Abstract
Recent evidence suggests a potential role for thrombospondin-2 (TSP-2), a matricellular glycoprotein, in the regulation of primary angiogenesis. To directly examine the biological effect of TSP-2 expression on tumor growth and angiogenesis, human A431 squamous cell carcinoma cells, which do not express TSP-2, were stably transfected with a murine TSP-2 expression vector or with vector alone. A431 cells expressing TSP-2 did not show an altered growth rate, colony-forming ability, or susceptibility to induction of apoptosis in vitro. However, injection of TSP-2-transfected clones into the dermis of nude mice resulted in pronounced inhibition of tumor growth that was significantly stronger than the inhibition observed in A431 clones stably transfected with a thrombospondin-1 (TSP-1) expression vector, and combined overexpression of TSP-1 and TSP-2 completely prevented tumor formation. Extensive areas of necrosis were observed in TSP-2-expressing tumors, and both the density and the size of tumor vessels were significantly reduced, although tumor cell expression of the major tumor angiogenesis factor, vascular endothelial growth factor, was maintained at high levels. These findings establish TSP-2 as a potent endogenous inhibitor of tumor growth and angiogenesis.
Thrombospondin-2 (TSP-2) is a member of a multigene family of five secreted, modular glycoproteins involved in the regulation of proliferation, adhesion, and migration of a number of normal and transformed cell types (1–3). TSP-2 has a high structural similarity to thrombospondin-1 (TSP-1); like TSP-1, TSP-2 is secreted as a disulfide-bonded homotrimer (2, 4–6) and interacts with a number of the same cell surface receptors, including the integrin αvβ3, low-density lipoprotein-related receptor protein, and heparan sulfate proteoglycans (7, 8). During embryonic development and in adult tissues, expression of TSP-2 is spatially and temporally different from TSP-1, with a predominant expression of TSP-2 in areas of chondrogenesis, osteogenesis, and in early connective tissues (9, 10). Moreover, TSP-2 expression was recently detected in the basal epidermal keratinocyte layer in normal human skin (11). In accordance with this distinct expression pattern, TSP-2 is characterized by a distinct regulation of gene expression by growth factors and hormones (1, 4, 12). Similar to TSP-1, however, TSP-2 is highly expressed in developing blood vessels, indicating a potential role in the regulation of primary angiogenesis (9, 13). Indeed, an inhibitory effect of TSP-2 on the angiogenic activity of basic fibroblast growth factor (14) and the formation of focal adhesions in bovine aortic endothelial cells (15) has been reported, and targeted disruption of the TSP-2 gene in mice resulted, among other abnormalities, in significantly increased numbers of small- and medium-sized blood vessels in several tissues including the skin (16).
In contrast to TSP-1, which has been demonstrated as an inhibitor of angiogenesis in vitro and in vivo (17) and as a potent suppressor of malignant growth (18–20), the biological role of TSP-2 for tumor growth and angiogenesis has remained unknown. To directly test the hypothesis that TSP-2 may play an important role as an endogenous inhibitor of tumor growth and angiogenesis, we stably transfected human A431 squamous cell carcinoma cells with TSP-2 cDNA. This cell line does not secrete TSP-2 and can be injected intradermally into nude mouse skin, providing an orthotopic model for cutaneous squamous cell carcinoma growth (20, 21). A431 cells expressing TSP-2 did not show an altered growth rate, colony-forming ability, or susceptibility to induction of apoptosis in vitro, as compared with vector-transfected control clones. Our in vivo study demonstrates that tumor cell production of TSP-2 potently inhibits malignant tumor growth in vivo, and that TSP-2 exerts a strong antiangiogenic effect on squamous cell carcinomas, significantly reducing the density and size of tumor blood vessels. Moreover, combined expression of TSP-2 and TSP-1 completely prevented tumor formation.
Materials and Methods
Cell Culture.
The human squamous cell carcinoma cell line A431 was obtained from the American Type Culture Collection and maintained in DMEM (GIBCO/BRL) containing 10% FBS, 4.5 mg/ml glucose, 2 mM l-glutamine, 100 units/ml penicillin G, and 100 μg/ml streptomycin (GIBCO/BRL). Human dermal microvascular endothelial cells (HDMEC) were isolated from neonatal foreskins and cultivated as described (22).
Cell Transfection.
The expression plasmid containing the murine TSP-2 coding sequence cDNA was prepared by ligation of a 3.6-kb EcoRI fragment into the EcoRI site of PIRES/Neo (CLONTECH), which contains a cytomegalovirus (CMV)-enhancer-promoter and a neomycin selection cassette. An expression plasmid containing the human TSP-1 cDNA was previously obtained (20) by ligation of a 3.6-kb EcoRI-fragment of human TSP-1 into pcDNA3.1Zeo(−) (Invitrogen), which contains a Zeocin selection cassette. A431 cells, already stably transfected with an unaltered pcDNA3.1Zeo(−) vector or the TSP-1 expression plasmid (20), were transfected with the TSP-2 expression plasmid or unaltered PIRES/Neo, by using the SuperFect transfection reagent (Qiagen, Chatsworth, CA) and 10 μg of each plasmid according to the manufacturer's recommendations. The cells were selected in medium containing 250 μg/ml Zeocin (Invitrogen) and 800 μg/ml G418 (Sigma) for 3–4 weeks, and 15 resistant clones were isolated and characterized for TSP-2 expression.
Northern Blot Analysis.
Total cellular RNA was isolated from confluent cell cultures by using the RNeasy kit (Qiagen) according to the manufacturer's instructions. RNA (10 μg) was fractionated by electrophoresis on a 1% agarose formaldehyde gel and transferred to Biotrans nylon-supported membranes (ICN) as described (23). 32P-radiolabeled cDNA probes were prepared with a random primed synthesis kit (Multiprime; Amersham International). mRNAs for TSP-1, TSP-2, and vascular endothelial growth factor (VEGF) were detected with a 4.1-kb human TSP-1 cDNA, a 3.6-kb mouse TSP-2 cDNA, or a 204-bp human VEGF cDNA that recognizes all known VEGF variants (24). A 2.0-kb human β-actin cDNA probe (CLONTECH) was used as a control for equal RNA loading. Blots were washed at high stringency and exposed to X-Omat MR film (Kodak) for varying times.
Western Blot Analysis.
Conditioned media were obtained from confluent cells grown for 48 h in serum-free DMEM. TSP-1 and TSP-2 were concentrated as described (20). Samples were boiled in denaturing sample buffer and 15 μl of each sample was electrophoresed on polyacrylamide gels (25) and blotted onto polyvinylidene difluoride membranes (Bio-Rad). Membranes were then incubated with anti-TSP-2 antibody R81939 (see below), incubated with horseradish peroxidase-conjugated anti-rabbit IgG, and analyzed by the enhanced chemiluminescence system (Amersham International). Membranes were stripped of bound antibody and then incubated with an antibody against human TSP-1 (clone 133; Genzyme).
Development of Anti-Human TSP-2 Antibody.
The 15-amino acid peptide DKDTTFDLFSISNIN, derived from the N-terminal sequence of the human TSP-2 coding region (amino acids 22–36), was used to immunize two rabbits with standard techniques. This sequence has only three amino acids in common with TSP-1, and is highly conserved (13/15 amino acids) between human and mouse TSP-2. A polyclonal antibody, R81939, was obtained that specifically detected two bands of approximately 180 and 135 kDa corresponding to human TSP-2 in protein lysates by Western blot. The specificity of the affinity-purified antibody was demonstrated by the lack of detection of natural human TSP-1 purified from human platelets (kindly provided by Jack Lawler, Harvard Medical School, Boston).
Cell Growth and Apoptosis Assays.
Anchorage-independent cell growth was measured with a soft-agar assay as described (20). Ten thousand control-transfected A431 cells or cells transfected with TSP-2, TSP-1, or both TSP-2 and TSP-1 were transferred into six 30-mm cell culture dishes with a 2-mm grid (Nunc), and colonies were counted after 8 days. Apoptosis induced by serum withdrawal was studied in subconfluent A431 cell clones after 6 days in serum-free medium. The percentage of apoptotic cells was determined as described (26) by using the Fluorescein-FragEL DNA fragmentation kit (Oncogene) according to the manufacturer's instructions, and a Beckton-Dickinson FACS-Scan.
Cell Migration Assay.
Transwell migration chambers (8-μm pore size; Costar) were coated on the underside with 10 μg/ml collagen type I (Collagen Corp., Palo Alto, CA); 1 × 105 HDMEC were added to the upper chamber in 300 μl of unconditioned DMEM medium or in conditioned medium obtained from control-transfected, TSP-1-transfected, or TSP-2-transfected A431 clones, supplemented with 10 mg/ml BSA. After 4 h, migrated cells were fixed and stained as described (27). Images of three different ×10 fields were captured from each membrane with a Spot digital camera (Diagnostic Instruments; Sterling Heights, MI) attached to a Nikon E-600 microscope, and the number of migrating cells was calculated per mm2 by using the ip-lab software (Scanalytics, Billerica, MA). All assays were performed in triplicate, and data were analyzed with the two-sided unpaired t test.
Tumorigenesis Assay.
A431 cells (2 × 106), stably transfected with TSP-1, TSP-2, TSP-1 and TSP-2, or with vector alone, were injected intradermally into both flanks of 8-weeks-old female BALB/c (nu/nu) mice (two sites per mouse and five mice per cell clone). Three different clones for each construct or control were studied. The smallest and largest tumor diameters were measured weekly with a digital caliper, and tumor volumes were calculated by using the following formula: Volume = [4/3] × π × (1/2 × smaller diameter)2 × 1/2 × larger diameter. Tumor data were analyzed by the two-sided unpaired t test. Mice were sacrificed after 3 weeks or earlier when the largest tumor diameter reached 20 mm. All animal studies were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
In Situ Hybridization and Immunohistochemistry.
In situ hybridization was performed on 6-μm paraffin sections of tumor xenografts as described (28). The sense and antisense single-stranded RNA probes for human VEGF were transcribed from a pGEM-3Zf(+) vector containing a 204-bp fragment common to all known VEGF-splicing variants. An RNA probe to mouse TSP-2 was transcribed from a pBluescript II KS+ vector containing a 290-bp fragment of the coding region of mouse TSP-2 (29). Immunohistochemical stainings were performed on 6-μm frozen or paraffin sections as described (30), by using monoclonal antibodies against human TSP-1 (Genzyme) and mouse CD31 (PharMingen), and rabbit antibody R81939 against human TSP-2. Representative 6-μm paraffin sections of tumor xenotransplants were stained with a monoclonal antibody against the proliferating cell nuclear antigen (Zymed), and quantitative analyses of positive tumor cells were performed on five different fields per section on the ip-lab software.
Computer-Assisted Morphometric Analysis of Tumor Vessels.
Cryostat sections (6 μm) were stained with an anti-mouse CD31 monoclonal antibody. Representative sections obtained from five tumors from each cell clone were analyzed with a Nikon E-600 microscope. Images were captured with a Spot digital camera, and morphometric analyses were performed on the ip- lab software. Three different fields in each section were examined at ×10 magnification, and the number of vessels per mm2, average vessel size, and relative area occupied by tumor blood vessels were determined. The two-sided unpaired t test was used to analyze differences in microvessel density and vascular size.
Results
Expression of TSP-2 in A431 Cells.
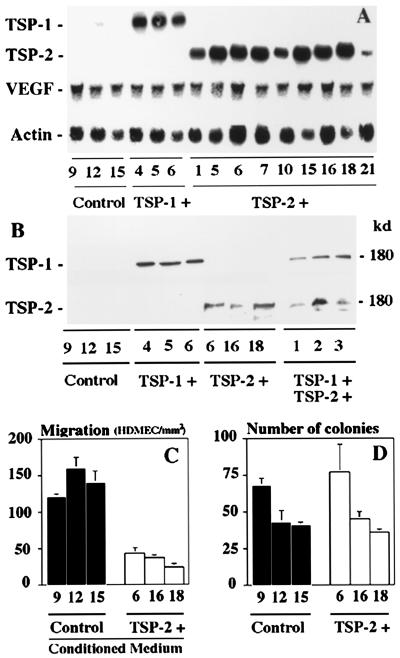
Fifteen neomycin-resistant clones derived from A431 squamous cell carcinoma cells transfected with the TSP-2-PIRES/Neo expression vector and 15 randomly selected cell clones transfected only with PIRES/Neo expression vector were characterized for TSP-2 mRNA expression and protein secretion. A431 cells transfected with empty expression vector only did not express any detectable TSP-2 mRNA or secrete TSP-2 (Fig. 1 A and B), whereas strong TSP-2 mRNA expression was detected in all TSP-2 transfected A431 cell clones (Fig. 1A). Clones 6, 16, and 18 were chosen for further characterization, and Western blot analyses of conditioned media confirmed efficient TSP-2 secretion by these clones (Fig. 1B). Little or no TSP-1 mRNA expression and no TSP-1 protein secretion were detected in TSP-2 transfected and control A431 cells (Fig. 1 A and B). In contrast, three previously characterized A431 clones, stably transfected to overexpress TSP-1 (20), strongly expressed TSP-1 mRNA and protein but not TSP-2 mRNA or protein (Fig. 1 A and B). We also established three A431 cell clones stably transfected with both TSP-1 and TSP-2 and confirmed efficient secretion of both proteins by Western blot analyses (Fig. 1B). Importantly, overexpression of TSP-2 or TSP-1 did not affect expression of the angiogenesis factor VEGF in tumor cells (Fig. 1A). To confirm the biological activity of transfected TSP-2, conditioned media (CM) were harvested from the TSP-2-overexpressing A431 clones 6, 16, and 18, and were tested for their ability to inhibit the in vitro migration of HDMEC, by using an established haplotaxis assay (27). All CM obtained from TSP-2-transfected A431 cells potently inhibited HDMEC migration, as compared with CM obtained from A431 cells transfected with vector only (P < 0.001; Fig. 1C).
Figure 1.
Expression of transfected TSP-2 and TSP-1 in A431 cells. (A) Northern blot analysis confirmed TSP-2 mRNA expression in A431 clones stably transfected with a TSP-2 expression vector (TSP-2 +) but not in vector control clones (Control) or in TSP-1-transfected clones (TSP-1 +). Little or no TSP-1 mRNA expression was detected in TSP-2-transfected or control clones and VEGF mRNA expression remained unchanged. The blot was also probed with a β-actin cDNA probe to control for loading. (B) Western blot analysis of conditioned media confirmed selective TSP-2 secretion by A431 clones stably transfected with a TSP-2 expression vector and selective TSP-1 secretion in TSP-1-transfected clones. (C) Inhibitory effect of CM obtained from TSP-2-transfected A431 clones on migration of HDMEC, as compared with control CM (P < 0.01). (D) No significant changes in soft-agar growth were observed in TSP-2-transfected A431 clones, as compared with vector-transfected controls. Mean values ± SD of two independent experiments.
Characterization of Tumor Cell Growth in Vitro.
To detect possible effects of TSP-2 overexpression on the growth of stable A431 transfectants, anchorage-independent cell growth was assessed by colony formation efficiency in soft agar, and the susceptibility to induction of apoptosis was studied after serum withdrawal for 6 days. No significant differences in soft agar colonization (Fig. 1D) or spontaneous and induced apoptosis rates (data not shown) were observed between control transfected and TSP-2-overexpressing A431 clones. Moreover, no alterations in cellular morphology and growth rates on plastic tissue culture dishes were detected (data not shown). Similar results were obtained with A431 clones stably expressing both TSP-2 and TSP-1.
TSP-2 Overexpression Potently Inhibits Squamous Cell Carcinoma Growth in Vivo.
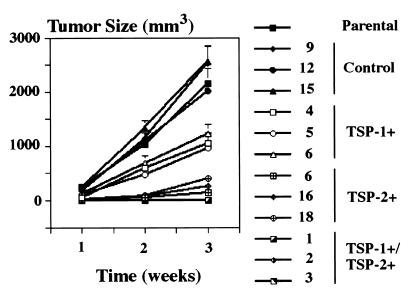
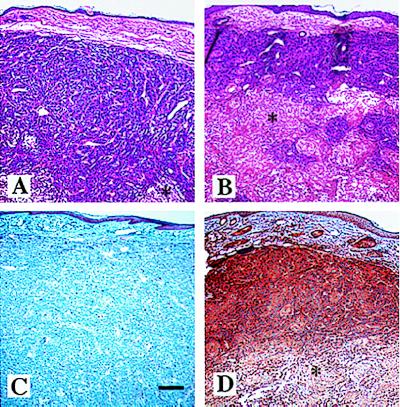
Mice were injected intradermally with cells from three TSP-2-transfected clones, three vector-transfected control clones, and the maternal A431 cell line. For comparison of TSP-2 effects with the previously described effects of the endogenous angiogenesis inhibitor TSP-1, mice were also injected with three TSP-1 transfected clones and with three clones of A431 cells that were transfected with both TSP-1 and TSP-2. A431 and vector-transfected control cells formed rapidly growing squamous cell carcinomas, reaching a volume of 2,000–3,000 mm3 within 3 weeks (Fig. 2). Overexpression of TSP-2 resulted in a dramatic inhibition of tumor growth by more than 90% (P < 0.001) in all three A431 clones tested (Fig. 2), and extensive areas of tumor cell necrosis were found in TSP-2-expressing tumors, whereas only small necrotic foci were found in control tumors (Fig. 3 A and B). The TSP-2-induced inhibition of squamous cell carcinoma growth was significantly more potent than the 40–50% inhibition observed in TSP-1-expressing tumors (P < 0.001; Fig. 2). None of the three clones cotransfected with both TSP-2 and TSP-1 formed any visible tumors over an observation period of up to 12 weeks (data not shown). It is of interest that the fraction of proliferating cells, as determined by staining for the proliferating cell nuclear antigen, remained unchanged within the viable tumor areas in TSP-2-overexpressing tumors as compared with control tumors (data not shown).
Figure 2.
Stable TSP-2 overexpression in A431 clones 6, 16, and 18 significantly (P < 0.01) inhibited intradermal tumor growth as compared with parental A431 cells or control transfected clones 9, 12, and 15. Combined overexpression of TSP-1 and TSP-2 completely prevented tumor formation. Data for TSP-1/TSP-2 clones 2 and 3 were identical to clone 1. TSP-1 expression alone (clones 4, 5, and 6) resulted in moderate tumor inhibition. Values represent means ± SEM for 10 tumors of each clone and time point.
Figure 3.
Extensive areas of necrosis (*) in TSP-2-overexpressing tumors (B and D) as compared with control transfected tumors (A and C). (A and B) Hematoxylin/eosin stain. (C and D) Immunohistochemistry for TSP-2. Strong TSP-2 immunoreactivity in TSP-2-overexpressing A431 tumors is shown in D. In control transfected tumors, TSP-2 was detected in the basal epidermal layer of adjacent normal skin but not in tumor cells (C). (Bar = 200 μm.)
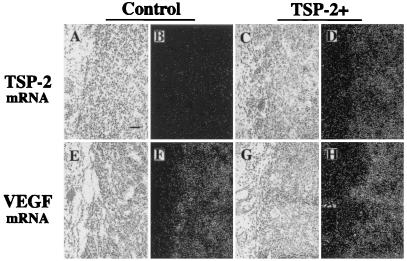
Immunohistochemical and in situ hybridization analyses confirmed that TSP-2-transfected clones maintained TSP-2 mRNA and protein expression after 3 weeks of tumor growth in vivo. TSP-2-transfected tumors showed strong TSP-2 immunoreactivity throughout the viable tumor tissue (Fig. 3D), whereas no staining for TSP-2 was detected in control tumors (Fig. 3C). Moreover, TSP-2 protein expression was detected in the basal layer of the epidermis overlying both control and TSP-2-expressing tumors and weakly in dermal blood vessels (Fig. 3 C and D). In accordance with these findings, TSP-2 mRNA expression was detected at high levels in TSP-2-transfected squamous cell carcinomas (Fig. 4 C and D) but not in control tumors (Fig. 4 A and B). It is of interest that no major differences in VEGF mRNA expression were found between TSP-2-transfected (Fig. 4 G and H) and control tumors (Fig. 4 E and F). Focally, high VEGF mRNA expression was observed adjacent to necrotic areas in all samples studied.
Figure 4.
Tumor expression of TSP-2 and VEGF mRNA. In situ hybridization confirmed strong TSP-2 mRNA expression in 3-week-old A431 tumors transfected with TSP-2 (C and D), whereas little or no TSP-2 mRNA expression was detected in vector-transfected control tumors (A and B) and in adjacent normal tissue. Strong VEGF mRNA expression was maintained both in control tumors (E and F) and TSP-2-overexpressing tumors (G and H), whereas little VEGF expression was detected in adjacent normal tissue. Bright-field (A, C, E, and G) and dark-field (B, D, F, and H) micrographs. (Bar = 100 μm.)
TSP-2 Overexpression in Squamous Cell Carcinomas Inhibits Tumor Angiogenesis.
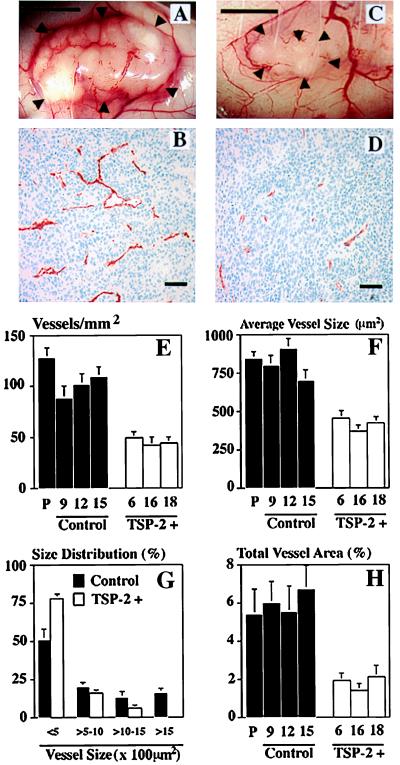
A significant rarefication of tumor-supplying blood vessels was found in TSP-2-overexpressing squamous cell carcinomas, as compared with control tumors (Fig. 5 A and C). Cryostat sections of five different tumors for each clone were stained for PECAM-1 (CD31), an endothelial junction molecule (31). These sections demonstrated a dramatic reduction of microvessels within TSP-2-expressing tumors (Fig. 5 B and D). The differences in tumor vascularity between control and TSP-2-expressing tumors were maintained even when tumors of the same size were evaluated (data not shown). To achieve a more detailed quantification of the effects of TSP-2 on tumor angiogenesis, the average vessel density, vessel size, and percentage of tissue area covered by vessels were determined by computer-assisted image analysis of representative digital images. Whereas control tumors demonstrated between 80 and 125 CD31 positive vessels per mm2 tumor area, the vascular density was reduced by more than 50% in TSP-2-expressing tumors (Fig. 5E). Moreover, the average vessel size was reduced by more than 45% in TSP-2-overexpressing tumors derived from clones 6, 16, and 18, as compared with control tumors derived from clones 9, 12, and 15 (Fig. 5F). In particular, TSP-2 expression resulted in the complete absence of blood vessels larger than 1,500 μm2, which represented 15% of all blood vessels in control tumors (Fig. 5G). In accordance with these data, the relative tumor area occupied by vessels was reduced by 70% in TSP-2-transfected tumors (P < 0.001; Fig. 5H).
Figure 5.
TSP-2 inhibits tumor angiogenesis. Rarefication of blood vessels supplying TSP-2-transfected A431 tumors (C) as compared with control tumors (A). ▸, Tumor borders. (Bar = 5 mm.) Immunostaining with an anti-CD31 monoclonal antibody demonstrated rarefication of small tumor blood vessels in TSP-2-transfected tumors (D) as compared with control tumors (B). (Bar = 100 μm.) (E–H) Quantitative computer-assisted image analysis revealed a significant inhibition of angiogenesis in TSP-2-transfected A431 tumors (P < 0.01), as measured by the number of blood vessels per mm2 tumor area (E). Significantly (P < 0.05) reduced average vessel area (F) with the absence of vessels of more than 1,500 μm2 in size (G) in TSP-2-transfected A431 tumors. (H) The relative tumor area covered by vessels was reduced by ≈70% in TSP-2-expressing A431 tumors, as compared with parental (P) A431 cell tumors or control-transfected tumors. CD31-stained blood vessels were evaluated in three different ×10 fields in sections obtained from five different tumors for each clone.
Discussion
There is increasing evidence that TSP-2 plays a major role in the regulation of primary angiogenesis, as demonstrated by its inhibitory activity on sprouting blood vessels in the chick chorioallantoic membrane assay (14), by the increased densities of blood vessels in several organs of TSP-2-deficient mice (16), and by the increased vascularization of foreign body capsules and the prolonged neovascularization of full-thickness wounds in TSP-2-deficient mice (32, 33). However, the biological role of TSP-2 for tumor growth and angiogenesis has remained unknown. We studied the effects of induced TSP-2 gene expression in an established orthotopic nude mouse model of cutaneous squamous cell carcinoma, by using the human squamous cell carcinoma cell line A431 stably transfected with a TSP-2 expression vector or with vector alone. The A431 cell line was chosen because of the complete absence of TSP-2 expression in these cells and because we previously found a marked antitumor and antiangiogenic effect of TSP-1 in this model (20). Our results demonstrate for the first time that tumor cell expression of TSP-2 potently inhibited the orthotopic growth of human squamous cell carcinomas, as compared with control tumors transfected with vector alone. The antitumoral effect of TSP-2 (more than 90% inhibition after 3 weeks) was stronger than the effect of TSP-1, which led to only 40–50% tumor inhibition in the identical experimental model. Importantly, combined expression of TSP-2 and TSP-1 in A431 cells completely prevented tumor growth during an observation period of 12 weeks, suggesting potential additive or synergistic effects of these two related proteins. Several lines of evidence strongly suggest that the inhibition of in vivo tumor growth by TSP-2 was not caused by a direct inhibition of tumor cell proliferation because we did not detect any significant differences in (i) the colony-forming efficiency of TSP-2-transfected A431 cells in soft agar, (ii) the susceptibility of TSP-2-transfected A431 cells to induction of apoptosis by serum withdrawal, and (iii) the tumor cell proliferation rate of TSP-2 expressing squamous cell carcinomas in vivo, as determined by the fraction of proliferating cell nuclear antigen-expressing tumor cells within viable tumor areas. Moreover, injection of control A431 cells (clone 15) together with an equal number of TSP-2-expressing A431 cells (clone 16) resulted in a marked inhibition of tumor growth (greater than 50%), as compared with injection of control A431 cells alone, demonstrating that TSP-2 can act to slow adjacent cell growth (data not shown).
Our results provide strong evidence that TSP-2-induced inhibition of squamous cell carcinoma growth was mediated by inhibition of tumor angiogenesis. By using an established in vitro assay to measure the migration of HDMEC (27), we found a pronounced inhibitory effect of conditioned media obtained from TSP-2-overexpressing A431 clones on HDMEC migration, as compared with conditioned media harvested from control transfectants. Moreover, computer-assisted image analyses of tumor sections stained for the endothelial junction molecule CD31 revealed a significant reduction of tumor microvessels in TSP-2-expressing squamous cell carcinomas, as measured by the average number of tumor blood vessels per mm2. Furthermore, the average size of tumor vessels was greatly diminished in TSP-2-expressing tumors, with a complete absence of larger vessels of more than 1,500 μm2 in size which accounted for more than 15% of all tumor vessels in control tumors. Similar differences were observed when tumors of the same size were evaluated. A combined evaluation of vessel densities and vessel sizes demonstrated that the relative area occupied by tumor vessels was dramatically reduced in TSP-2-expressing tumors, suggesting that assessment of relative vascular areas in tumor sections represents a more sensitive parameter for changes in tumor angiogenesis than the measurement of either vascular densities or individual vessel sizes alone. It remains to be established whether the inhibitory effects of TSP-2 on tumor angiogenesis apply to the majority of human malignancies or only to select types of cancer. Whereas no significant correlation between TSP-2 mRNA expression and angiogenesis was observed in invasive breast carcinomas (34), preliminary evidence suggests down-regulation of TSP-2 expression in human squamous cell carcinomas of the skin, as compared with normal human skin (ref. 11; M.D., M.S. & L.F.B., unpublished observations), and a correlation between TSP-2 gene expression and decreased vascularity was reported in non-small cell lung cancers (35) and colon cancers (36). Recent wound healing studies in mouse skin further support a role of TSP-2 in inhibiting in vivo angiogenesis. These studies demonstrate low expression of TSP-2 in early wounds when maximal vascular proliferation is observed, and strong TSP-2 expression in late wounds at the onset of and during vascular regression (33). Moreover, TSP-2-deficient mice were characterized by prolonged wound neovascularization, even in the presence of TSP-1 expression, thus clearly demonstrating a distinct antiangiogenic activity of TSP-2 in a genetic model (33).
In accordance with the pronounced inhibition of tumor angiogenesis, extensive areas of tumor cell necrosis were detected in TSP-2-expressing tumors. Importantly, in situ hybridization analyses revealed that the mRNA expression of VEGF, a major tumor angiogenesis factor (37), was maintained at equally high levels in TSP-2-expressing and control tumors. These findings excluded the possibility that the observed tumor necroses were caused by TSP-2-mediated down-regulation of VEGF gene expression, and demonstrate that TSP-2 inhibits tumor angiogenesis even in the presence of high tumor cell VEGF expression. In situ hybridization and immunohistochemical studies also confirmed that TSP-2 mRNA and protein expression were maintained at high levels in TSP-2-transfected tumor transplants and were absent in control tumors throughout the in vivo experiments, excluding a shift of TSP-2 gene expression in vivo.
In summary, the experiments reported here establish TSP-2 as a potent inhibitor of tumor growth and angiogenesis in vivo. The molecular mechanisms of TSP-2-mediated inhibition of tumor angiogenesis still need to be established. Previously, it was suggested that the antiangiogenic effect of the related molecule TSP-1 is partly mediated through interaction of the CSVTCG sequence, contained within the properdin-like type I repeats, with the CD36 receptor on endothelial cells (14, 38, 39). Although the importance of this interaction for the biological activity of TSP-2 still has to be established, it is of interest that TSP-2 also contains two CSVTCG domains within the type I repeats. In addition, analysis of proteolytic fragments of the TSP-1 molecule revealed antiangiogenic activity of peptides derived from the procollagen-like domain, in particular the peptide NGVQYRN (38). This sequence is absent from the procollagen-like domain of TSP-2, which shows relatively low homology with TSP-1, and we are currently investigating whether additional active sites reside within this domain of the TSP-2 molecule. Although it was previously reported that recombinant TSP-2 was less effective than TSP-1 in inhibiting endothelial cell migration in vitro and neovascularization of the rat cornea in vivo (14), our results demonstrate that TSP-2 was significantly more active than TSP-1 in inhibiting squamous cell carcinoma growth and angiogenesis. This finding may reflect differences in the molecular stability or protease resistance of the two molecules in vivo (8), or it may be related to differences in the responsiveness of endothelial cells of different organs. Indeed, the migration of microvascular endothelial cells derived from the human dermis was inhibited more potently by TSP-2 CM than by TSP-1 CM (data not shown). Moreover, mice deficient for TSP-2 demonstrate a more pronounced increase in the number of skin blood vessels (16), as compared with the number of skin blood vessels in TSP-1-deficient mice (29). Similar to the recently reported synergistic antitumoral effects of combined angiostatin and endostatin therapy (40), combined expression of TSP-1 and TSP-2 completely suppressed squamous cell carcinoma development, suggesting that a combination of angiogenesis inhibitors may provide better therapeutic efficiency than single agent therapy. However, further research is needed to evaluate the relative importance of distinct antiangiogenic factors and to establish optimized combinations of different classes of angiogenesis inhibitors (40) with or without additional established antitumoral therapies (41).
Acknowledgments
This work was supported by the National Institutes of Health/National Cancer Institute Grant CA69184 (M.D.), National Institutes of Health Grant AR45418 (P.B.), American Cancer Society Research Project Grant 99-23901 (M.D.), Deutscher Akademischer Austauschdienst (M.S.), Deutsche Forschungsgemeinschaft (T.H.), and the Cutaneous Biology Research Center through the Massachusetts General Hospital/Shiseido Co. Ltd. Agreement (M.D.).
Abbreviations
- TSP
thrombospondin
- VEGF
vascular endothelial growth factor
- HDMEC
human dermal microvascular endothelial cells
- CM
conditioned media
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bornstein P, Devarayalu S, Li P, Disteche C M, Framson P. Proc Natl Acad Sci USA. 1991;88:8636–8640. doi: 10.1073/pnas.88.19.8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bornstein P. FASEB J. 1992;6:3290–3299. doi: 10.1096/fasebj.6.14.1426766. [DOI] [PubMed] [Google Scholar]
- 3.Adams J, Lawler J. Curr Biol. 1993;3:188–190. doi: 10.1016/0960-9822(93)90270-x. [DOI] [PubMed] [Google Scholar]
- 4.Bornstein P, O'Rourke K, Wikstrom K, Wolf F W, Katz R, Li P, Dixit V M. J Biol Chem. 1991;266:12821–12824. [PubMed] [Google Scholar]
- 5.Bornstein P, Sage E. Methods Enzymol. 1994;245:62–85. doi: 10.1016/0076-6879(94)45006-4. [DOI] [PubMed] [Google Scholar]
- 6.Laherty C D, O'Rourke K, Wolf F W, Katz R, Seldin M F, Dixit V M. J Biol Chem. 1992;267:3274–3281. [PubMed] [Google Scholar]
- 7.Chen H, Sottile J, O'Rourke K M, Dixit V M, Mosher D F. J Biol Chem. 1994;269:32226–32232. [PubMed] [Google Scholar]
- 8.Chen H, Strickland D K, Mosher D F. J Biol Chem. 1996;271:15993–15999. doi: 10.1074/jbc.271.27.15993. [DOI] [PubMed] [Google Scholar]
- 9.Iruela-Arispe M L, Liska D J, Sage E H, Bornstein P. Dev Dyn. 1993;197:40–56. doi: 10.1002/aja.1001970105. [DOI] [PubMed] [Google Scholar]
- 10.Kyriakides T R, Zhu Y-H, Yang Z, Bornstein P. J Histochem Cytochem. 1998;46:1007–1015. doi: 10.1177/002215549804600904. [DOI] [PubMed] [Google Scholar]
- 11.Detmar, M. (2000) J. Invest. Dermatol. Symp. Proc., in press.
- 12.Lafeuillade B, Pellerin S, Keramidas M, Danik M, Chambaz E M, Feige J J. J Cell Physiol. 1996;167:164–172. doi: 10.1002/(SICI)1097-4652(199604)167:1<164::AID-JCP19>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 13.Reed M, Iruela-Arispe L, O'Brien E, Truong T, LaBell T, Bornstein P, Sage E. Am J Pathol. 1995;147:1068–1080. [PMC free article] [PubMed] [Google Scholar]
- 14.Volpert O V, Tolsma S S, Pellerin S, Feige J J, Chen H, Mosher D F, Bouck N. Biochem Biophys Res Commun. 1995;217:326–332. doi: 10.1006/bbrc.1995.2780. [DOI] [PubMed] [Google Scholar]
- 15.Murphy-Ullrich J E, Gurusidappa S, Frazier W A, Hook M. J Biol Chem. 1993;268:26784–26789. [PubMed] [Google Scholar]
- 16.Kyriakides T R, Zhu Y H, Smith L T, Bain S D, Yang Z, Lin M T, Danielson K G, Iozzo R V, LaMarca M, McKinney C E, et al. J Cell Biol. 1998;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson D W, Bouck N P. In: Antiangiogenic Agents in Cancer Therapy. Teicher B A, editor. Totowa, NJ: Humana; 1999. pp. 185–203. [Google Scholar]
- 18.Weinstat S D, Zabrenetzky V S, VanHoutte K, Frazier W A, Roberts D D, Steeg P S. Cancer Res. 1994;54:6504–6511. [PubMed] [Google Scholar]
- 19.Bleuel K, Popp S, Fusenig N, Stanbridge E, Boukamp P. Proc Natl Acad Sci USA. 1999;96:2065–2070. doi: 10.1073/pnas.96.5.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Streit M, Velasco P, Brown L F, Skobe M, Richard L, Riccardi L, Lawler J, Detmar M. Am J Pathol. 1999;155:441–452. doi: 10.1016/S0002-9440(10)65140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melnyk O, Shuman M A, Kim K J. Cancer Res. 1996;56:921–924. [PubMed] [Google Scholar]
- 22.Richard L, Velasco P, Detmar M. Exp Cell Res. 1998;240:1–6. doi: 10.1006/excr.1998.3936. [DOI] [PubMed] [Google Scholar]
- 23.Detmar M, Brown L F, Claffey K P, Yeo K-T, Kocher O, Jackman R W, Berse B, Dvorak H F. J Exp Med. 1994;180:1141–1146. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berse B, Brown L F, Van De Water L, Dvorak H F, Senger D R. Mol Biol Cell. 1992;3:211–220. doi: 10.1091/mbc.3.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Gorczyca W, Gong J, Darzynkiewicz Z. Cancer Res. 1993;53:1945–1951. [PubMed] [Google Scholar]
- 27.Senger D R, Ledbetter S R, Claffey K P, Papadopoulos-Sergiou A, Perruzzi C A, Detmar M. Am J Pathol. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- 28.Detmar M, Brown L F, Berse B, Jackman R W, Elicker B M, Dvorak H F, Claffey K P. J Invest Dermatol. 1997;108:263–268. doi: 10.1111/1523-1747.ep12286453. [DOI] [PubMed] [Google Scholar]
- 29.Lawler J, Sunday M, Thibert V, Duquette M, George E L, Rayburn H, Hynes R O. J Clin Invest. 1998;101:982–992. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Detmar M, Brown L F, Schön M P, Elicker B M, Velasco P, Richard L, Fukumura D, Monsky W, Claffey K P, Jain R K. J Invest Dermatol. 1998;111:1–6. doi: 10.1046/j.1523-1747.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- 31.Dejana E, Corada M, Lampugnani M G. FASEB J. 1995;9:910–918. [PubMed] [Google Scholar]
- 32.Kyriakides T R, Leach K J, Hoffman A S, Ratner B D, Bornstein P. Proc Natl Acad Sci USA. 1999;96:4449–4454. doi: 10.1073/pnas.96.8.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyriakides, T. R., Tam, J. W. Y. & Bornstein, P. (1999) J. Invest. Dermatol., in press. [DOI] [PubMed]
- 34.Bertin N, Clezardin P, Kubiak R, Frappart L. Cancer Res. 1997;57:396–399. [PubMed] [Google Scholar]
- 35.Oshika Y, Masuda K, Tokunaga T, Hatanaka H, Kamiya T, Abe Y, Ozeki Y, Kijima H, Yamazaki H, Tamaoki N, et al. Clin Cancer Res. 1998;4:1785–1788. [PubMed] [Google Scholar]
- 36.Tokunaga T, Nakamura M, Oshika Y, Abe Y, Ozeki Y, Fukushima Y, Hatanaka H, Sadahiro S, Kijima H, Tsuchida T, et al. Br J Cancer. 1999;79:354–359. doi: 10.1038/sj.bjc.6690056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown L F, Detmar M, Claffey K, Nagy J A, Feng D, Dvorak A M, Dvorak H F. EXS. 1997;79:233–269. doi: 10.1007/978-3-0348-9006-9_10. [DOI] [PubMed] [Google Scholar]
- 38.Tolsma S S, Volpert O V, Good D J, Frazier W A, Polverini P J, Bouck N. J Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dawson D W, Pearce S F, Zhong R, Silverstein R L, Frazier W A, Bouck N P. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergers G, Javaherian K, Lo K M, Folkman J, Hanahan D. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 41.Harris A L. Recent Res Cancer Res. 1998;152:341–352. doi: 10.1007/978-3-642-45769-2_33. [DOI] [PubMed] [Google Scholar]