Abstract
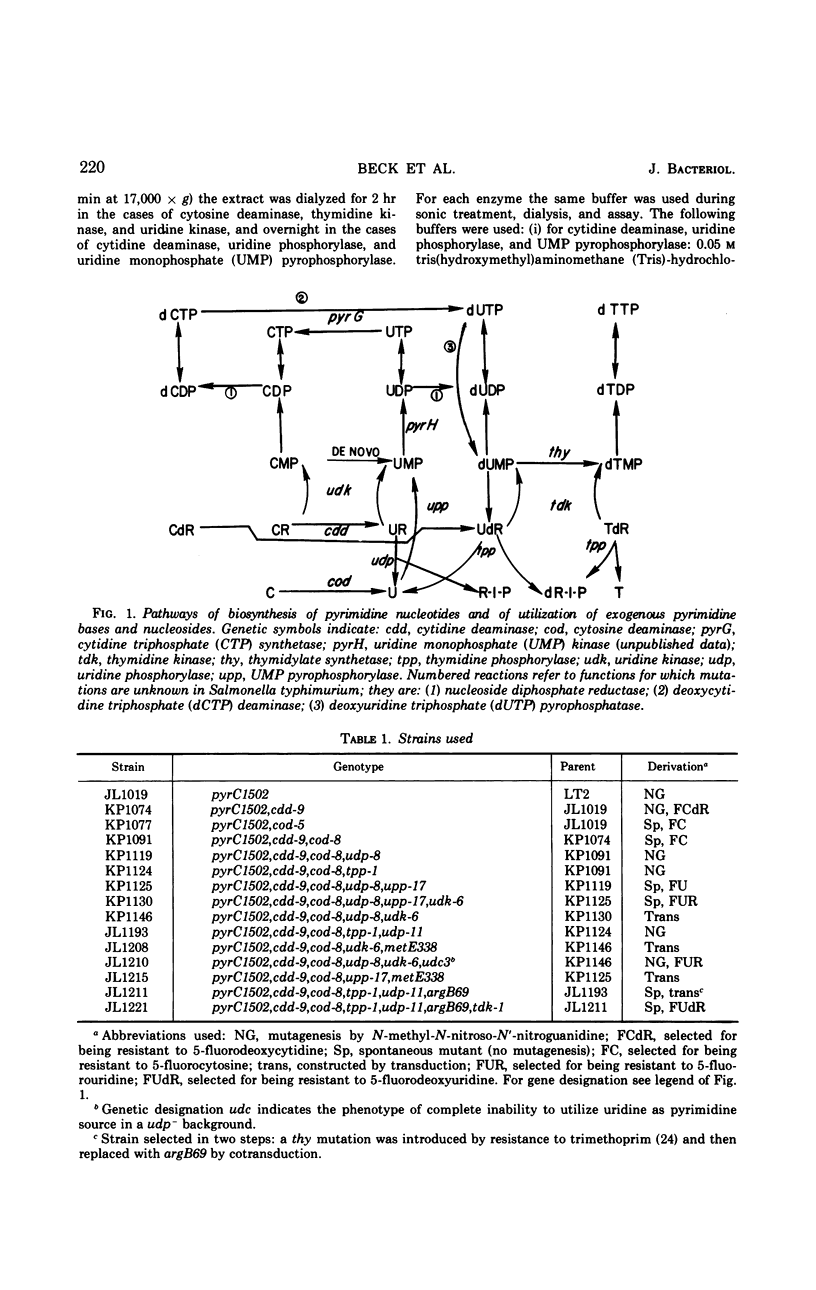
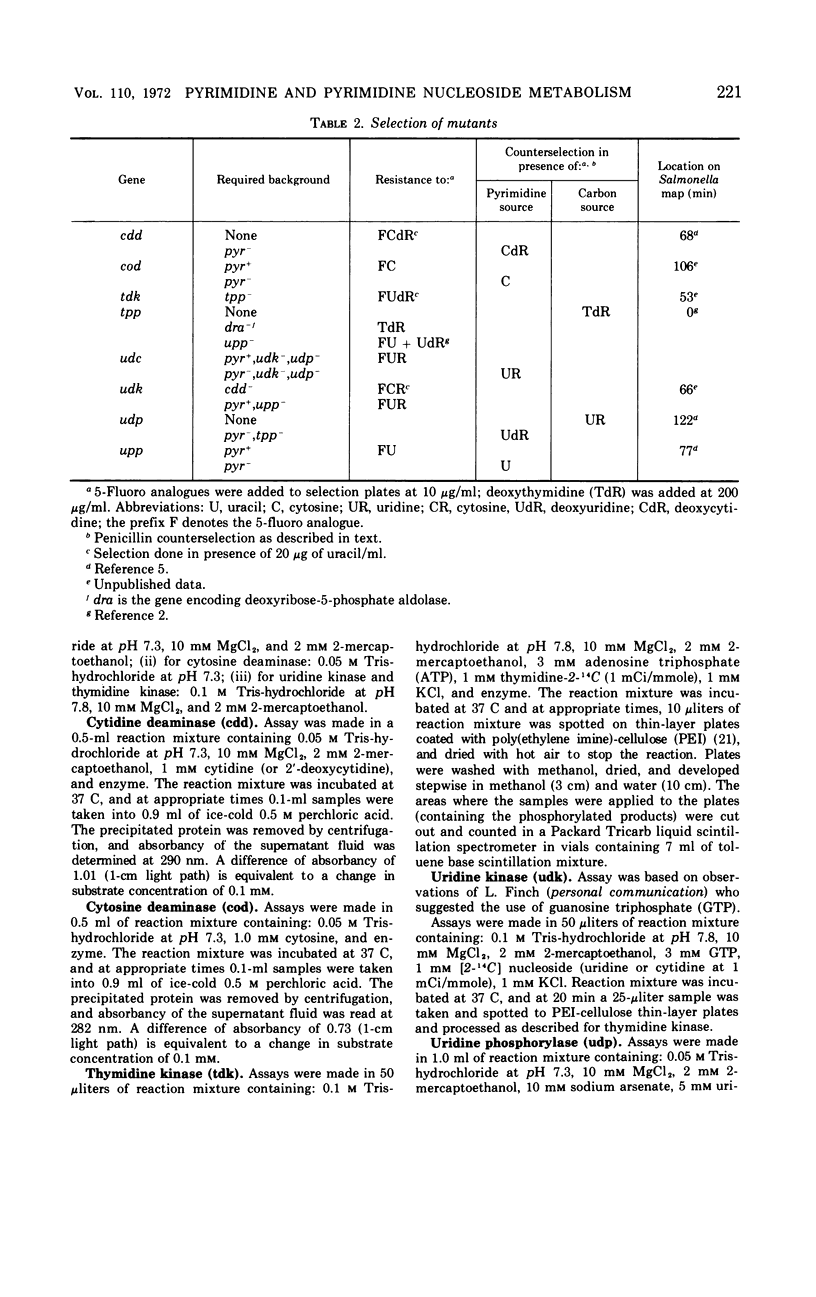
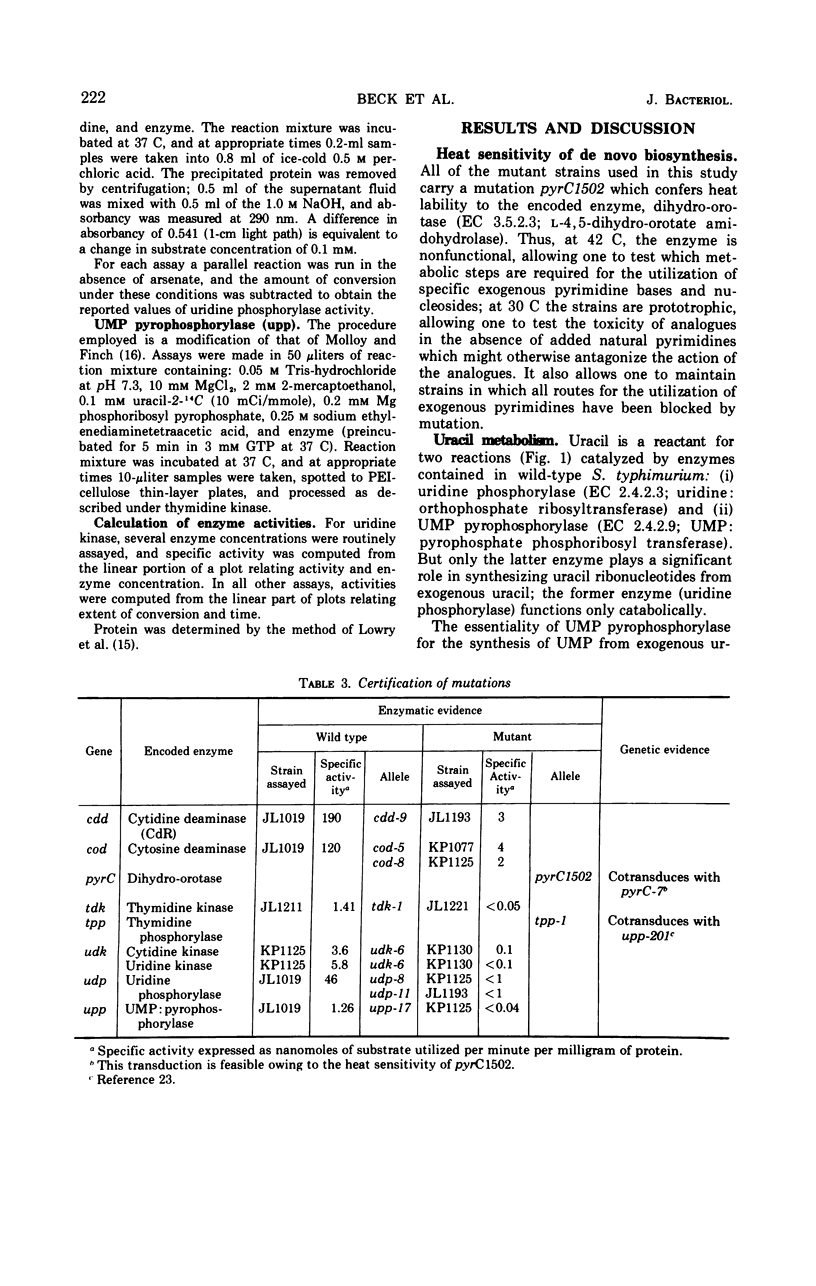
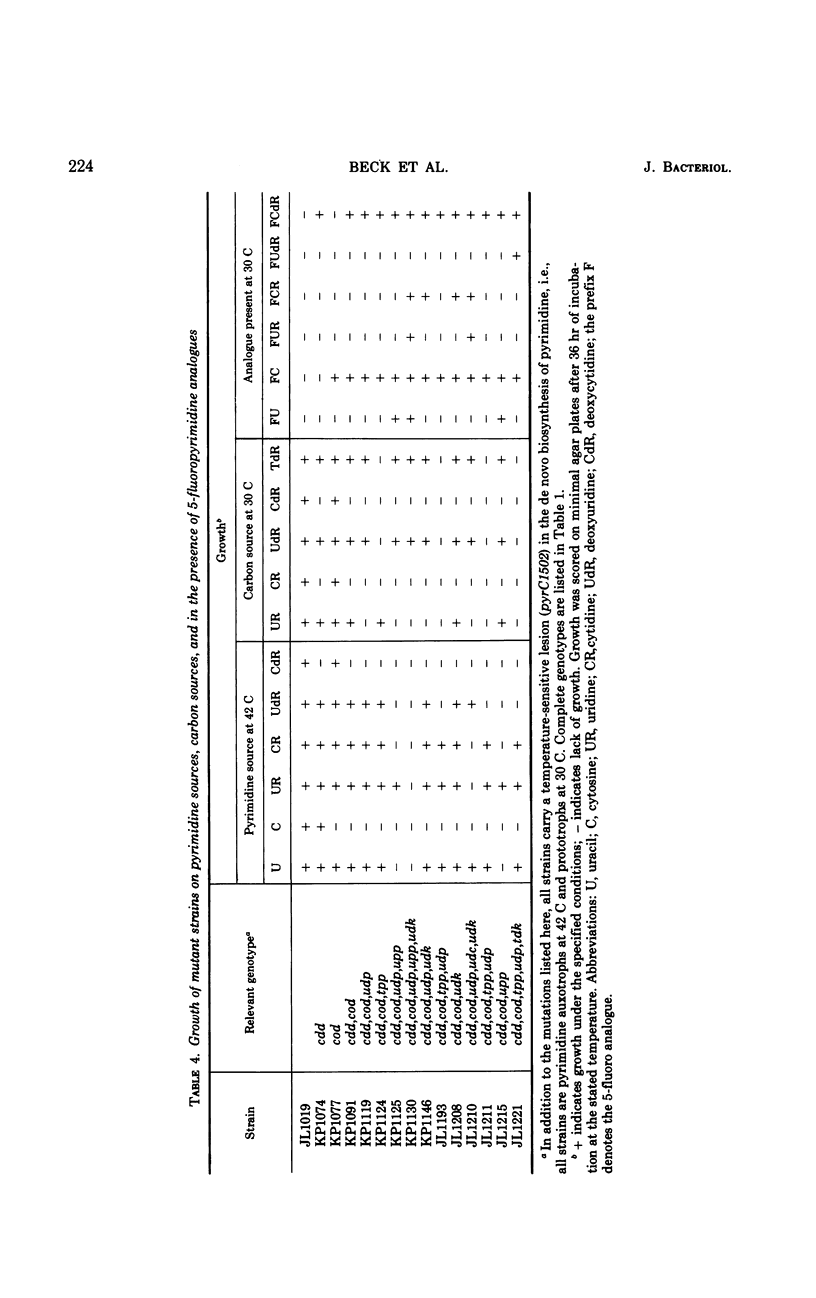
The pathways by which uracil, cytosine, uridine, cytidine, deoxyuridine, and deoxycytidine are metabolized by Salmonella typhimurium are established. The various 5-fluoropyrimidine analogues are shown to exert their toxic effects only after having been converted to the nucleotide level, and these conversions are shown to be catalyzed by the same enzymes which similarly convert the natural substrates. Methods for isolating mutant strains blocked in various steps of metabolism of pyrimidine bases and nucleosides are described.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON E. P., BROCKMAN R. W. FEEDBACK INHIBITION OF URIDINE KINASE BY CYTIDINE TRIPHOSPHATE AND URIDINE TRIPHOSPHATE. Biochim Biophys Acta. 1964 Nov 15;91:380–386. doi: 10.1016/0926-6550(64)90067-2. [DOI] [PubMed] [Google Scholar]
- Ahmad S. I., Pritchard R. H. A map of four genes specifying enzymes involved in catabolism of nucleosides and deoxynucleosides in Escherichia coli. Mol Gen Genet. 1969 Aug 15;104(4):351–359. doi: 10.1007/BF00334234. [DOI] [PubMed] [Google Scholar]
- BOLTON E. T., REYNARD A. M. Utilization of purine and pyrimidine compounds in nucleic acid synthesis by Escherichia coli. Biochim Biophys Acta. 1954 Mar;13(3):381–385. doi: 10.1016/0006-3002(54)90345-5. [DOI] [PubMed] [Google Scholar]
- Beacham I. R., Pritchard R. H. The role of nucleoside phosphorylases in the degradation of deoxyribonucleosides by thymine-requiring mutants of E. coli. Mol Gen Genet. 1971;110(4):289–298. doi: 10.1007/BF00438271. [DOI] [PubMed] [Google Scholar]
- Beck C. F., Ingraham J. L. Location on the chromosome of Salmonella typhimurium of genes governing pyrimidine metabolism. Mol Gen Genet. 1971;111(4):303–316. doi: 10.1007/BF00569782. [DOI] [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangman W. L. Specificity and efficiency of thymidine incorporation in Escherichia coli lacking thymidine phosphorylase. J Bacteriol. 1969 Sep;99(3):681–687. doi: 10.1128/jb.99.3.681-687.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M. The utilization of exogenous pyrimidines and the recycling of uridine-5'-phosphate derivatives in Saccharomyces cerevisiae, as studied by means of mutants affected in pyrimidine uptake and metabolism. Eur J Biochem. 1969 Dec;11(2):249–260. doi: 10.1111/j.1432-1033.1969.tb00767.x. [DOI] [PubMed] [Google Scholar]
- HOROWITZ J., CHARGAFF E. Massive incorporation of 5-fluorouracil into a bacterial ribonucleic acid. Nature. 1959 Oct 17;184:1213–1215. doi: 10.1038/1841213a0. [DOI] [PubMed] [Google Scholar]
- Hammer-Jespersen K., Munch-Petersen A., Schwartz M., Nygaard P. Induction of enzymes involed in the catabolism of deoxyribonucleosides and ribonucleosides in Escherichia coli K 12. Eur J Biochem. 1971 Apr 30;19(4):533–538. doi: 10.1111/j.1432-1033.1971.tb01345.x. [DOI] [PubMed] [Google Scholar]
- Karlström H. O. Inability of Escherichia coli B to incorporate added deoxycytidine, deoxyandenosine, and deoxyguanosine into DNA. Eur J Biochem. 1970 Nov;17(1):68–71. doi: 10.1111/j.1432-1033.1970.tb01135.x. [DOI] [PubMed] [Google Scholar]
- LICHTENSTEIN J., BARNER H. D., COHEN S. S. The metabolism of exogenously supplied nucleotides by Escherichia coli. J Biol Chem. 1960 Feb;235:457–465. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Molloy A., Finch L. R. Uridine-5'-monophosphate pyrophosphorylase activity from Escherichia coli. FEBS Lett. 1969 Nov 12;5(3):211–213. doi: 10.1016/0014-5793(69)80334-0. [DOI] [PubMed] [Google Scholar]
- Neuhard J., Ingraham J. Mutants of Salmonella typhimurium requiring cytidine for growth. J Bacteriol. 1968 Jun;95(6):2431–2433. doi: 10.1128/jb.95.6.2431-2433.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J. Pyrimidine nucleotide metabolism and pathways of thymidine triphosphate biosynthesis in Salmonella typhimurium. J Bacteriol. 1968 Nov;96(5):1519–1527. doi: 10.1128/jb.96.5.1519-1527.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. I. PURIFICATION AND SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1964 Jan;239:269–274. [PubMed] [Google Scholar]
- RANDERATH K., RANDERATH E. ION-EXCHANGE CHROMATOGRAPHY OF NUCLEOTIDES ON POLY-(ETHYLENEIMINE)-CELLULOSE THIN LAYERS. J Chromatogr. 1964 Oct;16:111–125. doi: 10.1016/s0021-9673(01)82445-6. [DOI] [PubMed] [Google Scholar]
- RAZZELL W. E., KHORANA H. G. Purification and properties of a pyrimidine deoxyriboside phosphorylase from Escherichia coli. Biochim Biophys Acta. 1958 Jun;28(3):562–566. doi: 10.1016/0006-3002(58)90519-5. [DOI] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Current linkage map of Salmonella typhimurium. Bacteriol Rev. 1970 Jun;34(2):176–193. doi: 10.1128/br.34.2.176-193.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Kessler D. P., Ingraham J. Cold-sensitive mutations in Salmonella typhimurium which affect ribosome synthesis. J Bacteriol. 1969 Mar;97(3):1298–1304. doi: 10.1128/jb.97.3.1298-1304.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



