social Science & Medicine. 1985;20(6):637–644. doi: 10.1016/0277-9536(85)90402-2. [DOI] [PubMed] [Google Scholar]
- 57.Carey MP, Kalra DL, Carey KB, Halperin S, Richards CS. Stress and unaided smoking cessation: A prospective investigation. Journal of Consulting and Clinical Psychology. 1993;61(5):831–838. doi: 10.1037//0022-006x.61.5.831. [DOI] [PubMed] [Google Scholar]
- 58.Steptoe A, Lipsey Z, Wardle J. Stress, hassles and variations in alcohol consumption, food choice and physical exercise: A diary study. British Journal of Health Psychology. 1998;3(1):51–63. [Google Scholar]
- 59.Oliver G, Wardle J, Gibson L. Stress and food choice: A laboratory study. Psychosomatic Medicine. 2000;62(6):853–865. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 60.McCann BS, Warnick GR, Knopp RH. Changes in plasma lipids and dietary intake accompanying shifts in perceived workload and stress. Psychosomatic Medicine. 1990;52(1):97–108. doi: 10.1097/00006842-199001000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Weidner G, Kohlmann C-W, Dotzauer E, Burns LR. The effects of academic stress on health behaviors in young adults. Anxiety, Stress & Coping: An International Journal. 1996;9(2):123–133. [Google Scholar]
- 62.Horwitz AV, White HR, Howell-White S. The use of multiple outcomes in stress research: A case study of gender differences in responses to marital dissolution. Journal of Health and Social Behavior. 1996;37(3):278–291. [PubMed] [Google Scholar]
- 63.Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. Journal of Psychosomatic Research. 2000;48(2):195–202. doi: 10.1016/s0022-3999(00)00076-3. [DOI] [PubMed] [Google Scholar]
- 64.Oliver G, Wardle J. Perceived effects of stress on food choice. Physiology and Behavior. 1999;66(3):511–515. doi: 10.1016/s0031-9384(98)00322-9. [DOI] [PubMed] [Google Scholar]
- 65.Rabin B. Stress, Immune Function, and Health: The connection. New York: Wiley-Liss; 1999. [Google Scholar]
- 66.Reiche EMV, Nunes SOV, Morimoto HK. Stress, depression, the immune system, and cancer. The Lancet Oncology. 2004;5(10):617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 67.Padgett DA, Glaser R. How stress influences the immune response. Trends in Immunology. 2003;24(8):444–448. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- 68.Bellinger DL, ThyagaRajan S, Lorton D, Madden KS, Tran L, Felten DL. Psychoimmunology today: mechanisms mediating the effects of psychological status on the immune function. In: Lewis CE, O'Brien RM, Barraclough J, editors. The Psychoimmunology of Cancer. New York: Oxford University Press; 2002. pp. 3–99. [Google Scholar]
- 69.Irwin M, Hauger RL, Jones L, Provencio M, Britton KT. Sympathetic nervous system mediates central corticotropin-releasing factor induced suppression of natural killer cytotoxicity. The Journal of Pharmacology and Experimental Therapeutics. 1990;255(1):101–107. [PubMed] [Google Scholar]
- 70.Mackinnon L. Exercise and Immunology. Champaign, IL: Human Kinetics Books; 1992. [Google Scholar]
- 71.Venjatraman JT, Fernandes G. Exercise, immunity and aging. Aging. 1997;9(1–2):42–56. doi: 10.1007/BF03340127. [DOI] [PubMed] [Google Scholar]
- 72.Emery CF, Kiecolt-Glaser JK, Glaser R, Malarkey WB, Frid DJ. Exercise accelerates wound healing among healthy older adults: A preliminary investigation. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60:1432–1436. doi: 10.1093/gerona/60.11.1432. [DOI] [PubMed] [Google Scholar]
- 73.Savard J, Miller SM, Mills M, O'Leary A, Harding H, Douglas SD, Mangan CE, Belch R, Winokur A. Association between subjective sleep quality and depression on immunocompetence in low-income women at risk for cervical cancer. Psychosomatic Medicine. 1999;61(4):496–507. doi: 10.1097/00006842-199907000-00014. [DOI] [PubMed] [Google Scholar]
- 74.Irwin M, Mascovich A, Gillin JC, Willoughby R, Pike J, Smith TL. Partial sleep deprivation reduces natural killer cell activity in humans. Psychosomatic Medicine. 1994;56(6):493–498. doi: 10.1097/00006842-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 75.Savard J, Laroche L, Simard S, Ivers H, Morin CM. Chronic insomnia and immune functioning. Psychosomatic Medicine. 2003;65(2):211–221. doi: 10.1097/01.psy.0000033126.22740.f3. [DOI] [PubMed] [Google Scholar]
- 76.Diaz LE, Montero A, Gonzalez-Gross M, Vallejo AI, Romeo J, Marcos A. Influence of alcohol consumption on immunological status: a review. European Journal of Clinical Nutrition. 2002;56 Suppl 3:S50–S53. doi: 10.1038/sj.ejcn.1601486. [DOI] [PubMed] [Google Scholar]
- 77.Latimer AE, Martin Ginis KA, Hicks AL. Buffering the Effects of Stress on Well-Being Among Individuals with Spinal Cord Injury: A Potential Role for Exercise. Therapeutic Recreation Journal. 2005;39(2):131–138. [Google Scholar]
- 78.Byrne DG. Cigarette smoking, psychological stress, and cardiovascular arousal. Australian Journal of Psychology. 2000;52(1):1–8. [Google Scholar]
- 79.Janeway C. Immunobiology. New York: Garland Science; 2004. [Google Scholar]
- 80.Herberman RB, Ortaldo JR. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214:24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- 81.Cohen S, Frank E, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Types of stressors that increase susceptibility to the common cold in healthy adults. Health Psychology. 1998;17(3):214–223. doi: 10.1037//0278-6133.17.3.214. [DOI] [PubMed] [Google Scholar]
- 82.Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346(8984):1194–1196. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- 83.Herbert TB, Cohen S. Stress and immunity in humans: A meta-analytic review. Psychosomatic Medicine. 1993;55:364–379. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 84.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behavior and Immunity. 2001;15(3):199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 85.Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boucher N, Dufeu-Duchesne T, Vicaut E, Farge D, Effros RB, Schachter F. CD28 expression in T cell aging and human longevity. Experimental Gerontology. 1998;33(3):267–282. doi: 10.1016/s0531-5565(97)00132-0. [DOI] [PubMed] [Google Scholar]
- 87.Wikby A, Johansson B, Ferguson F, Olsson J. Age-related changes in immune parameters in a very old population of Swedish people: a longitudinal study. Experimental Gerontology. 1994;29(5):531–541. doi: 10.1016/0531-5565(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 88.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: A potential role for leukocyte trafficking. Brain, Behavior, and Immunity. 1997;11(4):286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 89.Maes M, Van Bockstaele DR, Gastel A, Song C, Schotte C, Neels H, DeMeester I, Scharpe S, Janca A. The effects of psychological stress on leukocyte subset distribution in humans: evidence of immune activation. Neuropsychobiology. 1999;39(1):1–9. doi: 10.1159/000026552. [DOI] [PubMed] [Google Scholar]
- 90.Paik IH, Toh KY, Lee C, Kim JJ, Lee SJ. Psychological stress may induce increased humoral and decreased cellular immunity. Behavioral Medicine. 2000;26(3):139–141. doi: 10.1080/08964280009595761. [DOI] [PubMed] [Google Scholar]
- 91.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Annals of the New York Academy of Sciences. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 92.Gadek-Michalska A, Bugajski J. Repeated handling, restraint, or chronic crowding impair the hypothalamic-pituitary-adrenocortical response to acute restraint stress. Journal of Physiology and Pharmacology. 2003;54(3):449–559. [PubMed] [Google Scholar]
- 93.Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. Stress, loneliness, and changes in herpesvirus latency. Journal of Behavioral Medicine. 1985;8(3):249–260. doi: 10.1007/BF00870312. [DOI] [PubMed] [Google Scholar]
- 94.Glaser R, Kiecolt-Glaser JK, Stout JC, Tarr KL, Speicher CE, Holliday JE. Stress-related impairments in cellular immunity. Psychiatry Research. 1985;16(3):233–239. doi: 10.1016/0165-1781(85)90111-8. [DOI] [PubMed] [Google Scholar]
- 95.Miller GE, Dopp JM, Myers HF, Stevens SY. Psychosocial predictors of natural killer cell mobilization during marital conflict. Health Psychology. 1999;18(3):262–271. doi: 10.1037//0278-6133.18.3.262. [DOI] [PubMed] [Google Scholar]
- 96.Cohen F, Kearney KA, Zegans LS, Kemeny ME, Neuhaus JM, Stites DP. Differential immune system changes with acute and persistent stress for optimists vs pessimists. Brain, Behavior, and Immunity. 1999;13(2):155–174. doi: 10.1006/brbi.1998.0531. [DOI] [PubMed] [Google Scholar]
- 97.Esterling BA, Antoni MH, Kumar M, Schneiderman N. Defensiveness, trait anxiety, and Epstein-Barr viral capsid antigen antibody titers in healthy college students. Health Psychology. 1993;12(2):132–139. doi: 10.1037//0278-6133.12.2.132. [DOI] [PubMed] [Google Scholar]
- 98.Fredrikson M, Furst CJ, Lekander M, Rotstein S, Blomgren H. Trait anxiety and anticipatory immune reactions in women receiving adjuvant chemotherapy for breast cancer. Brain Behavior and Immunity. 1993;7(1):79–90. doi: 10.1006/brbi.1993.1008. [DOI] [PubMed] [Google Scholar]
- 99.Jamner LD, Schwartz GE, Leigh H. The relationship between repressive and defensive coping styles and monocyte, eosinophile, and serum glucose levels: Support for the opioid peptide hypothesis of repression. Psychosomatic Medicine. 1988;50(6):567–575. doi: 10.1097/00006842-198811000-00002. [DOI] [PubMed] [Google Scholar]
- 100.Kamen-Siegel L, Rodin J, Seligman ME, Dwyer J. Explanatory style and cell-mediated immunity in elderly men and women. Health Psychology. 1991;10(4):229–235. doi: 10.1037//0278-6133.10.4.229. [DOI] [PubMed] [Google Scholar]
- 101.Shea JD, Burton R, Girgis A. Negative affect, absorption, and immunity. Physiology and Behavior. 1993;53(3):449–457. doi: 10.1016/0031-9384(93)90138-6. [DOI] [PubMed] [Google Scholar]
- 102.Zorrilla EP, Redei E, DeRubeis RJ. Reduced cytokine levels and T-cell function in healthy males: relation to individual differences in subclinical anxiety. Brain Behavior and Immunity. 1994;8(4):293–312. doi: 10.1006/brbi.1994.1028. [DOI] [PubMed] [Google Scholar]
- 103.Stowell JR, Kiecolt-Glaser JK, Glaser R. Perceived stress and cellular immunity: When coping counts. Journal of Behavioral Medicine. 2001;24:323–339. doi: 10.1023/a:1010630801589. [DOI] [PubMed] [Google Scholar]
- 104.Goodkin K, Blaney NT, Feaster D, Fletcher MA, Baum MK, Mantero-Atienza E, Klimas NG, Millon C, Szapocznik J, Eisdorfer C. Active coping style is associated with natural killer cell cytotoxicity in asymptomatic HIV-1 seropositive homosexual men. Journal of Psychosomatic Research. 1992;36(7):635–650. doi: 10.1016/0022-3999(92)90053-5. [DOI] [PubMed] [Google Scholar]
- 105.Goodkin K, Fuchs I, Feaster D, Leeka J, Rishel DD. Life stressors and coping style are associated with immune easures in HIV-1 infection--a preliminary report. International Journal of Psychiatry in Medicine. 1992;22(2):155–172. doi: 10.2190/BD46-F4JD-K8TW-RUFH. [DOI] [PubMed] [Google Scholar]
- 106.Endresen IM, Vaernes R, Ursin H, Tonder O. Psychological stress-factors and concentration of immunoglobulins and complement components in Norwegian nurses. Work and Stress. 1987;1(4):365–375. [Google Scholar]
- 107.Segerstrom SC, Taylor SE, Kemeny ME, Fahey JL. Optimism is associated with mood, coping, and immune change in response to stress. Journal of Personality and Social Psychology. 1998;74(6):1646–1655. doi: 10.1037//0022-3514.74.6.1646. [DOI] [PubMed] [Google Scholar]
- 108.Tomaka J, Blascovich J, Kibler J, Ernst JM. Cognitive and physiological antecedents of threat and challenge appraisal. Journal of Personality and Social Psychology. 1997;73(1):63–72. doi: 10.1037//0022-3514.73.1.63. [DOI] [PubMed] [Google Scholar]
- 109.Matthews KA, Caggiula AR, McAllister CG, Berga SL, Owens JF, Flory JD, Miller AL. Sympathetic reactivity to acute stress and immune response in women. Psychosomatic Medicine. 1995;57:564–571. doi: 10.1097/00006842-199511000-00009. [DOI] [PubMed] [Google Scholar]
- 110.Cohen S, Hamrick N. Stable individual differences in physiological response to stressors: implications for stress-elicited changes in immune related health. Brain Behavior and Immunity. 2003;17(6):407–414. doi: 10.1016/s0889-1591(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 111.Brosschot JF, Godaert GL, Benschop RJ, Olff M, Ballieux RE, Heijnen CJ. Experimental stress and immunological reactivity: a closer look at perceived uncontrollability. Psychosomatic Medicine. 1998;60(3):359–361. doi: 10.1097/00006842-199805000-00024. [DOI] [PubMed] [Google Scholar]
- 112.Wetherell MA, Hyland ME, Harris JE. Secretory immunoglobulin A reactivity to acute and cumulative acute multi-tasking stress: relationships between reactivity and perceived workload. Biological Psychology. 2004;66(3):257–270. doi: 10.1016/j.biopsycho.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 113.Ellard DR, Barlow JH, Mian R. Perceived stress, health status, and the activity of neutrophils in undergraduates over one academic year. Stress and Health. 2005;21(4):245–253. [Google Scholar]
- 114.Miller GE, Cohen S, Pressman S, Barkin A, Rabin BS, Treanor JJ. Psychological stress and antibody response to influenza vaccination: when is the critical period for stress, and how does it get inside the body? Psychosomatic Medicine. 2004;66(2):215–223. doi: 10.1097/01.psy.0000116718.54414.9e. [DOI] [PubMed] [Google Scholar]
- 115.Burns VE, Drayson M, Ring C, Carroll D. Perceived stress and psychological well-being are associated with antibody status after meningitis C conjugate vaccination. Psychosomatic Medicine. 2002;64(6):963–970. doi: 10.1097/01.psy.0000038936.67401.28. [DOI] [PubMed] [Google Scholar]
- 116.Glaser R, Kiecolt-Glaser JK, Bonneau RH, Malarkey W, Kennedy S, Hughes J. Stress-induced modulation of the immune response to recombinant hepatitis B vaccine. Psychosomatic Medicine. 1992;54(1):22–29. doi: 10.1097/00006842-199201000-00005. [DOI] [PubMed] [Google Scholar]
- 117.Glaser R, Kiecolt-Glaser JK, Marucha PT, MacCallum RC, Laskowski BF, Malarkey WB. Stress-related changes in proinflammatory cytokine production in wounds. Archives of General Psychiatry. 1999;56(5):450–456. doi: 10.1001/archpsyc.56.5.450. [DOI] [PubMed] [Google Scholar]
- 118.Broadbent E, Petrie KJ, Alley PG, Booth RJ. Psychological stress impairs early wound repair following surgery. Psychosomatic Medicine. 2003;65(5):865–869. doi: 10.1097/01.psy.0000088589.92699.30. [DOI] [PubMed] [Google Scholar]
- 119.Locke SE, Kraus L, Leserman J, Hurst MW, Heisel JS, Williams RM. Life change stress, psychiatric symptoms, and natural killer cell activity. Psychosomatic Medicine. 1984;46:441–453. doi: 10.1097/00006842-198409000-00005. [DOI] [PubMed] [Google Scholar]
- 120.Cohen M, Klein E, Kuten A, Fried G, Zinder O, Pollack S. Increased emotional distress in daughters of breast cancer patients is associated with decreased natural cytotoxic activity, elevated levels of stress hormones and decreased secretion of Th1 cytokines. International Journal of Cancer. 2002;100(3):347–354. doi: 10.1002/ijc.10488. [DOI] [PubMed] [Google Scholar]
- 121.Linn BS, Linn MW, Klimas NG. Effects of psychophysical stress on surgical outcome. Psychosomatic Medicine. 1988;50(3):230–244. doi: 10.1097/00006842-198805000-00002. [DOI] [PubMed] [Google Scholar]
- 122.Lutgendorf SK, Garand L, Buckwalter KC, Reimer TT, Hong SY, Lubaroff DM. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. The Journals of Gerontology Series A: Biological sciences and medical sciences. 1999;54(9):M434–M439. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jabaaij L, Grosheide PM, Heijtink RA, Duivenvoorden HJ, Ballieux RE, Vingerhoets AJ. Influence of perceived psychological stress and distress on antibody response to low dose rDNA hepatitis B vaccine. Journal of Psychosomatic Research. 1993;37(4):361–369. doi: 10.1016/0022-3999(93)90138-6. [DOI] [PubMed] [Google Scholar]
- 124.Andersen BL, Farrar WB, Golden-Kreutz D, Kutz LA, MacCallum R, Courtney ME, Glaser R. Stress and immune responses after surgical treatment for regional breast cancer. Journal of the National Cancer Institute. 1998;90(1):30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miller GE, Cohen S. Psychological interventions and the immune system: a meta-analytic review and critique. Health Psychology. 2001 Jan;20:47–63. doi: 10.1037//0278-6133.20.1.47. [DOI] [PubMed] [Google Scholar]
- 126.Fawzy FI, Fawzy NW, Hyun CS, Elashoff R, Guthrie D, Fahey JL, Morton DL. Malignant melanoma. Effects of an early structured psychiatric intervention, coping, and affective state on recurrence and survival 6 years later. Archives of General Psychiatry. 1993;50:681–689. doi: 10.1001/archpsyc.1993.01820210015002. [DOI] [PubMed] [Google Scholar]
- 127.Fawzy FI, Kemeny ME, Fawzy NW, Elashoff R, Morton D, Cousins N, Fahey JL. A structured psychiatric intervention for cancer patients. II. Changes over time in immunological measures. Archives of General Psychiatry. 1990 Aug;47:729–735. doi: 10.1001/archpsyc.1990.01810200037005. [DOI] [PubMed] [Google Scholar]
- 128.Andersen BL, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, Shapiro CL, Carson WE., III Psychological, behavioral, and immune changes following a psychosocial intervention: A clinical trial. Journal of Clinical Oncology. 2004;17(1):3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Antoni MH, Cruess DG, Cruess S, Lutgendorf S, Kumar M, Ironson G, Klimas N, Fletcher MA, Schneiderman N. Cognitive-behavioral stress management intervention effects on anxiety, 24-hr urinary norepinephrine output, and T-cytotoxic/suppressor cells over time among symptomatic HIV-infected gay men. Journal of Consulting & Clinical Psychology. 2000;68(1):31–45. doi: 10.1037//0022-006x.68.1.31. [DOI] [PubMed] [Google Scholar]
- 130.Antoni MH, Baggett L, Ironson G, LaPerriere A, August S, Klimas N, Schneiderman N, Fletcher MA. Cognitive-behavioral stress management intervention buffers distress responses and immunologic changes following notification of HIV-1 seropositivity. Journal of Consulting and Clinical Psychology. 1991;59(6):906–915. doi: 10.1037//0022-006x.59.6.906. [DOI] [PubMed] [Google Scholar]
- 131.Coates TJ, McKusick L, Kuno R, Stites DP. Stress reduction for men with HIV. Advances. 1989;6(3):7–8. doi: 10.2105/ajph.79.7.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Locke SE, Ransil BJ, Covino NA, Toczydlowski J, Lohse CM, Dvorak HF, Arndt KA, Frankel FH. Failure of hypnotic suggestion to alter immune response to delayedtype hypersensitivity antigens. Annals of the New York Academy of Sciences. 1987;496:745–749. doi: 10.1111/j.1749-6632.1987.tb35841.x. [DOI] [PubMed] [Google Scholar]
- 133.Locke SE, Ransil BJ, Zachariae R, Molay F, Tollins K, Covino NA, Danforth D. Effect of hypnotic suggestion on the delayed-type hypersensitivity response. JAMA. 1994;272(1):47–52. [PubMed] [Google Scholar]
- 134.Lutgendorf S, Antoni MH, Schneiderman N, Fletcher MA. Psychosocial counseling to improve quality of life in HIV infection. Patient Education and Counseling. 1994;24(3):217–235. doi: 10.1016/0738-3991(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 135.O'Leary A, Shoor S, Lorig K, Holman HR. A cognitive-behavioral treatment for rheumatoid arthritis. Health Psychology. 1988;7(6):527–544. doi: 10.1037//0278-6133.7.6.527. [DOI] [PubMed] [Google Scholar]
- 136.Antoni MH. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003;6(3):173–188. doi: 10.1080/1025389031000156727. [DOI] [PubMed] [Google Scholar]
- 137.Elsesser K, Van Berkel M, Sartory G, Biermann-Gocke W, Ohl S. The effects of anxiety management training on psychological variables and immune parameters in cancer patients: A pilot study. Behavioural and Cognitive Psychotherapy. 1994;22(1):13–23. [Google Scholar]
- 138.Walker LG, Walker MB, Ogston K, Heys SD, Ah-See AK, Miller ID, Hutcheon AW, Sarkar TK, Eremin O. Psychological, clinical and pathological effects of relaxation training and guided imagery during primary chemotherapy. British Journal of Cancer. 1999;80(1–2):262–268. doi: 10.1038/sj.bjc.6690349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cohen JJ. Individual variability and immunity. Brain Behavior and Immunity. 1999;13(2):76–79. doi: 10.1006/brbi.1999.0561. [DOI] [PubMed] [Google Scholar]
- 140.Stone AA, Cox DS, Valdimarsdottir H, Jandorf L, Neale JM. Evidence that secretory IgA antibody is associated with daily mood. Journal of Personality and Social Psychology. 1987;52:988–993. doi: 10.1037//0022-3514.52.5.988. [DOI] [PubMed] [Google Scholar]
- 141.Shimamiya T, Terada N, Wakabayashi S, Mohri M. Mood Change and Immune Status of Human Subjects in a 10-Day Confinement Study. Aviation, Space, and Environmental Medicine. 2005;76(4):481–485. [PubMed] [Google Scholar]
- 142.Jamner LD, Leigh H. Repressive/defensive coping, endogenous opioids and health: How a life so perfect can make you sick. Psychiatry Research. 1999;85:17–31. doi: 10.1016/s0165-1781(98)00134-6. [DOI] [PubMed] [Google Scholar]


