Abstract
Fas activation triggers apoptosis in many cell types. Studies with anti-Fas antibodies have produced conflicting results on Fas signaling, particularly the role of the Bcl-2 family in this process. Comparison between physiological ligand and anti-Fas antibodies revealed that only extensive Fas aggregation, by membrane bound FasL or aggregated soluble FasL consistently triggered apoptosis, whereas antibodies could act as death agonists or antagonists. Studies on Fas signaling in cell lines and primary cells from transgenic mice revealed that FADD/MORT1 and caspase-8 were required for apoptosis. In contrast, Bcl-2 or Bcl-xL did not block FasL-induced apoptosis in lymphocytes or hepatocytes, demonstrating that signaling for cell death induced by Fas and the pathways to apoptosis regulated by the Bcl-2 family are distinct.
Keywords: APO-1, CD95, signal transduction
Fas (APO-1/CD95) is a member of the tumor necrosis factor-R (TNF-R) family, a group of type I transmembrane proteins (1). Fas and some members of this family (“death receptors”) have a “death domain” (DD) in their cytoplasmic region, which is essential for their induction of apoptosis. The only known physiological ligand of Fas, FasL (CD95L or APO-1L), belongs to the family of TNF-related cytokines (2). Like most of its relatives, FasL is synthesized as a transmembrane molecule and soluble FasL trimers can be generated through processing by a metalloprotease (3, 4).
Fas signaling plays a critical role in lymphocyte homeostasis. Repeated activation of antigen receptors on T cells induces FasL expression, leading to Fas-transduced apoptosis. Failure of this process, caused by mutations in Fas (lpr or Fas gene deletion) or FasL (gld), evokes lymphadenopathy and autoimmunity (2). This receptor also plays a role in regulating physiological death of hepatocytes because Fas-deficient mice develop mild liver hyperplasia (2).
Both biochemical experiments and protein-interaction screens have led to the identification of candidate Fas signal transducers. These experiments suggest the existence of several signaling pathways leading from Fas to apoptosis. Activated Fas DD forms a docking site for the DD in the cytoplasmic adapter protein FADD/MORT1 (5–7), which recruits procaspase-8 through its N-terminal “death effector domain” (7–9). The high local concentration of procaspase-8 is believed to promote its autocatalytic activation thereby initiating the proteolytic “death cascade” (10). Fas DD is also reported to associate with RIP kinase DD (11), which binds the adapter molecule RAIDD allowing it to recruit and activate procaspase-2 (12). In addition, Fas was reported to bind to DAXX, a DD-lacking molecule, that may trigger apoptosis by activating Jun kinase (13).
Whether Bcl-2 or its functional homologs can inhibit Fas-transduced apoptosis is controversial. Experiments with primary or cultured lymphoid cells showed that Bcl-2 or Bcl-xL overexpression had no impact on cell death induced by anti-Fas antibodies even though they efficiently inhibited apoptosis induced by other cytotoxic treatments (14, 15). However, it was reported that Bcl-2 could inhibit anti-Fas antibody-induced killing of hepatocytes in the whole animal (16, 17). A study in cell lines showed that Bcl-2 could inhibit Fas-induced apoptosis in some (type II) but not other cells (type I) (18). These studies also reported that Bcl-2-insensitive and -inhibitable Fas-signaling pathways can coexist within the same cell. A possible link between Fas signaling and Bcl-2 is provided by observations that caspase-8 can activate the pro-apoptotic BH3-only protein, Bid, to trigger mitochondrial cytochrome c release (19–21), a step probably regulated by Bcl-2 (22, 23).
Posttranslational modifications of FasL have profound influence on its activity because only membrane-bound or multimerized FasL induce cell death, whereas soluble FasL may inhibit this (3, 4). This result raises two critical questions about existing data on Fas signaling. Do antibodies to Fas reliably mimic membrane-bound FasL, soluble FasL, and could they act as death agonists or antagonists (24)? Does our current knowledge of Fas signaling, derived mainly from studies with antibodies, accurately reflect signaling by the physiological membrane anchored FasL, or could some of the findings be artifacts?
To resolve these issues, we compared the effects of different Fas inducers on a range of cells. We also tested the impact of the anti-apoptotic proteins Bcl-2 and Bcl-xL and inhibitors of Fas signal transducers, FADD and caspase-8, on Fas-induced apoptosis. These experiments revealed that only membrane-bound and aggregated FasL reliably induce apoptosis, whereas anti-Fas antibodies do not. Importantly, the physiological activation of Fas triggered apoptosis by a mechanism that required FADD and caspase-8 but was insensitive to Bcl-2 or Bcl-xL. These results reinforce the notion that the signaling pathway to cell death induced by Fas is distinct from those regulated by Bcl-2.
Materials and Methods
Mice.
Eμ-bcl-2–36, Eμ-bcl-2–25, lckpr-FADD-DN, and AAT-bcl-2 transgenic mouse strains have been described (17, 25, 26). All strains were backcrossed for >10 generations with C57BL/6 mice.
Expression Constructs, Cell Transfection, and Tissue Culture.
Expression constructs for Bcl-2, FLAG- Bcl-xL, -crmA, and -FADD-DN and the generation of stable cell lines have been described (14, 26, 27).
Single-cell suspensions of thymus, spleen, or lymph nodes were prepared in a balanced salt solution containing 10% FCS (Trace BioScience, Castlehill, NSW, Australia). Primary mouse lymphocytes or cell lines were cultured as described (14, 27).
Immunofluorescence Staining, Flow Cytometric Analysis, and Cell Sorting.
Cells were stained with fluorescein isothiocyanate, R-phycoerytherin- or biotin-conjugated mAb (Molecular Probes). Biotinylated mAbs were detected with phycoerytherin-streptavidin (Caltag, South San Francisco, CA). Staining with hamster anti-mouse Fas Jo2 (PharMingen) was detected with fluorescein isothiocyanate-conjugated mouse anti-hamster IgG (PharMingen). Staining with mouse anti-human Fas APO-1 (P. Krammer, Heidelberg, Germany) was revealed by fluorescein isothiocyanate-coupled goat anti-mouse IgG (Southern Biotechnology Associates). Cells excluding propidium iodide were analyzed in a FACScan (Becton Dickinson).
T cells were purified by negative cell sorting as described (26). Staining of the enriched population with a biotinylated anti-Thy-1 plus phycoerytherin-streptavidin (Caltag) revealed that the purity was >95%.
Expression of Bcl-2 or FLAG-Bcl-xL, -CrmA, or -FADD-DN was detected by cytoplasmic immunofluorescence staining and flow cytometric analysis, as described, using mouse mAbs anti-human Bcl-2 (Bcl-2–100) or anti-FLAG M2 (Sigma) (14).
Recruitment of FADD and Procaspase-8 to the Plasma Membrane.
To detect translocation of signaling molecules, cells were treated with 0.1 μg/ml APO-1 mAb ± 0.1 μg/ml protein A for 5 min or with 0.5 μg/ml FasL ± 1 μg/ml anti-FLAG for 2 hr. Cells were harvested in hypotonic lysis buffer (10 mM Tris⋅HCl (pH 7.5)/20 mM NaCl containing 0.5 μg/ml Pefabloc/100 μg/ml soybean trypsin inhibitor/1 μg/ml each of leupeptin, aprotinin, and pepstatin/50 mM NaF/5 mM Na3VO4) (Sigma or Roche) and lyzed by dounce homogenization. Cellular debris and nuclei were removed by centrifugation at 10 g, and the P100 fraction was obtained by centrifugation at 100 g for 1 h at 4°C (Beckman TL-100). The pellet was suspended in the same buffer also containing 1% Triton X-100 and 10% glycerol. Lysates from 106 cells were fractionated by SDS/PAGE and transferred to nitrocellulose membranes (Amersham Pharmacia BioTech). The membranes were probed with mouse mAbs anti-FADD cl.A66–2 and anti-caspase-8 cl.B-9 (PharMingen). Bound mAbs were detected with horseradish peroxidase-conjugated sheep anti-mouse Ig (AMRAD BioTech, Boronia, Vic, Australia) followed by enhanced chemiluminescence (Amersham Pharmacia BioTech).
Cell Survival and Apoptosis Assays.
Cell viability was determined by staining cells with propidium iodide or annexin V-fluorescein isothiocyanate and analysis on a FACScan. To induce cell death in vitro, we used membrane-anchored mouse FasL expressed on Neuro2A cells, 1–100 ng/ml recombinant human FLAG FasL (4), 1–103 ng/ml anti-mouse Fas mAb Jo2 (PharMingen) or anti-human Fas mAb APO-1 (28), and staurosporine (Sigma). Recombinant FasL was aggregated by 1 μg/ml anti-FLAG, and Fas mAbs were crosslinked with 100 ng/ml protein A (Amersham Pharmacia BioTech).
Mice were anesthetized and intra-thymic injections performed as described (29). One lobe was injected with 10 μg of FasL plus 20 μg of anti-FLAG; the other with anti-FLAG alone. In other experiments, mice were injected into the tail vein with 12 μg of FasL and 25 μg of anti-FLAG and killed when moribund. Serum levels of alanine aminotransferase and aspartate aminotransferase were determined as described (30). Livers were excised and tissue fragments embedded in Tissue-Tek or fixed overnight in PBS/4% paraformaldehyde at 4°C before embedding in paraffin. Sections were stained with hematoxylin and eosin, and the numbers of dead cells in each section were determined by microscopic examination.
Results
Membrane-Bound or Aggregated FasL Potently Induce Apoptosis.
We undertook a comparison between membrane-anchored FasL, recombinant FasL (soluble or aggregated trimers), and mAbs to Fas. The source of physiological membrane-bound FasL was Neuro2A neuroblastoma cells expressing FasL (Neuro2A-FasL). They only express membrane-anchored FasL because they lack the metalloprotease required for FasL processing (4). Neuro2A cells transfected with the parental vector (Neuro2A-neo) served as the control. The recombinant FasL was engineered to contain a FLAG tag, so that ligand aggregation by anti-FLAG mAb could be used to mimic membrane-anchored FasL (4). As target cells, we chose the human B lymphoid line SKW6 (type I), two human T lymphoma lines, Jurkat and CEM (both type II), the mouse B lymphoma line CH1 (type I), the mouse mastocytoma line P815 (type II), and nontransformed mouse T cells. All of these cells express readily detectable levels of Fas (14, 18).
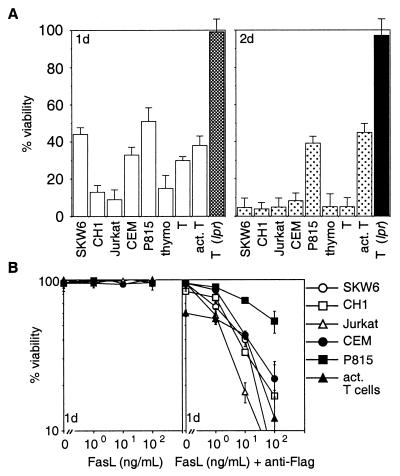
Coculture with Neuro2A-FasL cells led to rapid apoptosis of all cell types tested. With the exception of P815 cells or activated T lymphoblasts, <10% of the cells survived after 48 hr (Fig. 1A). In contrast, coculture with control cells did not induce significant cell death. The death signal from Neuro2A-FasL cells was transmitted via Fas on the target cells because lpr T cells were resistant (Fig. 1A).
Figure 1.
Membrane-anchored and aggregated FasL induce apoptosis. (A) SKW6, CH1, Jurkat, CEM, P815 cells and mouse thymocytes, purified resting or activated T cells were cocultured with Neuro2A-FasL or control cells. (B) Cells were treated with 0–100 ng/ml FasL trimers without (Left) or with (Right) 1 μg/ml anti-FLAG mAb. Cell viability was determined by flow cytometric analysis after 1–2 days of culture. Data shown represent arithmetic means ± SD of more than or equal to three independent experiments.
Addition of recombinant soluble FasL alone did not kill any of these tumor cell lines or normal mouse T cells, but aggregated FasL triggered significant apoptosis in all cell types (Fig. 1B). There was little difference in sensitivity or kinetics of cell killing between the various cell types. Most cells were efficiently killed by 100 ng/ml aggregated FasL and maximal cell death occurred within 24 hr.
These results show that extensive aggregation of Fas molecules, achieved physiologically by membrane-bound FasL or by aggregating soluble FasL, is required to induce apoptosis efficiently.
Antibodies to Fas Do Not Reliably Mimic FasL.
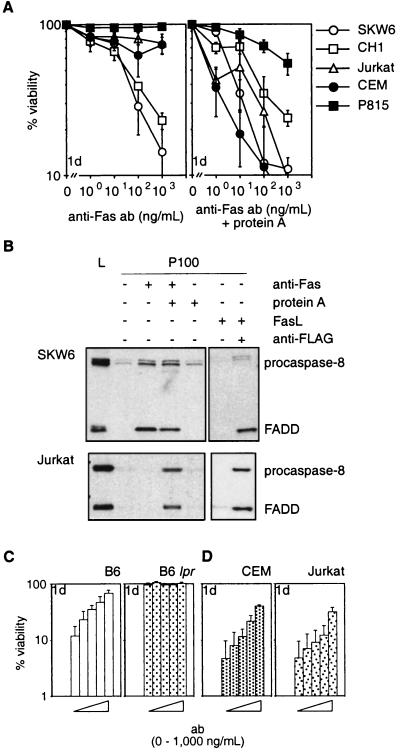
Dramatic differences became apparent when cells were treated with mAbs to Fas. The mAbs alone killed type I (SKW6, CH1) cells efficiently but type II (Jurkat, CEM, P815) or normal mouse T cells were resistant to concentrations up to 10 μg/ml (Fig. 2A and not shown). Because aggregation of FasL was required to trigger apoptosis, we speculated that the mAbs alone elicited insufficient receptor crosslinking to kill some (type II) cells. Consistent with this idea, Jurkat and CEM cells (type II) were highly sensitive to anti-Fas mAbs crosslinked by protein A (Fig. 2A). Both types of cells had comparable sensitivity to treatment with crosslinked mAb and died at a similar rate. As little as 10 ng/ml of crosslinked mAb triggered apoptosis in the majority of cells within 24 hr. Treatment with protein A alone or a control mAb and protein A had no effect (not shown).
Figure 2.
Anti-Fas antibodies do not reliably mimic FasL. (A) SKW6, CH1, Jurkat, CEM, and P815 cells were treated with 0–103 ng/ml anti-Fas mAbs (APO-1 for human or Jo2 for mouse cells) without (Left) or with (Right) 100 ng/ml protein A. (B) Recruitment of FADD and procaspase-8 to the plasma membrane in SKW6 (Upper) and Jurkat (Lower) cells. Gels were loaded with total lysate (L) or equivalent pellet fractions (P100) from untreated cells or cells treated with anti-Fas mAb (APO-1) ± protein A or recombinant FasL ± anti-FLAG. (C) Purified mouse T cells from C57BL/6 (Left) or C57BL/6 lpr mice (Right) were incubated for 2 hr with Jo2 anti-mouse Fas mAb (bars from left to right: 0, 1, 10, 100, or 1,000 ng/ml) and then cocultured with Neuro2A-FasL or control cells. (D) After preincubation with APO-1 mAb (bars from left to right: 0, 1, 10, 100, or 1,000 ng/ml), CEM (Left), or Jurkat cells (Right) were cocultured with Neuro2A-FasL or control cells. Viability of cells cocultured with Neuro2A-FasL cells relative to cells cultured with control Neuro2A cells was determined after 1 day. Data shown represent arithmetic means ± SD of three independent experiments.
Moreover, only those modes of Fas ligation, which triggered apoptosis efficiently, promoted recruitment of FADD and procaspase-8 to the plasma membrane to assemble the death-inducing signaling complex. In SKW6 cells (type I), mobilization of FADD and procaspase-8 was evident after treatment with aggregated FasL or anti-Fas mAbs, alone or crosslinked (Fig. 2B). In Jurkat cells (type II), the signaling molecules were only recruited when treated with aggregated FasL or crosslinked mAbs, but not after treatment with mAbs alone (Fig. 2B). No recruitment of FADD and procaspase-8 was seen in either cell line after addition of soluble FasL alone. Thus, efficient induction of apoptosis by Fas correlated with recruitment of FADD and procaspase-8 to the plasma membrane.
Because mAbs to Fas were unable to kill some cells (type II), we reasoned that they might act as antagonists in certain scenarios. Normal mouse T cells and CEM or Jurkat (type II) cells were preincubated with Jo2 (anti-mouse Fas) and APO-1 (anti-human Fas) mAbs, respectively, and the cells were then cocultured with Neuro2A-FasL cells. At higher concentrations, both mAbs significantly inhibited Neuro2A-FasL-induced apoptosis (Fig. 2 C and D). These mAbs were inhibitory even when added 2 hr after coculture of target cells with Neuro2A-FasL cells was started.
These results demonstrate that mAbs to Fas, which were thought to act exclusively as agonists, can antagonize Fas-transduced apoptosis under certain conditions. Furthermore, Fas-induced apoptosis can be mimicked by crosslinked mAbs to Fas but not reliably by anti-Fas mAbs alone.
FADD and Caspase-8 Are Essential for FasL-Induced Apoptosis.
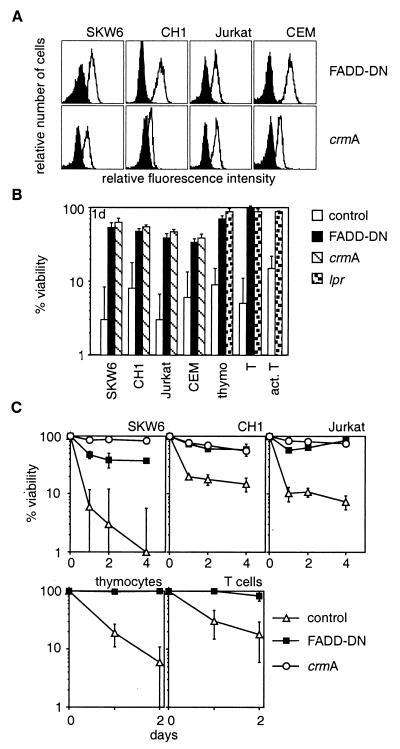
Experiments with anti-Fas mAbs indicated that the adapter protein FADD and caspase-8 are essential for Fas-transduced apoptosis (31–33). We investigated whether FADD and caspase-8 are also needed for cell death induced by the ligand. These experiments were performed with cell lines and nontransformed transgenic mouse T cells expressing a dominant-interfering mutant of FADD (FADD-DN) or the cowpox virus serpin CrmA (Fig. 3A), an inhibitor of caspase-8 (34). Cells expressing FADD-DN or CrmA were resistant to membrane-anchored FasL, aggregated FasL, and crosslinked mAbs to Fas but were normally sensitive to removal of serum or treatment with cytotoxic agents (Fig. 3 B and C and not shown). These results demonstrate that FADD and caspase-8 are essential for Fas-induced cell death but dispensable for other pathways to apoptosis.
Figure 3.
FADD and caspase-8 are essential for FasL-induced apoptosis. SKW6, CH1, Jurkat, and CEM cells were stably transfected with expression constructs encoding a FLAG-tagged dominant-interfering mutant of FADD (FADD-DN), a vector encoding FLAG-tagged CrmA, or with a control vector. Expression of the proteins was determined by anti-FLAG staining. Staining of parental cells is shown by the filled histograms (A). Cell lines, thymocytes and purified resting or activated T cells from control, lpr or FADD-DN transgenic mice were cocultured with Neuro2A-FasL or control cells (B) or with 100 ng/ml recombinant FasL + 1 μg/ml anti-FLAG (C). Cell viability was determined after 1–4 days. Data shown represent arithmetic means ± SD of more than or equal to three independent experiments.
Bcl-2 or Bcl-xL Do Not Protect Lymphocytes or Hepatocytes Against FasL-Induced Apoptosis.
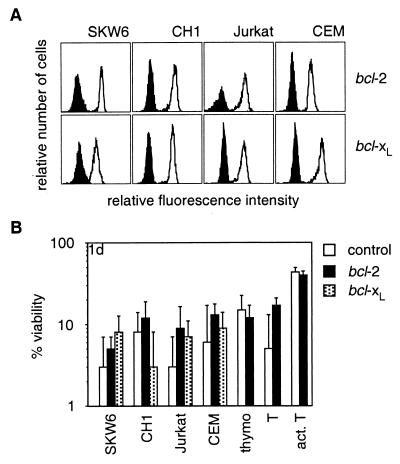
Because mAbs to Fas have produced conflicting results as to the role of Bcl-2 or Bcl-xL in Fas-transduced apoptosis, we tested the impact of Bcl-2 and Bcl-xL on FasL-induced apoptosis in cell lines and in nontransformed T cells. Bcl-2- and Bcl-xL-overexpressing cells were as sensitive to membrane-bound or aggregated FasL (at 1, 10, or 100 ng/ml) as control cells (Fig. 4B and not shown). The levels of Bcl-2 and Bcl-xL in these cells were functional because they conferred resistance against serum deprivation or cytotoxic agents (not shown).
Figure 4.
Bcl-2 and Bcl-xL do not inhibit FasL-induced apoptosis. (A) SKW6, CH1, Jurkat, and CEM cells were stably transfected with expression constructs encoding Bcl-2, FLAG-Bcl-xL, or a control vector. Expression of Bcl-2 or FLAG-Bcl-xL was determined by anti-Bcl-2 or anti-FLAG staining; staining of parental cells is shown by the filled histograms (A). Thymocytes and purified resting or activated T cells from bcl-2 transgenic mice or control mice also were examined. (B) Cells were cocultured with Neuro2A-FasL or control cells. Cell viability was determined after 1 day. Data shown represent arithmetic means ± SD of more than or equal to three independent experiments.
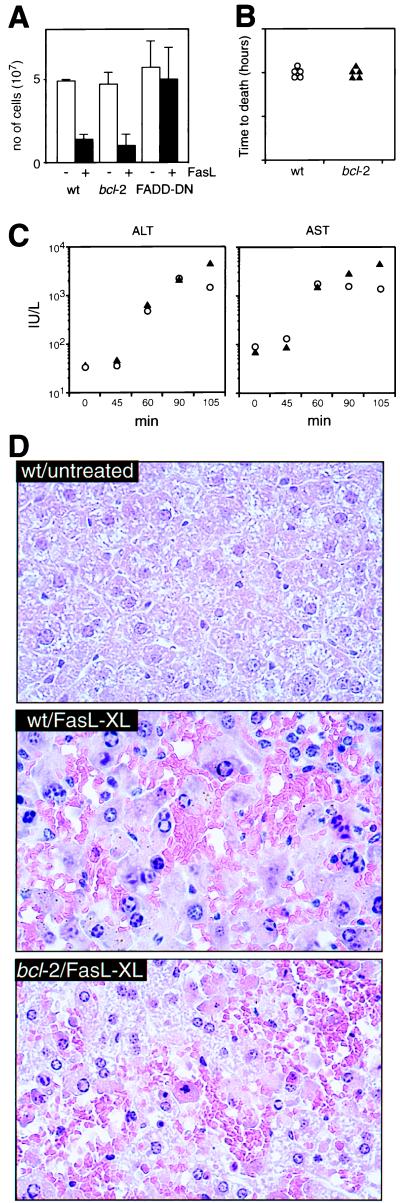
We also assessed the impact of Bcl-2 on FasL-induced apoptosis in vivo. One thymic lobe from control, Bcl-2 or FADD-DN expressing transgenic mice was injected with FasL and anti-FLAG, the other with anti-FLAG alone. Aggregated FasL killed >80% of normal and bcl-2 transgenic thymocytes. In contrast, FADD-DN transgenic cells were completely resistant to FasL-induced apoptosis (Fig. 5A).
Figure 5.
Bcl-2 does not inhibit FasL-induced apoptosis within the whole animal. (A) Thymi from bcl-2 or FADD-DN transgenic or control mice were injected with 10 μg of recombinant FasL + 20 μg of anti-FLAG (filled bars) in one lobe or with anti-FLAG alone in the other lobe (open bars). Mice were killed after 20 hr, and the cellularity of each lobe was determined. Data shown represent arithmetic means ± SD from three mice of each genotype. Mice expressing a bcl-2 transgene in hepatocytes were injected iv with 12 μg of recombinant FasL + 25 μg of anti-FLAG and killed when moribund (B). Serum levels of the liver enzymes alanine aminotransferase or aspartate aminotransferase (C) were measured 45–100 min after the injections. (D) Representative hematoxylin/eosin-stained liver sections (magnification, ×50) from untreated control and control and bcl-2 transgenic mice 90 min after injection.
Hepatocytes are the only nontransformed cell type for which Bcl-2 has been documented to inhibit Fas-transduced apoptosis (16, 17). These experiments were performed with the Jo2 mAb, which may not reliably mimic FasL (Fig. 2). There were also discrepancies between the two studies: one reported that Bcl-2 protected the mice against lethal hepatic injury caused by mAb injection (16), while the other reported that Bcl-2 did not (17). Furthermore, in mice rescued by Bcl-2 expression, the number of apoptotic hepatocytes and the levels of liver enzymes in the serum were comparable to those found in dying control mice (16). We investigated the impact of Bcl-2 on FasL-induced hepatocyte apoptosis in vivo by studying mice expressing a bcl-2 transgene in hepatocytes. Both transgenic and control mice died of liver injury within 2 hr of injection with aggregated FasL (Fig. 5B). The magnitude and rate of increase in serum liver enzymes were indistinguishable between the bcl-2 transgenic and control mice (Fig. 5C) and histological examination revealed that they had sustained comparable hepatocyte destruction (Fig. 5D).
These results show that Bcl-2 and Bcl-xL cannot inhibit FasL-induced apoptosis and challenge the notion that there are type I or II cells (18).
Discussion
A broad range of physiological or experimentally applied stimuli induce apoptosis. They trigger independent signaling pathways that converge upon activation of caspases leading to degradation of vital cellular constituents and eventual collapse of the cell. The results presented here demonstrate that physiological cell deaths activated by FasL and those regulated by Bcl-2 are subject to distinct control.
Apoptosis triggered by FasL requires extensive aggregation of Fas as trimerization of receptors by soluble FasL is insufficient (Figs. 1 and 2; refs. 3 and 4). Under physiological conditions, related death receptors may also trigger apoptosis only upon extensive multimerization. For example, soluble TNF does not induce apoptosis in vivo. Mice injected with ConA suffered severe hepatocyte destruction, caused by membrane-bound TNF on activated T cells, only when an inhibitor of the metalloprotease that releases soluble TNF was coinjected (35).
Most data on sensitivity of different cell types to Fas have been derived from studies with mAbs alone. Some of these studies are contradicted by experiments performed with the FasL. For example, resting T cells were reported to be resistant (36, 37), but we find them highly sensitive to membrane-bound or aggregated FasL (Fig. 1). Consequently, studies based on mAbs alone, which do not reliably elicit the same biological responses as the ligand (Figs. 1 and 2), may be misleading. Cellular responses to Fas activation should be studied with membrane-bound and/or recombinant FasL.
Our analyses in cell lines demonstrate that Bcl-2 and Bcl-xL do not block Fas-induced apoptosis when cell death was assessed by vital dye exclusion, phosphatidylserine exposure, or DNA fragmentation (Figs. 4 and 5 and data not shown). These results agree with previous studies showing that Bcl-2 or its homologs do not block anti-Fas mAb-induced death (14, 15, 27) or that triggered by enforced expression of FADD (38) or caspase-8 (39). Others reported, however, that Bcl-2 and Bcl-xL can inhibit anti-Fas mAb-induced apoptosis in some (type II) but not other lymphoid cell lines (type I) (18). We found that type II cells are insensitive to anti-Fas mAb alone, even without expression of Bcl-2 or Bcl-xL, but were highly sensitive to crosslinked antibody. In contrast, type I cells are sensitive to both types of stimulation (Fig. 2). When Fas was stimulated by membrane-bound or aggregated FasL, no difference in sensitivity or recruitment of FADD and procaspase-8 was observed. Importantly, no protection by Bcl-2 or Bcl-xL was achieved (Figs. 4 and 5). This result demonstrates identical Fas signaling in type I and II cells when Fas is stimulated physiologically.
The sensitivity of type I cells to the antibody alone may reflect increased receptor density (SKW6 cells have higher levels of Fas than Jurkat cells) or expression of molecules that promote Fas aggregation. Interestingly, anti-Fas mAbs potently kill hepatocytes in vivo where FcγR-expressing Kupffer cells are in close proximity, yet kill cultured hepatocytes only when protein synthesis was blocked (40). Alternatively, type II cells might express molecules blocking Fas aggregation, such as homologs of SODD, a cytoplasmic inhibitor of TNF-R1 aggregation (41).
As defective apoptosis can lead to transformation (42), immortalized cells commonly used to study cell death signaling may harbor mutations in cell death regulatory genes and this may affect the experimental outcome. This problem can avoided by studying nontransformed cells whenever possible. Our experiments with primary cells clearly show that Bcl-2 cannot inhibit FasL-induced apoptosis, and this result is consistent when bcl-2 or bcl-xL transgenic mice were compared with lpr or gld mice. Expression of Bcl-2, or Bcl-xL, in lymphocytes does not cause lpr/gld-like lymphadenopathy (25, 43, 44) but is synergistic with loss of Fas or FasL (14, 45, 46). These observations demonstrate that Bcl-2 and Bcl-xL regulate pathways to cell death distinct from those activated by FasL.
Supportive evidence for the existence of distinct pathways to apoptosis comes from the study of viruses. Many viral genomes encode Bcl-2 homologs as well as specific inhibitors of death receptor signaling. For example, human γ-herpes virus HHV-8 express a Bcl-2-like protein (KS-Bcl-2) and an inhibitor of Fas-induced apoptosis, v-FLIP (47). Because viruses are under evolutionary pressure to minimize the size of their genomes, they probably express two classes of inhibitors because each can independently enhance virus persistence and replication by blocking distinct apoptotic pathways in host cells.
These and other data support a model for two independent but ultimately converging, physiological pathways signaling for apoptosis in mammals. One induced by radiation or cytotoxic drugs is mediated by Apaf-1 and caspase-9. This pathway can be inhibited by Bcl-2 or its homologs (22) but is dispensable for Fas killing (48–51). Instead Fas-induced cell killing requires FADD and caspase-8 (31–33). Although caspase-8 can activate Bid, this step may serve primarily to amplify the signal (52). Bid is not absolutely essential for Fas-induced apoptosis because some Bid-deficient mice still die after injection with anti-Fas mAb and their lymphocytes are normally sensitive to FasL (21).
Acknowledgments
We thank Drs. S. Cory, J. Adams, A. Harris, P. Vassalli, P. Krammer, S. Nagata, and V. Dixit for gifts of transgenic mice and reagents. We acknowledge Dr. K. Kelly for intra-thymic injections; Dr. F. Battye, D. Kaminaris, V. Lapatis, J. Parker, S. Novakovic, L. Cullen, and S. Muljana for expert technical assistance; E. Shomali and F. Beerman for animal husbandry; and J. Tyers for editorial assistance. We are grateful to Drs. D. Vaux, A. Harris, J. Adams, and S. Cory for discussions and critical review of the manuscript. A.V. and K.N. are supported by an Austrian Science Fund postdoctoral fellowship and a Melbourne University Ph.D. scholarship, respectively. D.C.S.H. is a Special Fellow, and A.S. a Scholar, of the Leukemia Society of America. This work was supported by the Cancer Research Institute, the Dr. Josef Steiner Cancer Foundation, National Health and Medical Research Council (Canberra, Reg. Key 973002), and the Swiss National Science Foundation.
Abbreviations
- DD
death domain
- TNF
tumor necrosis factor
References
- 1.Ashkenazi A, Dixit V M. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 2.Nagata S. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka M, Itai T, Adachi M, Nagata S. Nat Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 4.Schneider P, Holler N, Bodmer J L, Hahne M, Frei K, Fontana A, Tschopp J. J Exp Med. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinnaiyan A M, O'Rourke K, Tewari M, Dixit V M. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 6.Boldin M P, Varfolomeev E E, Pancer Z, Mett I L, Camonis J H, Wallach D. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 7.Medema J P, Scaffidi C, Kischkel F C, Shevchenko A, Mann M, Krammer P H, Peter M E. EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muzio M, Chinnaiyan A M, Kischkel F C, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, et al. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 9.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 10.Muzio M, Stockwell B R, Stennicke H R, Salvesen G S, Dixit V M. J Biol Chem. 1998;273:2926–2930. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 11.Stanger B Z, Leder P, Lee T-H, Kim E, Seed B. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 12.Duan H, Dixit V M. Nature (London) 1997;385:86–89. doi: 10.1038/385086a0. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Khosravi-Far R, Chang H Y, Baltimore D. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strasser A, Harris A W, Huang D C S, Krammer P H, Cory S. EMBO J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Memon S A, Moreno M B, Petrak D, Zacharchuk C M. J Immunol. 1995;115:4644–4652. [PubMed] [Google Scholar]
- 16.Lacronique V, Mignon A, Fabre M, Viollet B, Rouquet N, Molina T, Porteu A, Henrion A, Bouscary D, Varlet P, et al. Nat Med. 1996;2:80–86. doi: 10.1038/nm0196-80. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez I, Matsuura K, Khatib K, Reed J C, Nagata S, Vassalli P. J Exp Med. 1996;183:1031–1036. doi: 10.1084/jem.183.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K-M, Krammer P H, Peter M E. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo X, Budlhardjo I, Zou H, Slaughter C, Wang X. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Zhu H, Xu C-J, Yuan J. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 21.Yin X-M, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth K A, Korsmeyer S J. Nature (London) 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 22.Adams J M, Cory S. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 23.Gross A, McDonnell J M, Korsmeyer S J. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 24.Strasser A, O'Connor L. Nat Med. 1998;4:21–22. doi: 10.1038/nm0198-021. [DOI] [PubMed] [Google Scholar]
- 25.Strasser A, Harris A W, Cory S. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 26.Newton K, Harris A W, Bath M L, Smith K G C, Strasser A. EMBO J. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang D C S, Cory S, Strasser A. Oncogene. 1997;14:405–414. doi: 10.1038/sj.onc.1200848. [DOI] [PubMed] [Google Scholar]
- 28.Trauth B C, Klas C, Peters A M J, Matzku S, Moller P, Falk W, Debatin K-M, Krammer P H. Science. 1989;245:301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- 29.Kelly K, Scollay R. Int Immunol. 1990;2:419–425. doi: 10.1093/intimm/2.5.419. [DOI] [PubMed] [Google Scholar]
- 30.Hahne M, Kataoka T, Schršter M, Hofmann K, Irmler M, Bodmer J-L, Schneider P, Bornand T, Holler N, French L E, et al. J Exp Med. 1998;188:1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Cado D, Chen A, Kabra N H, Winoto A. Nature (London) 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 32.Yeh W C, Pompa J L, McCurrach M E, Shu H B, Elia A J, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, et al. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 33.Varfolomeev E E, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann J S, Mett I L, Rebrikov D, Brodianski V M, Kemper O C, Kollet O, et al. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 34.Srinivasula S M, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri E S. Proc Natl Acad Sci USA. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ksontini R, Colagiovanni D B, Josephs M D, Edwards C K, III, Tannahill C L, Solorzano C C, Norman J, Denham W, Clare-Salzler M, MacKay S L D, et al. J Immunol. 1998;160:4082–4089. [PubMed] [Google Scholar]
- 36.Owen-Schaub L B, Yonehara S, Crump W L I, Grimm E A. Cell Immunol. 1992;140:197–205. doi: 10.1016/0008-8749(92)90187-t. [DOI] [PubMed] [Google Scholar]
- 37.Klas C, Debatin K-M, Jonker R R, Krammer P H. Int Immunol. 1993;5:625–630. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- 38.Hsu H, Shu H-B, Pan M-G, Goeddel D V. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 39.Martin D A, Siegel R M, Zheng L, Lenardo M J. J Biol Chem. 1998;273:4345–4349. doi: 10.1074/jbc.273.8.4345. [DOI] [PubMed] [Google Scholar]
- 40.Ni R, Tomita Y, Matsuda K, Ichihara A, Ishimura K, Ogasawara J, Nagata S. Exp Cell Res. 1994;215:332–337. doi: 10.1006/excr.1994.1349. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Y, Woronicz J D, Liu W, Goeddel D V. Science. 1999;283:543–546. doi: 10.1126/science.283.5401.543. [DOI] [PubMed] [Google Scholar]
- 42.Strasser A, Harris A W, Bath M L, Cory S. Nature (London) 1990;348:331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 43.Sentman C L, Shutter J R, Hockenbery D, Kanagawa O, Korsmeyer S J. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 44.Grillot D A M, Merino R, Nuñez G. J Exp Med. 1995;182:1973–1983. doi: 10.1084/jem.182.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reap E A, Leslie D, Abrahams M, Eisenberg R A, Cohen P L. J Immunol. 1995;154:936–943. [PubMed] [Google Scholar]
- 46.Traver D, Akashi K, Weissman I L, Lagasse E. Immunity. 1998;9:47–57. doi: 10.1016/s1074-7613(00)80587-7. [DOI] [PubMed] [Google Scholar]
- 47.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J-L, Schršter M, et al. Nature (London) 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 48.Kuida K, Haydar T F, Kuan C-Y, Gu Y, Taya C, Karasuyama H, Su M S-S, Rakic P, Flavell R A. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 49.Hakem R, Hakem A, Duncan G S, Henderson J T, Woo M, Soengas M S, Elia A, de la Pompa J L, Kagi D, Khoo W, et al. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 50.Cecconi F, Alvarez-Bolado G, Meyer B I, Roth K A, Gruss P. Cell. 1998;94:727–737. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida H, Kong Y-Y, Yoshida R, Elia A J, Hakem A, Hakem R, Penninger J M, Mak T W. Cell. 1998;94:739–750. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]
- 52.Gross A, Yin X-M, Wang K, Wei M C, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer S J. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]