Abstract
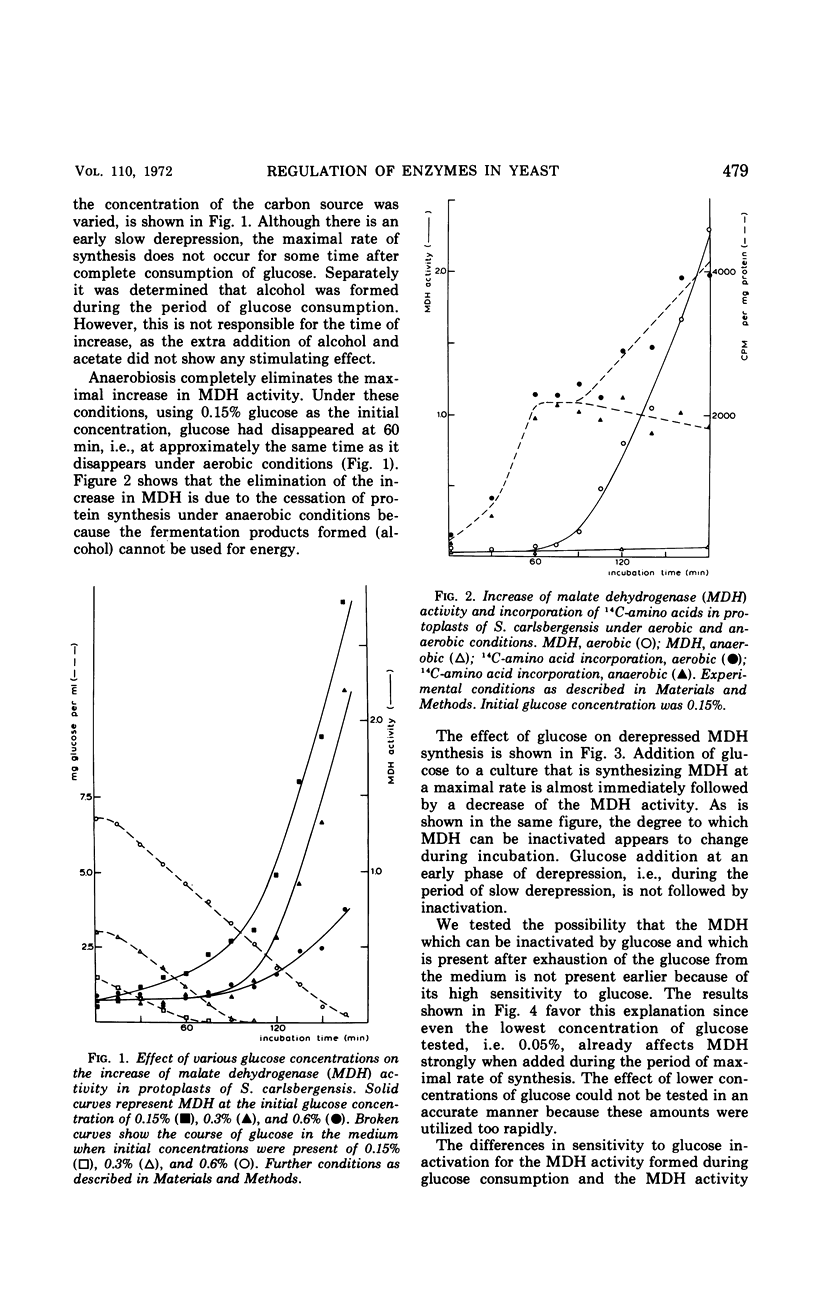
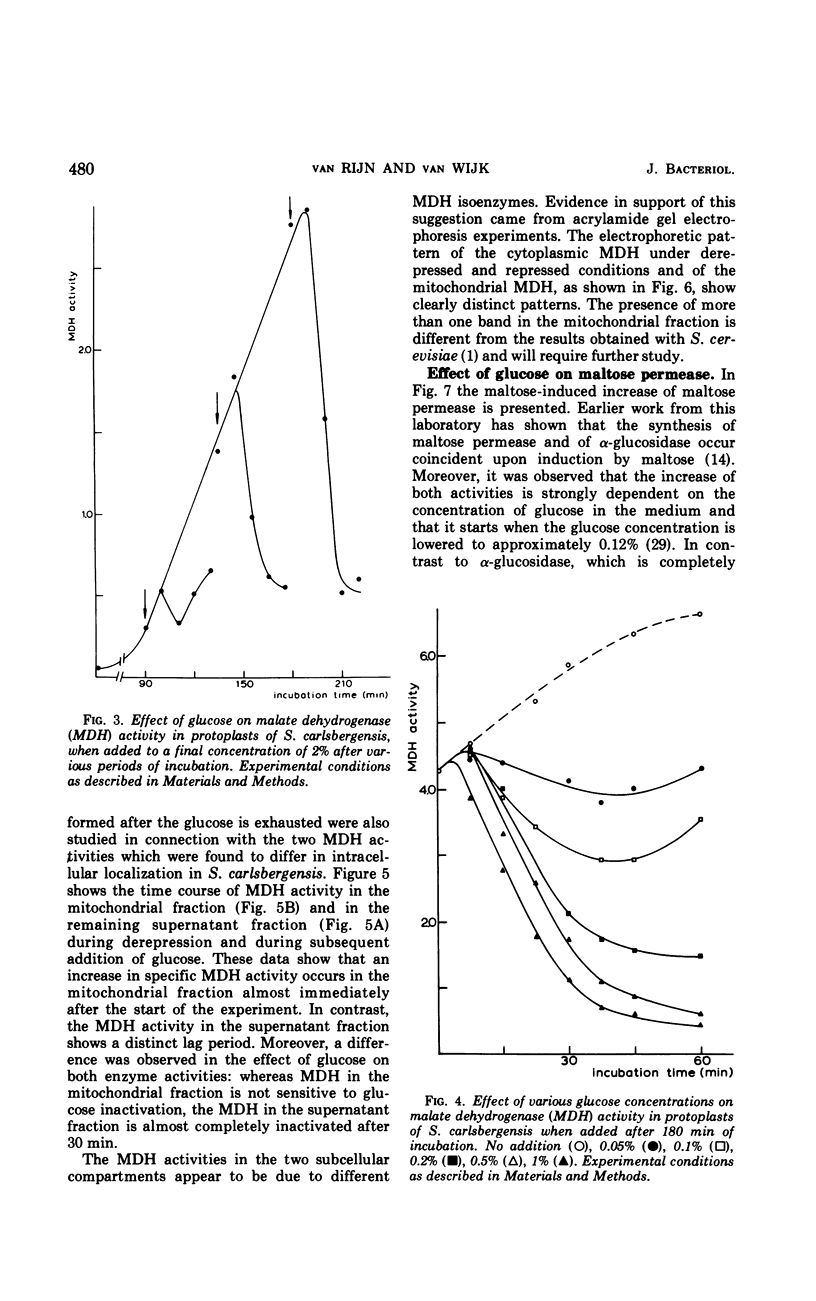
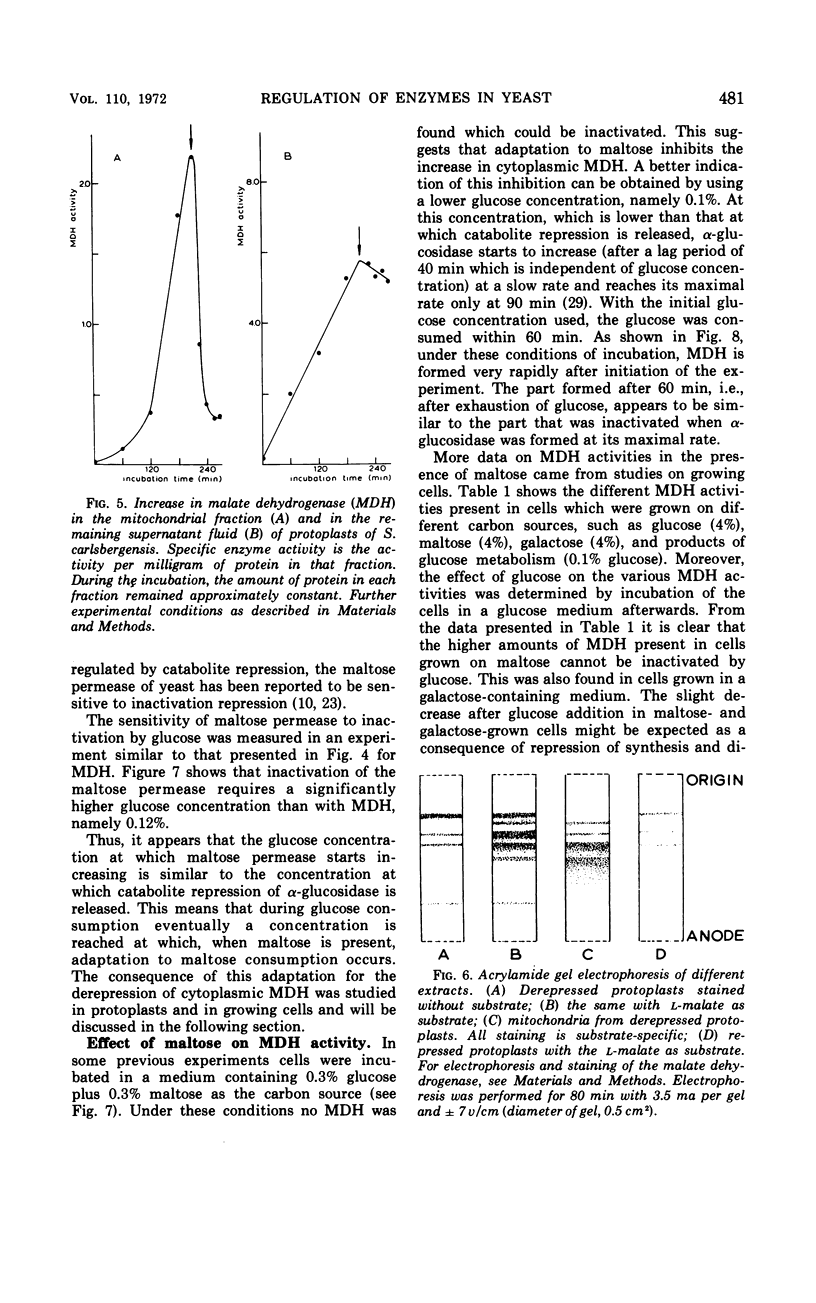
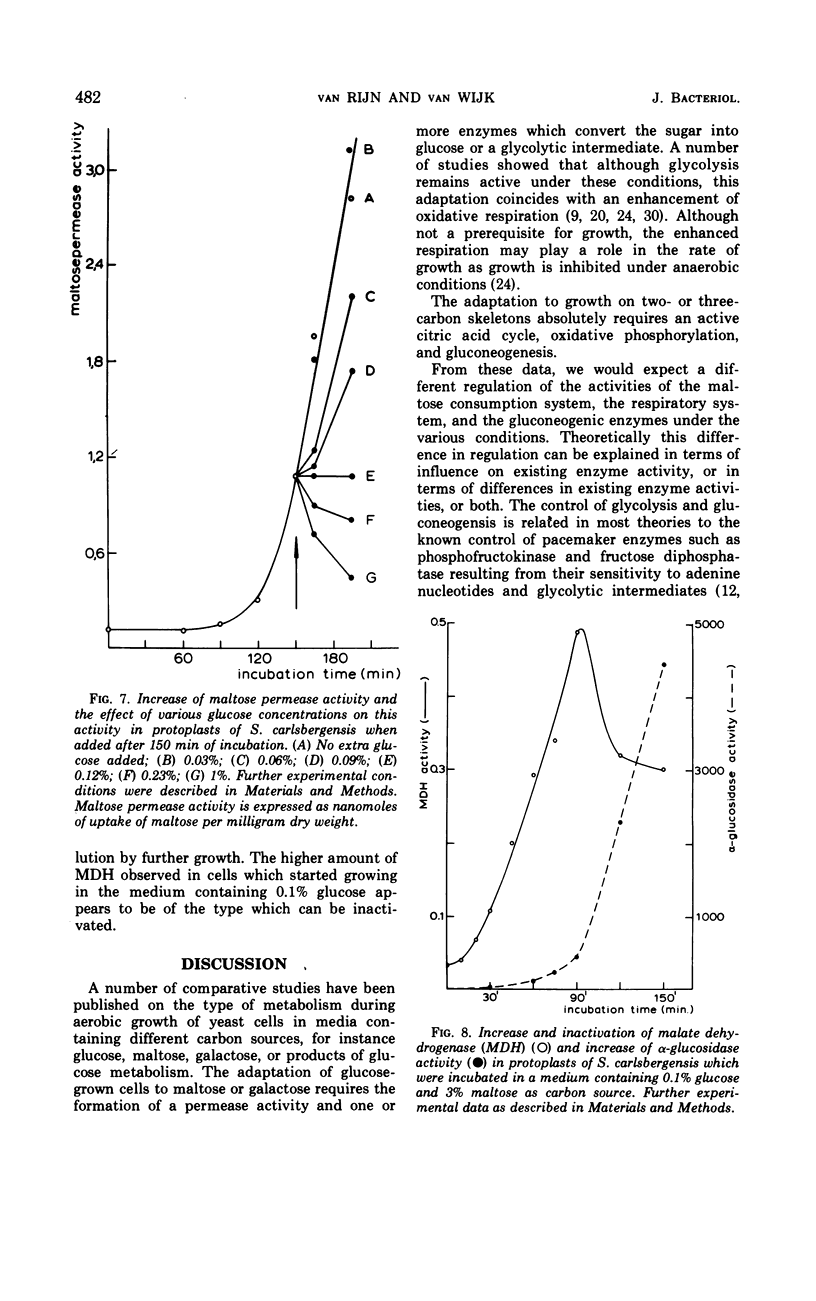
In Saccharomyces carlsbergensis the two malate dehydrogenase activities, which are localized in different compartments of the cell, were found to differ in their response to glucose. The cytoplasmic malate dehydrogenase activity appears to be sensitive to inactivation by very low concentrations of glucose. The mitochondrial malate dehydrogenase activity is only repressed at a higher glucose concentration. Maltose permease is also sensitive to inactivation by glucose. Conditions were found such that the maltose permease was present while the cytoplasmic malate dehydrogenase was inactivated. The different sensitivities of the two malate dehydrogenases and maltose permease to the effect of glucose may explain the preferential use of glucose, maltose, and products of glucose metabolism (2- and 3-carbon skeletons) as carbon sources for growth in the order as mentioned.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atzpodien W., Gancedo J. M., Duntze W., Holzer H. Isoenzymes of malate dehydrogenase in Saccharomyces cerevisiae. Eur J Biochem. 1968 Dec;7(1):58–62. doi: 10.1111/j.1432-1033.1968.tb19573.x. [DOI] [PubMed] [Google Scholar]
- Chapman C., Bartley W. Adenosine phosphates and the control of glycolysis and gluconeogenesis in yeast. Biochem J. 1969 Mar;111(5):609–613. doi: 10.1042/bj1110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman C., Bartley W. The kinetics of enzyme changes in yeast under conditions that cause the loss of mitochondria. Biochem J. 1968 Apr;107(4):455–465. doi: 10.1042/bj1070455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUELL E. A., INOUE S., UTTER M. F. ISOLATION AND PROPERTIES OF INTACT MITOCHONDRIA FROM SPHEROPLASTS OF YEAST. J Bacteriol. 1964 Dec;88:1762–1773. doi: 10.1128/jb.88.6.1762-1773.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duntze W., Neumann D., Gancedo J. M., Atzpodien W., Holzer H. Studies on the regulation and localization of the glyoxylate cycle enzymes in Saccharomyces cerevisiae. Eur J Biochem. 1969 Aug;10(1):83–89. doi: 10.1111/j.1432-1033.1969.tb00658.x. [DOI] [PubMed] [Google Scholar]
- Ferguson J. J., Jr, Boll M., Holzer H. Yeast malate dehydrogenase: enzyme inactivation in catabolite repression. Eur J Biochem. 1967 Mar;1(1):21–25. doi: 10.1007/978-3-662-25813-2_4. [DOI] [PubMed] [Google Scholar]
- Gancedo C. Inactivation of fructose-1,6-diphosphatase by glucose in yeast. J Bacteriol. 1971 Aug;107(2):401–405. doi: 10.1128/jb.107.2.401-405.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görts C. P. Effect of glucose on the activity and the kinetics of the maltose-uptake system and of alpha-glucosidase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1969 Jul 30;184(2):299–305. doi: 10.1016/0304-4165(69)90032-4. [DOI] [PubMed] [Google Scholar]
- HALVORSON H., ELLIAS L. The purification and properties of an alpha-glucosidase of Saccharomyces italicus Y1225. Biochim Biophys Acta. 1958 Oct;30(1):28–40. doi: 10.1016/0006-3002(58)90237-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Newsholme E. A., Gevers W. Control of glycolysis and gluconeogenesis in liver and kidney cortex. Vitam Horm. 1967;25:1–87. doi: 10.1016/s0083-6729(08)60033-3. [DOI] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J. 1965 Oct;97(1):284–297. doi: 10.1042/bj0970284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W., Meek G. A. Changes in the activities of respiratory enzymes during the aerobic growth of yeast on different carbon sources. Biochem J. 1965 Oct;97(1):298–302. doi: 10.1042/bj0970298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W., Meek G. A. Changes in the structure and enzyme activity of Saccharomyces cerevisiae in response to changes in the environment. Biochem J. 1964 Feb;90(2):369–374. doi: 10.1042/bj0900369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON J. J., HALVORSON H. O. The components of maltozymase in yeast, and their behavior during deadaptation. J Bacteriol. 1957 Feb;73(2):186–198. doi: 10.1128/jb.73.2.186-198.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustanoff E. R., Bartley W. Development of respiration in yeast grown anaerobically on different carbon sources. Biochem J. 1964 Jun;91(3):595–600. doi: 10.1042/bj0910595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wijk R., Ouwehand J., van den Bos T., Koningsberger V. V. Induction and catabolite repression of alpha-glucosidase synthesis in protoplasts of Saccharomyces carlsbergensis. Biochim Biophys Acta. 1969 Jul 22;186(1):178–191. doi: 10.1016/0005-2787(69)90501-2. [DOI] [PubMed] [Google Scholar]
- Vary M. J., Edwards C. L., Stewart P. R. The biogenesis of mitochondria. IX. Formation of the soluble mitochondrial enzymes malate dehydrogenase and fumarase in Saccharomyces cerevisiae. Arch Biochem Biophys. 1969 Mar;130(1):235–243. doi: 10.1016/0003-9861(69)90029-0. [DOI] [PubMed] [Google Scholar]
- Witt I., Kronau R., Holzer H. Isoenzyme der malatdehydrogenase und ihre regulation in Saccharomyces cerevisiae. Biochim Biophys Acta. 1966 Oct 17;128(1):63–73. [PubMed] [Google Scholar]
- Witt I., Kronau R., Holzer H. Repression von Alkoholdehydrogenase, Malatdehydrogenase, Isocitratlyase und Malatsynthase in Hefe durch Glucose. Biochim Biophys Acta. 1966 Jun 15;118(3):522–537. [PubMed] [Google Scholar]
- de KLOET S., van WERMESKERKEN R., KONINGSBERGER V. V. Studies on protein synthesis by protoplasts of Saccharomyces carlsbergensis. I. The effect of ribonuclease on protein synthesis. Biochim Biophys Acta. 1961 Feb 12;47:138–143. doi: 10.1016/0006-3002(61)90838-1. [DOI] [PubMed] [Google Scholar]
- de Kroon R. A., Koningsberger V. V. An inducible transport system for alpha-glucosides in protoplasts of Saccharomyces carlsbergensis. Biochim Biophys Acta. 1970 Apr 15;204(2):590–609. doi: 10.1016/0005-2787(70)90178-4. [DOI] [PubMed] [Google Scholar]
- van Wijk R. Alpha-glucosidase synthesis, respiratory enzymes and catabolite repression in yeast. 3. The correlation between the synthesis of alpha-glucosidase and that of some respiratory enzymes. Proc K Ned Akad Wet C. 1968;71(2):137–151. [PubMed] [Google Scholar]
- van Wijk R. Alpha-glucosidase synthesis, respiratory enzymes and catabolite repression in yeast. I. The effects of glucose and maltose on inducible alpha-glucosidase synthesis in protoplasts of S. carlsbergensis. Proc K Ned Akad Wet C. 1968;71(1):60–71. [PubMed] [Google Scholar]
- van Wijk R. Alpha-glucosidase synthesis, respiratory enzymes and catabolite repression in yeast. IV. A. Studies on the level of protein synthesis at which repression and induction of alpha-glucosidase synthesis occur. Proc K Ned Akad Wet C. 1968;71(3):293–301. [PubMed] [Google Scholar]


