Abstract
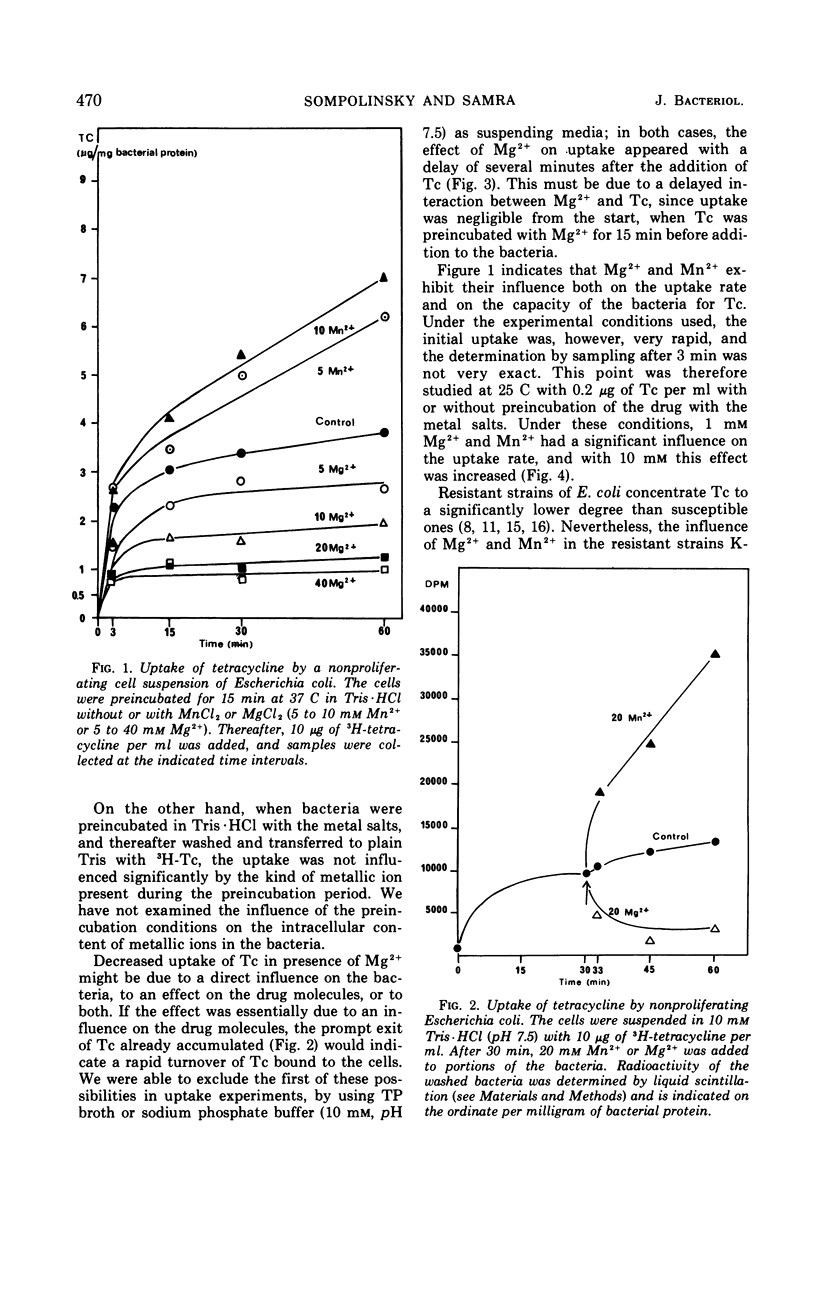
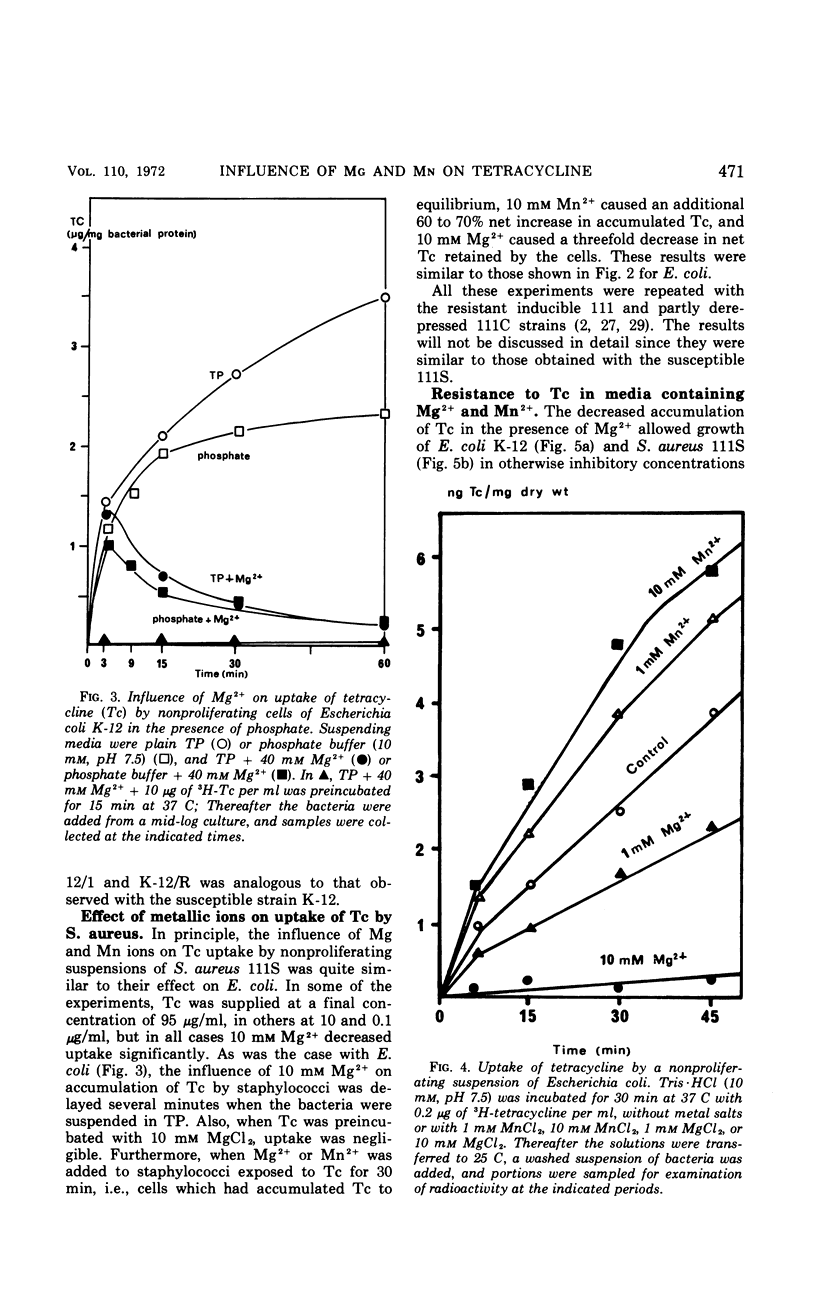
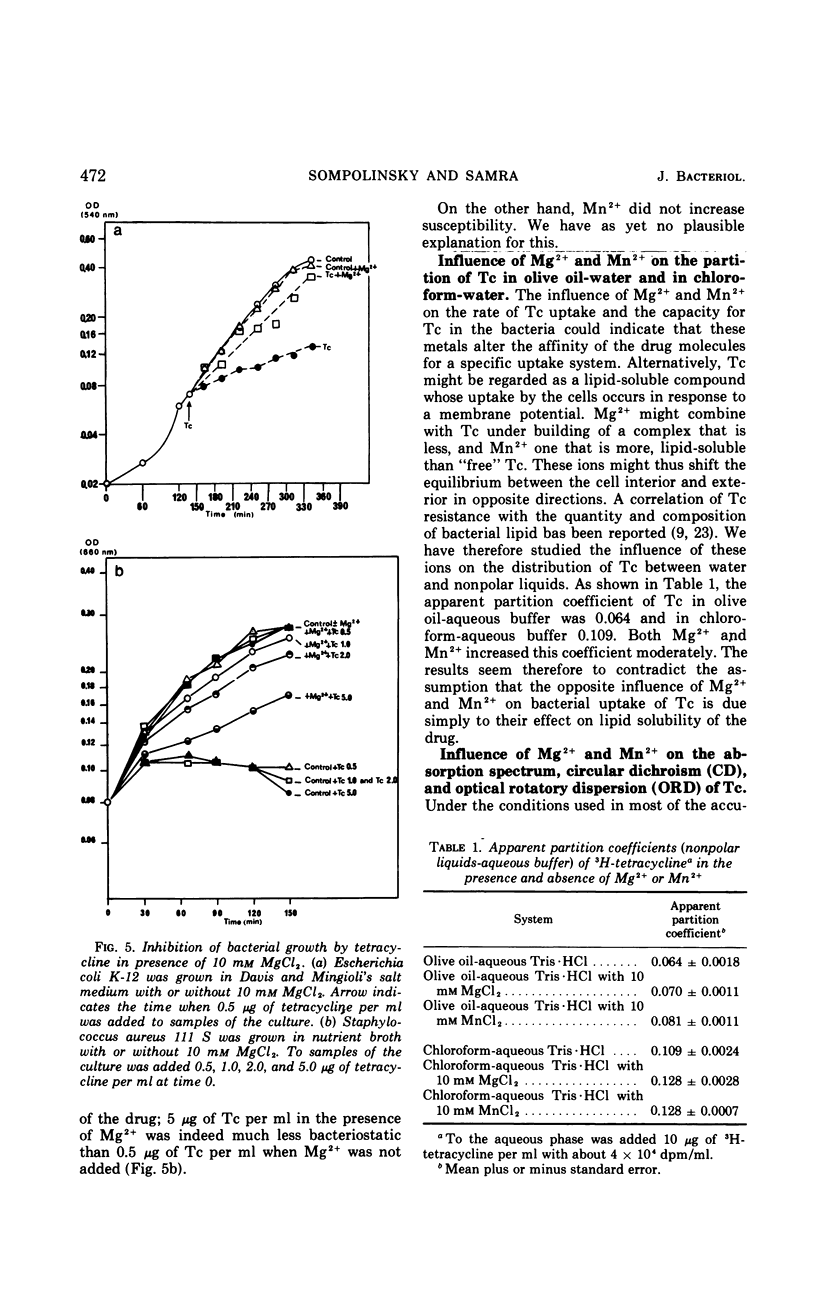
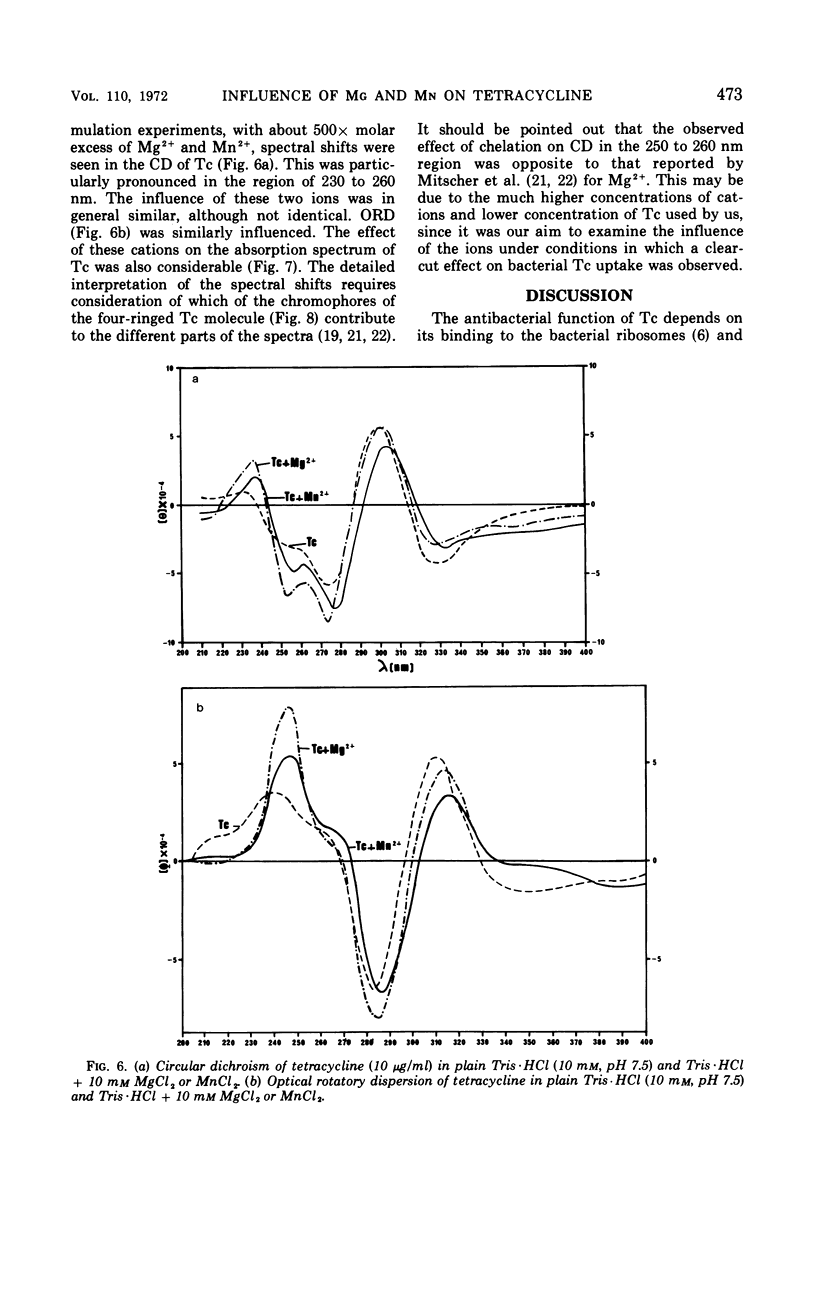
Accumulation of 3H-tetracycline in nonproliferating cells of susceptible and resistant strains of Escherichia coli and Staphylococcus aureus in tris(hydroxymethyl)aminomethane (Tris) buffer (10 mm, pH 7.5) was significantly decreased in the presence of 5 to 40 mm MgCl2 and increased in the presence of 5 to 10 mm MnCl2. When the bacteria first accumulated 3H-tetracycline in plain Tris·HCl, and the metal salts were thereafter added, a prompt decrease or increase in radioactivity of the cells was observed after the addition of Mg2+ or Mn2+, respectively. In phosphate buffer (10 mm, pH 7.5), the effect of Mg2+ was delayed. Three minutes after addition of 3H-tetracycline, uptake was as in the control cell suspension, but thereafter it dropped rapidly. When 3H-tetracycline was incubated with Mg2+ before addition to the bacterial suspension, uptake was scarcely measurable. The addition of Mg2+ to growing cultures of S. aureus and E. coli caused a marked decrease in susceptibility; in contrast, no increase in susceptibility could be demonstrated when Mn2+ was added. It was also demonstrated that Mg2+ and Mn2+ had distinct influences on the absorption spectrum, the optical rotatory dispersion, the circular dichroism, and the lipid solubility of tetracycline.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERT A., REES C. W. Avidity of the tetracyclines for the cations of metals. Nature. 1956 Mar 3;177(4505):433–434. doi: 10.1038/177433a0. [DOI] [PubMed] [Google Scholar]
- Avtalion R. R., Ziegler-Schlomowitz R., Pearl M., Wojdani A., Sompolinsky D. Depressed resistance to tetracycline in Staphylococcus aureus. Microbios. 1971 Mar;3(10):165–180. [PubMed] [Google Scholar]
- CONNAMACHER R. H., MANDEL H. G. BINDING OF TETRACYCLINE TO THE 30S RIBOSOMES AND TO POLYURIDYLIC ACID. Biochem Biophys Res Commun. 1965 Jun 18;20:98–103. doi: 10.1016/0006-291x(65)90954-x. [DOI] [PubMed] [Google Scholar]
- Colaizzi J. L., Klink P. R. pH-Partition behavior of tetracyclines. J Pharm Sci. 1969 Oct;58(10):1184–1189. doi: 10.1002/jps.2600581003. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zeeuw J. R. Accumulation of tetracyclines by Escherichia coli. J Bacteriol. 1968 Feb;95(2):498–506. doi: 10.1128/jb.95.2.498-506.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick J. K., O'Leary W. M. Correlation of bacteria lipid composition with antibiotic resistance. J Bacteriol. 1970 Mar;101(3):892–900. doi: 10.1128/jb.101.3.892-900.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J., Higginson B. Active accumulation of tetracycline by Escherichia coli. Biochem J. 1970 Jan;116(2):287–297. doi: 10.1042/bj1160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J. Resistance of Escherichia coli to tetracyclines. Changes in permeability to tetracyclines in Escherichia coli bearing transferable resistance factors. Biochem J. 1967 Oct;105(1):371–378. doi: 10.1042/bj1050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY W. D., HILL R. T., WINNE R., CUNNINGHAM R. W. The enhancement of chlortetracycline absorption by citric acid. J Pharmacol Exp Ther. 1954 Mar;110(3):327–333. [PubMed] [Google Scholar]
- Hutchings B. L. Tetracycline transport in Staphylococcus aureus H. Biochim Biophys Acta. 1969 Feb 18;174(2):734–748. doi: 10.1016/0005-2787(69)90302-5. [DOI] [PubMed] [Google Scholar]
- IZAKI K., ARIMA K. EFFECT OF VARIOUS CONDITIONS ON ACCUMULATION OF OXYTETRACYCLINE IN ESCHERICHIA COLI. J Bacteriol. 1965 May;89:1335–1339. doi: 10.1128/jb.89.5.1335-1339.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Kiuchi K., Arima K. Specificity and mechanism of tetracycline resistance in a multiple drug resistant strain of Escherichia coli. J Bacteriol. 1966 Feb;91(2):628–633. doi: 10.1128/jb.91.2.628-633.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin A. I., May Chan W. Inhibition by tetracyclines of polyuridylic acid directed phenylalanine incorporation in Escherichia coli cell-free systems. Biochem Biophys Res Commun. 1964;14:137–142. doi: 10.1016/0006-291x(64)90243-8. [DOI] [PubMed] [Google Scholar]
- Mitscher L. A., Bonacci A. C., Slater-Eng B., Hacker A. K., Sokoloski T. D. Interaction of various tetracyclines with metallic cations in aqueous solutions as measured by by circular dichroism. Antimicrob Agents Chemother (Bethesda) 1969;9:111–115. [PubMed] [Google Scholar]
- Mitscher L. A., Bonacci A. C., Sokoloski T. D. Circular dichroism and solution conformation of the tetracycline antibiotics. Tetrahedron Lett. 1968 Oct;(51):5361–5364. doi: 10.1016/s0040-4039(00)75384-6. [DOI] [PubMed] [Google Scholar]
- Mitscher L. A., Bonacci A. C., Sokoloski T. D. Circular dichroism and solution conformation of the tetracycline antibiotics. Antimicrob Agents Chemother (Bethesda) 1968;8:78–86. [PubMed] [Google Scholar]
- SAZ A. K., SLIE R. B. Reversal of aureomycin inhibition of bacterial cell-free nitro reductase by manganese. J Biol Chem. 1954 Sep;210(1):407–412. [PubMed] [Google Scholar]
- SONCIN E. Fenomeni di interferenza tra elettroliti e antibiotici. III. Ione magnesio e aureomicina, terramicina, cloramfenicolo. Arch Int Pharmacodyn Ther. 1953 Jul;94(3):346–352. [PubMed] [Google Scholar]
- Sompolinski D., Ben-Yakov M., Aboud M., Boldur I. Transferable resistance factors with mutator effect in Salmonella typhi. Mutat Res. 1967 Mar-Apr;4(2):119–127. doi: 10.1016/0027-5107(67)90063-2. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Krawitz T., Zaidenzaig Y., Abramova N. Inducible resistance to tetracycline in Staphylococcus aureus. J Gen Microbiol. 1970 Aug;62(3):341–349. doi: 10.1099/00221287-62-3-341. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Samra Z. Mechanism of high-level resistance to chloramphenicol in different Escherichia coli variants. J Gen Microbiol. 1968 Jan;50(1):55–66. doi: 10.1099/00221287-50-1-55. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Zaidenzaig Y., Ziegler-Schlomowitz R., Abramova N. Mechanism of tetracycline resistance in Staphylococcus aureus. J Gen Microbiol. 1970 Aug;62(3):351–362. doi: 10.1099/00221287-62-3-351. [DOI] [PubMed] [Google Scholar]
- VAN METER J. C., SPECTOR A., OLESON J. J., WILLIAMS J. H. In vitro action of aureomycin on oxidative phosphorylation in animal tissues. Proc Soc Exp Biol Med. 1952 Oct;81(1):215–217. doi: 10.3181/00379727-81-19825. [DOI] [PubMed] [Google Scholar]


