Abstract
Reorganization of motor circuits in the cerebral cortex is thought to contribute to recovery following stroke. These can be examined with transcranial magnetic stimulation (TMS) using measures of corticospinal tract integrity and intracortical excitability. However, little is known about how these changes develop during the important early period post-stroke and their influence on recovery. We used TMS to obtain multiple measures bilaterally in a group of 10 patients during the early days and weeks and up to 6 months post-stroke, in order to examine correlations with tests of hand function. Ten age-matched healthy subjects were also studied. After stroke, day-to-day variation in performance was unrelated to physiological measures in the first 3 weeks. Measures of corticospinal integrity averaged over the same period correlated well with hand function, but this relationship became weaker at 3 months. In contrast, most intracortical excitability measures did not correlate acutely but did so strongly at 3 months. Thus in the acute stage, patients’ performance is limited by damage to corticospinal output. Improved performance at 3 months may depend on reorganization in alternative cortical networks to maximize the efficiency of remaining corticospinal pathways—intracortical disinhibition may aid recovery by promoting access to these networks.
Keywords: intracortical inhibition, reorganization, stroke, transcranial magnetic stimulation
Introduction
Ischemic stroke frequently leads to impairment of upper limb motor function, after which a variable degree of motor recovery is seen (Twitchell 1957). Functional imaging in humans (Ward et al. 2003a, 2004) and physiological observations in animal models (Jones and Schallert 1994; Nudo and Milliken 1996) suggest that recovery of function is associated with extensive reorganization of the motor system at the cortical level, presumably to maximize control of remaining motor output. Transcranial magnetic stimulation (TMS) has also been used in human stroke patients to probe corticospinal and intracortical physiology. Reduced corticospinal excitability from the affected hemisphere (AH) reflects damage to the corticospinal connection (Traversa et al. 1998; Byrnes et al. 2001), whereas increased intracortical excitability in both hemispheres (Liepert, Hamzei, and Weiller 2000; Liepert, Storch, et al. 2000; Manganotti et al. 2002) reflects changes in intrinsic circuits of the cortex.
There are, however, important gaps in our knowledge. First, physiological data acquired during the first weeks after stroke have not provided consistent results: motor thresholds in the AH were raised in some (Liepert, Storch et al. 2000; Manganotti et al. 2002) but not all (Delvaux et al. 2003) studies. Likewise, corticospinal hyperexcitability in the unaffected hemisphere (UH) was observed in some studies (Cicinelli et al. 1997; Traversa et al. 1998; Delvaux et al. 2003) but not in others (Manganotti et al. 2002). For many patients, the early days and weeks after stroke are likely to be a period of great clinical and physiological change. Although several studies have performed TMS assessments during this early period, many have made only a single assessment. The greatest number within the first month is 3 measures of corticospinal excitability (D'Olhaberriague et al. 1997; Delvaux et al. 2003) and 2 measures of intracortical excitability (Manganotti et al. 2002). Given the variability in many physiological parameters found by previous studies, it seems likely that more frequent early measurements would provide a more accurate assessment of early neurophysiological changes after stroke.
Secondly, little is known about the clinical significance of these abnormalities. Reduced corticospinal excitability of the AH in the first 5 days after stroke predicts poorer motor outcome later on (Trompetto et al. 2000) and is known to be associated with poor function when studied in the chronic stage (Thickbroom et al. 2002). Likewise, intracortical disinhibition of the AH and UH (at 1 month) is seen in patients with greater motor impairment (Manganotti et al. 2002). Thus, although some neurophysiological parameters appear to be related to motor impairment in these cross-sectional studies, it is not clear whether a longitudinal relationship exists. Furthermore, these measures assess different aspects of neurophysiological function each of which might be more or less important for recovery at different times after stroke. It is therefore important to know whether the relationship between motor impairment and these parameters changes during the days, weeks, and months after stroke.
We present experiments in which we acquired detailed longitudinal neurophysiological and clinical data over the first few weeks and months after first-ever ischemic stroke. Our patient group had a relatively wide range of functional impairment, allowing the possibility to examine correlations with clinical scores. Single-pulse TMS measures (resting motor thresholds [rMTs], active motor thresholds [aMTs], and motor-evoked potential [MEP] recruitment curves [RCs]) provide information about corticospinal excitability, specifically of the remaining original projection from the primary motor cortex (M1) to the spinal cord. The 3 paired pulse measures used (short-interval intracortical inhibition [SICI] and long-interval intracortical inhibition [LICI], and intracortical facilitation [ICF]) are well-described parameters that provide information about intracortical excitability. These intracortical interactions are thought to play a role in regulating the output of the motor cortex. We reasoned that if dynamic changes occur in how the motor output is organized during the course of recovery, these would likely be reflected in corresponding changes in the relationships between physiological parameters and behavioral measures.
We included multiple time points during the early poststroke period to provide information about the variability of neurophysiological parameters at this stage, the degree to which early abnormalities might resolve, and the time course over which this might occur. Our aim was to provide a picture of the changes in cortical physiology occurring after stroke in this group of 10 patients and to relate these changes to upper limb function. On the basis of data from this group, we propose a model, for further testing, of how cortical reorganization may facilitate functional recovery.
Materials and Methods
Subjects
Ten patients were recruited consecutively from the Acute Stroke and Brain Injury Unit of the National Hospital for Neurology and Neurosurgery, London. All patients had suffered from first-ever ischemic stroke causing upper limb weakness (4 or less on the Medical Research Council [MRC] scale in at least one upper limb muscle group) lasting more than 48 h. Exclusion criteria consisted of 1) history of major psychiatric or previous neurological disease, including seizures or previous stroke; 2) cognitive impairment or dysphasia sufficient to affect informed consent; and 3) major comorbidity. All patients received multidisciplinary post-stroke care appropriate to their clinical needs, and in all a magnetic resonance imaging (MRI) head scan was performed as part of routine clinical practice in order to localize the infarction. The age-matched control group of 10 subjects was recruited from a database of volunteers held at the Institute of Neurology, London. They were right handed according to the Edinburgh Inventory of handedness (Oldfield 1971), with a mean handedness score of +19.1, and reported no history of neurological or psychiatric illness. Full written consent was obtained from all subjects in accordance with the Declaration of Helsinki. The study was approved by the Joint Research Ethics Committee of the National Hospital for Neurology and Neurosurgery and Institute of Neurology, University College of London Hospitals National Health Service Trust, London.
Behavioral Evaluation
On each occasion, patients were evaluated using a battery of standardized outcome measures designed to assess both upper limb function (action research arm test [ARAT] and nine-hole peg test [NHPT]) and more global aspects of recovery (Motricity Index, National Institutes of Health Stroke Scale [NIHSS], timed 10-m walk, Barthel Index, and modified Rankin Score). In the ARAT, patients are asked to manipulate objects of varying sizes with the affected arm, assessing 4 aspects of arm function: grasp, grip, pinch movements, and gross arm movements. The NHPT involves measuring the time taken to place 9 pegs consecutively into individual holes, using 1 hand. If the task was not completed within a minute, then the number of pegs placed within that time was scored. Each hand was examined 3 times at each evaluation and the mean score recorded as pegs per second for that hand. The ARAT and NHPT were each assessed on both the affected and unaffected sides and were subsequently corrected within each patient by expressing the score for the affected side as a percentage of that for the unaffected side, such that no impairment at all would result in a score of 100%. Although the ARAT tests a range of aspects of arm and hand function, including proximal movements, the NHPT depends largely on the fine control of finger movements.
Radiological Localization
Routine clinical MRI comprising T1, T2, fluid-attenuated inversion recovery, and diffusion-weighted sequences was performed in all patients within a week of the stroke. Only patients with ischemic infarction were included as the vascular pathology of intracerebral hemorrhage may result in different pathophysiological changes during the acute period studied. Patients were not selected on the basis of lesion site (see Table 1).
Table 1.
Patient characteristics
| Patient | Age (years) | Sex | Affected arm | Lesion site | First Assessed (days post) | Initial severity (MRC) | Initial Barthel score | Previous medical history | Medication |
| 1 | 67 | M | L | R MCA | 8 | 2 | 10 | Hypertension, PUD, bladder surgery | Clopidogrel, Lisinopril, Propranolol, Amlodipine |
| 2 | 82 | F | L | R striatocapsular | 19 | 3 | 13 | AF, thyrotoxicosis, IHD | Warfarin, Atenolol, Thyroxine, Ramipril, Digoxin |
| 3 | 67 | F | L | Isolated R M1 | 15 | 3 | 20 | Hypertension, asthma, CAH | Aspirin, Amlodipine, Azathioprine, Prednisolone |
| 4 | 50 | M | R | L striatocapsular | 10 | 4 | 20 | AF, hypertension, liver transplant, Hepatitis C, IDDM | Warfarin, Insulin, Mycophenolate, Thiamine, Citalopram |
| 5 | 19 | F | L | R MCA | 7 | 0 | 15 | DVT, postinfectious arthritis | Aspirin, Simvastatin |
| 6 | 65 | M | L | R MCA | 8 | 4 | 13 | IHD, PUD, head injury (no surgery) | Clopidogrel, Atenolol, Atorvastatin, Imdur, Codeine, Phosphate |
| 7 | 59 | M | L | R MCA (sparing cortex) | 10 | 1 | 5 | Appendicectomy, skin neoplasm | Aspirin, Atorvastatin, Omeprazole |
| 8 | 57 | M | L | R MCA (sparing cortex) | 11 | 3 | 18 | Nil | Aspirin, Atorvastatin, Temazepam (nocte) |
| 9 | 58 | M | R | L pons | 6 | 1 | 10 | Hypertension, NIDDM, gout | Aspirin, Simvastatin Diltiazem, Gliclazide Metformin, Omeprazole |
| 10 | 55 | F | L | L striatocapsular | 7 | 4 | 20 | Hypertension, COPD, raised cholesterol, mild depression, paroxysmal hemicrania | Aspirin, Ezetimibe, Candesartan, Pizotifen, Sertraline, Omeprazole, Salbutamol (inhaler) |
Note: Initial severity describes the weakest upper limb muscle group (MRC scale) at the time of maximum weakness. M, male; F, female; L, left; R, right; MCA, middle cerebral artery; PCA, posterior cerebral artery; PUD, peptic ulcer disease; AF, atrial fibrillation; IHD, ischemic heart disease; CAH, chronic active hepatitis; IDDM, insulin-dependent diabetes mellitus; DVT, deep vein thrombosis; NIDDM, noninsulin-dependent diabetes mellitus; COPD, chronic obstructive pulmonary disease.
Transcranial Magnetic Stimulation
TMS was performed using 2 MAGSTIM 200 stimulators (Magstim, Dyfed, UK) connected via a Y-cable to a single figure-of-eight–shaped coil with an internal wing diameter of 7 cm. During stimulation, the coil was held with the handle pointing posterolaterally at an angle of 45 degrees to the midline, such that the current induced in the brain was in an anterior direction. Surface electromyographic (EMG) recordings in a belly-to-tendon montage were made from the first dorsal interosseus (FDI) muscles bilaterally. On each side, the position was identified at which stimulation produced optimal MEPs in the contralateral FDI and these positions were used for the remainder of the session. The raw EMG signal was amplified and filtered with a band-pass filter of 30 Hz to 1 kHz (Digitimer Ltd, Welwyn Garden City, UK). Signals were digitized at 2 kHz (CED Power1401, Cambridge Electronic Design, Cambridge, UK) and stored on a laboratory computer for off-line analysis.
In the UH and AH, the following parameters were assessed: rMT, aMT, MEP RCs, SICI, ICF, and LICI.
Motor Thresholds
Both forms of motor threshold reflect the membrane excitability of pyramidal cell axons. The rMT is also susceptible to synaptic influences at the cortical and spinal levels, both of which may be altered following stroke and are unknown when tested at rest. The aMT, by contrast, is measured during contraction when such synapses are at or near firing potential: this measure may thus be less susceptible to tonic changes in these influences (Talelli et al. 2006). The rMT was defined as the lowest stimulation intensity required to evoke an MEP in the resting FDI of >50 μV in 5 out of 10 trials. The aMT was assessed during mild tonic contraction of the FDI (10–20% of maximum) and was defined as the lowest stimulation intensity required to evoke an MEP of >200 μV in 5 out of 10 trials. If no MEP could be evoked at maximum stimulator output, then the threshold was described as 100%.
Recruitment Curves
MEP RCs test the availability of corticospinal pyramidal cells for excitation across a range of intensities and thus provide a measure of the functional integrity of the corticospinal tract. In order to assess MEP recruitment, MEPs were recorded in turn at 90%, 110%, 130%, and 150% of the rMT for that hemisphere, at least 10 trials being recorded at each stimulus intensity. If due to raised motor thresholds it was not possible to stimulate at 150% of the rMT, then evenly spaced stimulus intensities were used of which 3 were above the rMT.
SICI and ICF
SICI reflects activity in cortical inhibitory interneurons and is at least partly mediated by γ-aminobutyric acid A (GABAA) receptors (Ziemann et al. 1996). ICF is thought to reflect activity in glutamatergic excitatory interneurons, although spinal excitability may also be modulated (Di Lazzaro et al. 2006). These 2 parameters were assessed in the same experiment by the paired pulse method described by Kujirai et al. (1993), with a subthreshold conditioning stimulus (CS) preceding a suprathreshold test stimulus (TS). For both of these measures, the CS was fixed at 80% of aMT and the TS was adjusted at the start of the experiment to evoke an unconditioned MEP in the contralateral FDI of approximately 1 mV amplitude. During each trial a TS was either given on its own or preceded by a CS at an interval of 2, 3, 10, or 15 ms, such that there were 5 conditions. Thus SICI was assessed at interstimulus intervals of 2 and 3 ms, whereas ICF was assessed at 10 and 15 ms. These conditions were randomly intermixed and presented 10 times each, so that a total of 50 trials were performed in each experiment. If there is normal SICI, then MEPs are of reduced amplitude when the CS is present: small MEPs in this context therefore denote strong inhibition. If there is normal ICF, then MEPs are enhanced when the CS is present: large MEPs in this context therefore denote strong facilitation.
Long-Interval Intracortical Inhibition
The measurement of LICI is thought to reflect GABAB-mediated activity in intracortical interneurons and may also depend on recurrent axonal collaterals (Werhahn et al. 1999; Orth and Rothwell 2004). This was measured with 2 identical suprathreshold stimuli separated by 100 ms. The stimulus intensity was adjusted at the start of the experiment to a level evoking an MEP in the contralateral FDI of approximately 1 mV amplitude. If there is normal LICI, then the amplitude of the second MEP is considerably reduced compared with the first.
For both the SICI/ICF and the LICI experiments, if it was not possible to evoke an MEP of 1-mV amplitude due to impaired MEP recruitment, then the lowest stimulus intensity resulting in an MEP of stable size was used.
Study Design
We aimed to begin the assessments as soon as possible after stroke in order to gain insight into the pathophysiology of the acute period. TMS studies as described, along with clinical assessments, were performed as close to daily as possible for the first week after recruitment. Thereafter assessments occurred weekly until 1 month after the stroke and were then repeated at 3 and 6 months. The exact timings were dictated by the practicalities associated with the patient's care: in some cases, fewer sessions were possible during the first week of participation. One patient was unavailable for assessment at the 3-month time point and another was unavailable at 6 months; otherwise complete data sets were obtained in all 10 patients.
Data Analysis and Statistics
In order to ensure that TMS parameters (other than aMTs) were assessed in the resting state, individual trials were examined off-line: those showing any background muscle activity prior to the TMS stimulus artifact were discarded. Peak-to-peak MEP amplitudes were measured in the remaining trials and outliers removed (defined as more than 3 standard deviations [SDs] from the mean for that condition).
When examining correlations between motor thresholds and clinical scores, it was desirable to minimize the effect of the considerable between-subject variability commonly observed in the healthy population. We therefore normalized thresholds in the AH to those in the UH at each data point because natural variability is commonly symmetrical. The formula used was (AH/UH − 1), such that raised thresholds in the AH would result in a positive value, whereas the converse situation would result in a negative value.
For the RC data, the mean MEP amplitude was calculated at each stimulus intensity and a curve was plotted of MEP amplitude versus stimulus intensity. If different stimulus intensities were used (due to a high motor threshold), then their relation to rMT was calculated and the RC plotted in the usual way. The RC gradient was determined for each hemisphere by calculating the gradient of the line of best fit. As only 4 stimulus intensities were tested, a linear fit was applied using the least squares method, in which a line y = mx + b is determined using the formula:
Thus, the calculated RC gradient represents a measure of corticospinal excitability in that hemisphere normalized for the rMT. When determining SICI and ICF, the mean MEP amplitude was calculated for each state and this MEP was then expressed as a percentage of that resulting from a single pulse (conditioned/unconditioned). SICI for the hemisphere being examined was defined as the mean of these values for the 2- and 3-ms interstimulus intervals, whereas ICF was defined as the mean of the 10 and 15 ms values. In order to calculate LICI, the mean MEP amplitude resulting from the second (test) stimulus was expressed as a percentage of that resulting from the first (conditioning) stimulus. Thus for these 3 intracortical parameters, a value less than 100% denotes inhibition, whereas a value greater than 100% denotes facilitation.
Considerable within-subject variability was observed in the physiological parameters during the first month after stroke (see Results). It was therefore desirable to define a measure representative of this period for each patient, in order that clinical correlations from this period could be examined for each parameter. We defined the “acute period” measure, in each patient, as the mean of all values obtained for that parameter within the first 3 weeks after stroke. This time period was chosen so that the early weeks may be effectively represented while maintaining a gap of at least a week from the 1-month time point, thus improving the chance of detecting any time effects present. It was important to ensure that the choice of time interval was not responsible for the results obtained. We therefore also retested all clinical correlations using 2 alternative measures for this period: each patient's first-ever physiological assessment (i.e., a single value) and a mean value across 4 weeks. The results obtained were very similar, providing reassurance that the precise choice of time interval did not determine the results. Group means were calculated in each parameter for this acute value and for all subsequent time points.
We tested whether physiological parameters covaried with ARAT scores across the acute period. In order to do this, it was important to eliminate baseline differences across the group, such that any observed correlations would reflect longitudinal covariance rather than cross-sectional correlation. For each patient, mean values were 1st determined for both the physiological parameter in question and the ARAT score during the acute period. Individual time points were then expressed relative to the patient's mean value: (individual value/mean value). This process allowed data points from different patients to be combined in order to test for longitudinal correlations across the acute period.
All data sets were tested for a normal distribution, using the Kolmogorov–Smirnoff test: of 58 data sets tested, 6 deviated from normal. In order to avoid inconsistent treatment of the data at different time points, we have therefore used parametric statistics throughout. Changes with time in each physiological parameter in each hemisphere were examined using a repeated measures analysis of variance (ANOVA) with the factors time and hemisphere. Differences between the UH and AH were tested using paired t-tests, whereas differences from the healthy control group were tested using unpaired t-tests, Bonferroni corrected for multiple comparisons. Correlations were tested using linear regression analysis between physiological and behavioral measures. Values are not corrected for multiple comparisons, but we have supplied all correlation coefficients and corresponding P values (Table 3).
Table 3.
Correlation coefficients
| Acute | 3 months | 6 months | |||||
| ARAT | NHPT | ARAT | NHPT | ARAT | NHPT | ||
| rMT | −0.720 (0.0094) | −0.620 (0.0281) | −0.292 (0.2236) | −0.597 (0.0449) | −0.837 (0.0025) | −0.572 (0.0539) | |
| aMT | −0.740 (0.0073) | −0.657 (0.0196) | −0.090 (0.4061) | −0.466 (0.1032) | −0.837 (0.0025) | −0.696 (0.0186) | |
| RC gradient | UH | 0.077 (0.4162) | 0.148 (0.3416) | 0.403 (0.1412) | 0.259 (0.2509) | −0.150 (0.3502) | −0.393 (0.1477) |
| AH | 0.754 (0.0059) | 0.774 (0.0043) | 0.449 (0.1128) | 0.535 (0.0687) | 0.545 (0.0648) | 0.454 (0.1100) | |
| SICI | UH | −0.224 (0.2667) | −0.437 (0.1033) | −0.895 (0.0006) | −0.686 (0.0206) | −0.078 (0.4212) | −0.220 (0.2845) |
| AH | −0.020 (0.4830) | −0.038 (0.4680) | −0.526 (0.0728) | −0.550 (0.0627) | −0.418 (0.1314) | −0.134 (0.3758) | |
| ICF | UH | 0.160 (0.3298) | 0.030 (0.4670) | −0.613 (0.0395) | −0.693 (0.0192) | 0.179 (0.3228) | 0.464 (0.1234) |
| AH | −0.096 (0.4186) | 0.098 (0.4174) | −0.237 (0.2695) | −0.329 (0.1939) | −0.287 (0.2269) | 0.077 (0.4350) | |
| LICI | UH | −0.377 (0.1589) | −0.415 (0.1334) | −0.845 (0.0041) | −0.737 (0.0117) | 0.258 (0.2687) | 0.412 (0.1354) |
| AH | −0.908 (0.0024) | −0.907 (0.0024) | −0.656 (0.0387) | −0.636 (0.0327) | −0.225 (0.2958) | −0.315 (0.2240) | |
Note: Correlation coefficients are shown for the plots of each physiological parameter against the clinical scores shown. The first 3 parameters are plotted as semilog plots, as shown in Figure 4A. P values are shown in brackets; Significant correlations are shown in bold.
Results
Patients: Clinical Details
Clinical details of the patients are given in Table 1. The group contained 6 males and 4 females, aged between 19 and 82 (mean ± SD 58.0 ± 16.2 years). One patient was unavailable for assessment at 3 months after the stroke and one other at 6 months. The control group consisted of 10 healthy volunteers, 7 males and 3 females, aged between 23 and 80 (56.2 ± 15.4 years).
Results of Behavioral Tests
Upper limb function was assessed by the ARAT and NHPT. Figure 1A shows results from every assessment within the first 3 months, whereas Figure 1B shows data at the principal time points. There were significant improvements in both scores by 1 month (paired t-tests of initial assessment vs. 1 month: ARAT, P = 0.007; NHPT, P = 0.042) but no further significant changes (1 month vs. 3 months, 3 months vs. 6 months). Floor and ceiling effects were noted with the NHPT and ARAT, respectively. Thus heterogeneity was better represented by the ARAT in the first month after stroke and by the NHPT at 3–6 months.
Figure 1.
Clinical scores describing upper limb function. Scores in the ARAT and NHPT are shown as performance in the affected limb as a percentage of that in the intact limb. (A) Individual scores in the ARAT (A1) and NHPT (A2) are shown for every assessment performed in the first (ca.) 3 months. (B) Scores are displayed for each patient at the principal time points, along with group means. Both ARAT (B1) and NHPT (B2) were significantly improved by 1 month (paired t-tests P < 0.05 vs. initial assessment) and changes beyond this point were not significant. A number of patients had NHPT scores of 0 (or near 0) at the first assessment, whereas many had a maximum ARAT score by 3 months—thus, the ARAT is more informative of the 2 tests in the early period, whereas the NHPT becomes more sensitive later on.
Compared with initial assessment, improvements were significant by 1 month after stroke in Motricity Index, NIHSS, 10-m walk, and Barthel index (paired t-tests: P values, 0.035, 0.002, 0.031, and 0.033, respectively) and almost significant in the modified Rankin score (P = 0.051) (see Supplementary Fig. 1). From 1 month to 3 months, there were further significant improvements in 10-m walk (P = 0.027) and Barthel index (P = 0.0499) but in no other tests. There were no further significant changes beyond 3 months. Most recovery of upper limb function was thus observed in the first month, with some additional improvement in global scores up to 3 months.
Motor Thresholds
Figure 2A shows group means at each time point for rMTs and aMTs. The value for the acute period represents a mean, within each patient, of all assessments made within 3 weeks of the stroke (see Materials and Methods). Absolute values at each time point are given in Table 2, and an illustration of all data collected in the first 40 days can be seen in Supplementary Figure 2A.
Figure 2.
Corticospinal excitability. (A) Group means values for resting (A1) and active (A2) motor thresholds in the UH and AH are shown at the principal time points. Thresholds are shown here as the logarithm of percentage of maximum stimulator output. The value described as acute has been determined in each patient as the mean of all values within the first 3 weeks. Time has differing effects on rMT in the 2 hemispheres—thresholds are initially raised in the AH and subsequently reduce but do not significantly change in the UH. For aMTs, there is a trend reduction in the AH from the acute period to 3 months but no time × hemisphere interaction and no Time effect across all 4 time points (see text for ANOVA details). Both rMT and aMT are significantly higher in the AH than the UH during the acute period (paired t-tests: * P < 0.05), but this difference is not significant later. rMT values in the AH are raised compared with the healthy group only during the acute period, whereas aMT values are also raised at 3 months (unpaired t-tests: † P < 0.05, corrected for multiple comparisons). (B) Group means for the first 3 measurements of aMT in each patient are shown—these were taken 10.1 (±1.3), 13.3 (±1.4), and 17.3 (±1.9) days after the stroke (mean ± standard error). There is a significant time × hemisphere interaction across these 3 time points, explained by a significant increase in aMT in the AH between the first and third measurements (paired t-test: * P = 0.009—see text for ANOVA details). Thus, an increase in aMT in this early period is followed by a reduction in thresholds between months 1 and 3 (Fig. 2A). (C) Group means are shown for RC gradients in either hemisphere at the principal time points. There is a significant time × hemisphere interaction across the 4 time points, but no effect of Time on either hemisphere alone (significant effect of Hemisphere). From the acute period to 6 months, there is also a significant equivalent interaction, explained by a significant decrease in excitability in the UH (paired t-test: * P < 0.05) and a trend increase in the AH (P = 0.059). Excitability in the AH is significantly reduced with respect to the UH at the first 2 time points (paired t-tests: * P < 0.05, ** P < 0.01), but this difference is not significant at later time points. Compared with the healthy control group, RC gradients are significantly reduced in the AH at all time points (unpaired t-tests: †† P < 0.01, † P < 0.05, corrected for multiple comparisons), whereas those in the UH are not significantly different from normal.
Table 2.
Motor thresholds
| Acute period | 1 month | 3 months | 6 months | |
| Resting thresholds (%) | ||||
| UH | 42.3 ± 2.0 | 43.4 ± 2.6 | 45.0 ± 1.5 | 44.8 ± 2.9 |
| AH | 64.3 ± 7.8 | 57.4 ± 6.6 | 51.0 ± 3.6 | 57.4 ± 6.5 |
| Active thresholds (%) | ||||
| UH | 33.9 ± 1.9 | 33.7 ± 1.5 | 34.7 ± 1.1 | 34.4 ± 1.7 |
| AH | 55.2 ± 8.5 | 46.5 ± 6.7 | 41.8 ± 4.1 | 48.2 ± 7.7 |
Note: Group means (±standard error) are shown at each time point for the rMTs and aMTs, as percentage of maximum stimulator output.
Early Variability
There was considerable within- and between-subject variability in motor thresholds during the first 3 weeks after stroke. We examined the combined data from this early period for longitudinal correlations with clinical scores: the observed variability did not reflect fluctuations in clinical scores (combined correlations with ARAT scores for the first 3 weeks: rMT, r = −0.025 [not significant, NS]; aMT, r = −0.011 [NS]). This suggests that such physiological fluctuations are not related in a simple way to early changes in clinical status and must be explained in another way.
The Effect of Time on Resting Thresholds Was Different in the 2 Hemispheres
For resting thresholds, there was a significant time × hemisphere interaction (2-way ANOVA: F3,21 = 8.24, P = 0.022) that was due to the fact that thresholds decreased in the AH (1-way ANOVA: F3,21 = 7.84, P = 0.025) but did not change in the UH (1-way ANOVA: F3,21 = 1.57, P = 0.307). Such a reduction in resting thresholds implies that there was some recovery of corticospinal excitability in the AH during this period. However, the same was not true of aMTs, for which there was no significant difference in the effects of time on the 2 hemispheres (2-way ANOVA: time × hemisphere F3,21 = 3.41, P = 0.110; trend effect of hemisphere F1,7 =4.06, P = 0.084; no effect of Time).
Motor Thresholds Were Raised in the AH during the Acute Period
Both rMT and aMT were significantly higher in the AH than the UH during the acute period (paired t-tests: rMT, P = 0.017; aMT, P = 0.030), but this difference was not significant at later time points. Comparison with the healthy control group revealed that rMT was significantly raised during the acute period but not later (unpaired t-tests, values Bonferroni corrected for multiple comparisons: acute, P = 0.038; 1 month, P = 0.140; 3 months, P = 0.160; and 6 months, P = 0.115), whereas aMT was significantly raised during the acute period and at 3 months (acute, P = 0.015; 1 month, P = 0.051; 3 months, P = 0.039; and 6 months, P = 0.058). Corticospinal excitability as assessed by motor thresholds was thus impaired during the acute period.
We Observed an Early Rise in aMTs
Close inspection of the earliest time points (shown in Supplementary Fig. 2A) gives the impression that, contrary to the overall trend for thresholds to reduce with time, there was an initial increase over the first few assessments. In order to address this possibility, the first 3 measurements of motor thresholds were analyzed separately (Fig. 2B). There was a small variation in the time of the earliest measurements between patients, with the mean number of days after stroke being 10.1 ± 1.3, 13.3 ± 1.4, and 17.3 ± 1.9 (mean ± standard error). There was a significant time × hemisphere interaction (2-way ANOVA: F2,18 =13.02, P = 0.003). This was due to the fact that there was a significant increase in aMT from the first to third assessment in the AH (paired t-test, P = 0.009), but not in the UH. Corticospinal excitability as measured by aMT thus declined further at this early stage, contrary to the longer-term pattern of improvement; there was no equivalent effect for rMT.
Recruitment Curves
Figure 2C shows group means at each time point for RC gradients. An illustration of all data collected in the first 40 days can be seen in Supplementary Figure 2B.
Early Variability
RC gradients in the UH and AH over the first 3 weeks revealed considerable variation between patients with some showing substantial recovery of excitability, whereas in others gradients remained low. This early variability did not reflect fluctuations in clinical scores (combined correlations with ARAT scores during the acute period: UH, r = 0.023 [NS]; AH, r = 0.059 [NS]). This supports the idea that fluctuations in corticospinal excitability do not relate simply to clinical variability during this period.
The Effect of Time on RC Gradients Was Different in the 2 Hemispheres
There was a significant time × hemisphere interaction (2-way ANOVA: F3,21 = 9.08, P = 0.018; significant effect of hemisphere F1,7 = 7.05, P = 0.033), suggesting that this measure of corticospinal excitability changes in opposite directions with time in the 2 hemispheres. Follow-up 1-way ANOVAs were performed separately for each hemisphere: although there was no significant change across all 4 time points in either hemisphere, there is a tendency for the UH gradients to decline (decreasing UH excitability) and the AH gradients to increase (increasing AH excitability). When the acute period was directly compared with 6 months, these effects were more marked with a stronger time × hemisphere interaction (2-way ANOVA: F1,8 = 15.56, P = 0.004; significant effect of hemisphere F1,8 = 14.64, P = 0.005), a significant excitability reduction in the UH (paired t-test, P = 0.042), and a trend increase in the AH (P = 0.059).
RC Gradients in the AH Remained Reduced Compared with Normal
RC gradients were significantly lower in the AH than the UH during the acute period and at 1 month (paired t-tests, P = 0.001 and P = 0.035, respectively), but this difference was not significant later. Comparison with the healthy control group revealed that excitability was reduced in the AH at all time points (unpaired t-tests, values Bonferroni corrected for multiple comparisons: acute, P < 0.001; 1 month, P = 0.011; 3 months, P = 0.033; and 6 months, P = 0.001). Despite the trend for excitability in the AH to increase across the period studied, it did not, therefore, recover to normal levels. RC gradients in the UH did not differ from the control group.
Measures of Intracortical Excitability
Figure 3 shows group means at each time point for SICI, ICF, and LICI. An illustration of all data collected in the first 40 days can be seen in Supplementary Figure 3.
Figure 3.
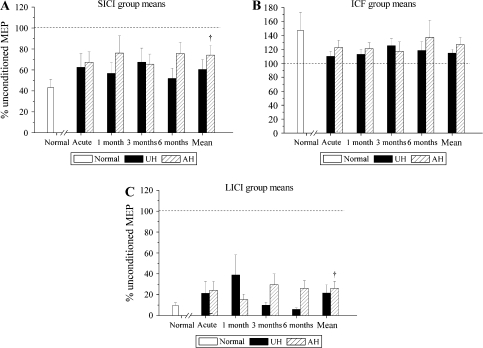
Intracortical Excitability. Mean values at the principal time points are shown for 3 measures of intracortical excitability: (A) SICI, (B) ICF, and (C) LICI. The value shown in each case represents the percentage change of the response to a TS in the presence of a CS: thus 100% would denote no inhibition or facilitation (shown as a dotted line). None of these values were significantly different from the healthy control group at individual time points (unpaired t-tests, values corrected for multiple comparisons). No parameter showed a significant time × hemisphere interaction. A mean value across all time points was thus calculated in each patient (and each parameter and hemisphere). This mean value is significantly raised compared with the normal group in the AH for SICI and LICI (unpaired t-tests: * P < 0.05). This suggests that these forms of intracortical inhibition are weak in the AH (i.e., increased excitability to paired pulse stimuli). The values for SICI and LICI also appear raised in the UH but are not significantly different from the healthy control group.
Early Variability
As with other data collected in this period, there is substantial variation both within and between subjects for the AH and the UH. There is no clear trend to normalization or worsening. Variability during the 3 weeks after stroke did not reflect fluctuations in clinical scores for any of the intracortical excitability parameters in either hemisphere (combined correlations with ARAT scores during the acute period: r values for the UH between −0.099 and 0.352 [NS]; r values for the AH between −0.123 and 0.216 [NS]). As described above for corticospinal excitability, it therefore appears that fluctuations in intracortical excitability likewise do not relate in a simple manner to clinical variability during this early period.
There Were No Time Effects. Inhibition (SICI and LICI) Was Weak in the AH
No clear trends were observed in these parameters up to 6 months. The 2-way time × hemisphere ANOVAs for each parameter showed no significant main or interaction effects. In view of the lack of time effects, a mean value for each parameter across all assessments was calculated for every patient and in each hemisphere (shown in Fig. 3) to allow comparison with values from the healthy control subjects. SICI and LICI (but not ICF) were significantly reduced in the AH when compared with the healthy control group (unpaired t-tests: SICI, P = 0.015; LICI, P = 0.029), but were not significantly reduced in the UH (SICI, P = 0.157; LICI, P = 0.123). Mean values for SICI were 74.1 ± 9.3% of MEP amplitude after a single pulse (patients, AH), 60.4 ± 9.5% (patients, UH), and 44.0 ± 5.6% (healthy volunteers). Mean values for LICI were 26.1 ± 6.8% of MEP amplitude after a single pulse (patients, AH), 21.6 ± 8.0% (patients, UH), and 7.8 ± 2.1% (healthy volunteers). The significantly larger conditioned MEPs (relative to unconditioned) in the patient group imply that these 2 forms of inhibition are weak in the AH.
Relating Physiological Measures to Clinical Performance
At each of the principal time points, correlations were examined between the TMS measures and upper limb clinical scores (ARAT and NHPT). Two examples of such plots are shown in Figure 4 (individual correlation plots can also be seen in Supplementary Figs 4–8); a graphical summary of changes in these correlations over time is displayed in Figure 5 (the complete set of correlation coefficients is given in Table 3).
Figure 4.
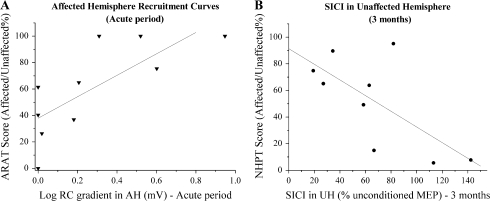
Correlations with clinical status—2 examples. The relationships between measures of clinical performance and 2 physiological parameters are shown, each point representing a patient. (A) There is a significant positive correlation between AH RC gradients in the acute period and ARAT scores at initial assessment (r = 0.754, P = 0.006). (B) SICI in the UH is negatively correlated with NHPT scores when the patients are assessed at 3 months, such that weaker inhibition (i.e., increased excitability to paired pulse TMS) is associated with poor clinical status at this stage (r = −0.686, P = 0.021).
Figure 5.
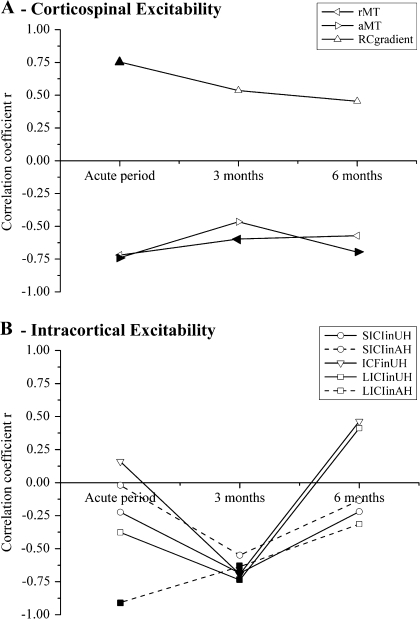
Correlations with clinical status—changes with time. Correlation coefficients between the physiological parameters measured and clinical outcome scores are presented for the 3 phases of stroke recovery studied. Significant correlations are denoted by filled symbols (P < 0.05). Because of the respective floor and ceiling effects seen in NHPT and ARAT scores in the early and late stages, the correlations in the acute period are with ARAT scores and those at later time points are with NHPT scores. The complete correlation coefficients are given in Table 3, and the plots can be seen in Supplementary Figures 4–8. (A) Physiological parameters reflecting integrity of the corticospinal tract are shown. rMTs and aMTs show negative clinical correlations, whereas RC gradients show positive correlations. Thus, poor clinical performance is associated with depressed RC gradients and raised motor thresholds. These correlations are significant for both resting and active thresholds in the acute period and for rMT at 3 months and aMT at 6 months. RC gradients show significant correlations in the acute period. (B) Measures of intracortical excitability are shown, as assessed by paired pulse TMS in each hemisphere . No significant/trend clinical correlations were seen with ICF in the AH, which is not shown. In the acute period, LICI in the AH showed a negative clinical correlation—weaker inhibition associated with worse clinical status—but no other measures showed significant correlations. At 3 months, however, all these measures showed significant negative clinical correlations except for SICI in the AH (which showed a trend correlation, P = 0.063)—weaker SICI/LICI or stronger ICF at this stage was associated with poorer motor function. By 6 months, none of the intracortical excitability measures showed significant or trend clinical correlations. Thus, although clinical status relates closely to corticospinal excitability in the acute period (but less so beyond), intracortical excitability measures become important by 3 months—this relationship is lost by 6 months.
Different Changes in Clinical Correlations with Time Were Observed for Measures of Corticospinal and Intracortical Excitability
Figure 5A shows that in the acute period there were strong relationships between clinical scores and all 3 measures of corticospinal excitability in the AH (rMT, aMT, and RC gradients): small RC gradients or raised motor thresholds were associated with poor clinical status. At 3 and 6 months, this relationship had weakened for RC gradients and was slightly weaker for motor thresholds. By contrast, as shown in Figure 5B, measures of intracortical excitability (SICI, ICF, and LICI), apart from ICF in the AH that was not correlated at any stage (data not shown), behaved very differently. Relationships to clinical scores were weak in the acute period (except for LICI in the AH) but strong at 3 months: at this time raised intracortical excitability to paired pulse TMS (weak inhibition or strong facilitation) was associated with poor clinical status. At 3 months, all the intracortical excitability measures shown in Figure 5B correlated significantly with NHPT scores (except for SICI in the AH: P = 0.063). This relationship had disappeared at 6 months.
To summarize, correlations between clinical status and measures of corticospinal or intracortical excitability changed over time after stroke: correlations with corticospinal excitability were highest in the acute stage, whereas correlations with intracortical excitability were initially poor but then increased strongly at 3 months.
Discussion
The present data provide a longitudinal picture of bilateral cortical physiology in this patient group over a 6-month period after stroke. The results confirm several previously described neurophysiological abnormalities but additionally describe their evolution. We also report changes in LICI after stroke for the first time. Most importantly, we have been able to correlate physiological parameters with clinical function at several time points. The results suggest that in the period following stroke corticospinal and intracortical excitability evolve differently in their relationship to motor function. We argue that these changes in clinical correlation over the 6-month period reflect shifts in how a motor output to the paretic hand is generated.
The Patient Group
Patients were recruited consecutively from an acute stroke unit and were heterogeneous with regard to impairment and lesion site. A relatively wide range of impairment reduces the statistical power of comparisons with healthy controls and may therefore give false negatives when testing for abnormalities. However, this range has the complementary advantage of allowing correlations with functional status to be examined, providing insights into the clinical relevance of the parameters tested. It is also worth noting that there was a wide age range in the patient group, with a mean patient age (58 years) that is somewhat younger than mean age at stroke onset in the wider population. Although this is a potential source of physiological variance, it would not be expected to contribute to the changes observed with time.
Although all patients studied had a single infarction involving the corticospinal tract, the M1 was involved in 4 out of 10, the other 6 having purely subcortical infarctions. The inclusion of patients with cortical involvement raises the possibility that disruption of transcallosal neural populations may have altered the observed pathophysiology in some patients. Although Liepert et al. (2005) found differences in intracortical inhibition depending on whether M1 was involved in chronic stroke patients, other studies with mixed groups have observed no such distinction in the acute (Manganotti et al. 2002) or chronic (Cicinelli et al. 2003) periods. It is difficult to know whether cortical involvement affected the changing relationships that we have described without studying 2 larger groups. However, it is by no means clear that patients classified as having “cortical infarcts,” on the basis of damage involving M1, can be regarded as homogenous. As well as the involvement or otherwise of transcallosal fibers, such a categorization would have to make allowance for the extent of damage to ipsilesional nonprimary cortical areas and their topographically distinct underlying white matter pathways (Newton et al. 2006) as well as deeper structures in the basal ganglia and thalamus. A simple distinction between cortical and subcortical may therefore not be useful in this context. Even among a homogeneous patient group with only subcortical infarctions the pattern of reorganization is determined crucially by the degree of corticospinal disruption (Ward et al. 2006, 2007). A similar observation has been made using a diffusion tensor imaging measure of corticospinal tract disruption, in a homogeneous group, and relating this measure to motor function (Stinear et al. 2007). We observed such a relationship here, between intracortical excitability measures and clinical function, in a more heterogeneous group. It may be therefore that impaired corticospinal output, rather than lesion location per se, is the primary determinant of altered excitability in the distributed cortical network. Further studies that are designed to test this hypothesis specifically are required.
It should be noted that 3 patients took neuroactive medications. Citalopram (P4) can increase SICI but only in subjects with the long/long polymorphism within the promoter region of the serotonin transporter gene, 5-HTTLPR (Eichhammer et al. 2003)—such genetic information is unavailable for this patient. Temazepam (P8), a Benzodiazepine, can increase SICI (Ziemann et al. 1996) but was taken here in the evening—it has a short effect, reaching peak plasma concentration in 45 min (Wang and Devane 2003), so is unlikely to affect SICI the following day. Sertraline (P10) can increase MEP amplitudes for up to 2 h and reduce ICF for up to 6 h. With testing performed in the early afternoon, it is unlikely that MEP amplitudes were affected, but the possibility cannot be excluded that ICF was reduced. None of these medications were changed during the course of the study, however, so that any potential distortion of physiological measures should not explain the time effects observed.
Behavioral Measures
Scores in all behavioral tests were significantly improved by 1 month (except for the modified Rankin score). The tests of upper limb function (ARAT and NHPT) did not show further significant change beyond this point but differed as to when they most usefully described the clinical heterogeneity: the NHPT showed a floor effect during the acute period, whereas the ARAT showed a ceiling effect by 3 months. This observation is consistent with previous reports of the respective characteristics of these tests (Wade 1992). The ARAT is partly dependent on proximal power and simple grasp movements, whereas the NHPT relies also on the ability to perform fractionated finger movements, to hold a peg between thumb and finger, and to pronate the wrist (in order to insert the peg). The differing time courses for improved performance of these 2 tests reflect recovery of proximal upper limb function before hand function and is a reminder that in order to accurately describe clinical heterogeneity it is important to choose a behavioral test appropriate to the stage of recovery.
The upper limb scores were expressed relative to those obtained in the unaffected arm, in order to minimize the impact of any global factors on test performance. This is likely to be particularly important during the early weeks—crucial for this study—when patients are subject to fluctuations in a variety of systemic factors which may reduce effort globally. This approach has been used in a number of other publications in the field of stroke recovery (Ward et al. 2003b, 2006; Murase et al. 2004; Talelli et al. 2007). It is certainly true that the unaffected arm may perform suboptimally on tests of dexterity, when compared with a healthy control group. Such deficits are seen chiefly in the context of left-hemispheric cortical strokes, in association with the resulting cognitive deficit (Sunderland et al. 1999, 2000), whereas all of the patients with cortical involvement in our study had infarctions on the right side. However, subtle deficits have recently also been reported ipsilateral to subcortical stroke (Noskin et al. 2007; Nowak et al. 2007).
TMS Measurements in the Acute Period
TMS data from either hemisphere at multiple time points within the first month revealed great within-subject variability. Unexpectedly, there was no relationship between the observed physiological variations and motor performance in this period. The reason for this is unclear. It may be argued that fatigue in the early weeks after stroke could exert a fluctuating global influence on the parameters measured. Alternatively, changes may result from the developing effects of diaschisis as the functional loss of the affected region exerts a distributed effect. Finally, fluctuations in cerebral perfusion in the post-stroke period, resulting from disturbed cerebrovascular autoregulation, may well affect penumbral brain regions and thus affect physiological measures. Whatever the reason, our findings demonstrate that single physiological measurements made in the first 3 weeks after stroke have little clinical use on their own. In the present study, we therefore averaged measurements acquired during the first 3 weeks in order to obtain a reliable measure of early post-stroke cortical physiology. This time interval was chosen in order to encompass the early weeks, although remaining temporally distinct from the 1-month assessment. In order to ensure that the choice of time interval did not affect the observed pattern of results, we recalculated the clinical correlations for this period using a 4-week period and also using only the first TMS session in each patient. Correlations for all the TMS parameters were remarkably similar using these alternative acute measures to those presented here, providing reassurance that the precise choice of time interval did not alter the results.
Corticospinal Excitability
Motor Thresholds
Both rMT and aMT were higher in the AH than in the UH (and higher than the healthy control group) during the acute period, while aMT was also raised at 3 months. Thresholds in the AH reduced with time, significantly for the rMTs but not for the aMTs. Correlations with clinical scores persisted to 3 and 6 months in both measures but were strongest in the acute period.
Raised motor thresholds in the AH have been described in several reports (Heald et al. 1993; Catano et al. 1996, 1997a; Traversa et al. 2000; Nardone and Tezzon 2002). Here, the resting thresholds in the AH had largely normalized by 1 month, although active thresholds were still raised at 3 months. Examining the individual patient data (Supplementary Fig. 2A) also suggests that a plateau had been reached by approximately a month, which is in agreement with previous work (Catano et al. 1996; Traversa et al. 2000). However, the degree to which thresholds normalized varied across the group, and power analyses suggest that abnormally raised active thresholds may have been detected with a larger group at 1 month and 6 months. Furthermore, there was still a significant correlation between aMT and functional status at 6 months, suggesting that this measure remained abnormal in more affected patients.
Reports of clinical correlations with motor thresholds have been variable and have generally been described at single time points. In the acute period, an association of raised motor thresholds with poor clinical status has been demonstrated at 1 day after stroke (Heald et al. 1993) and within 14 days (Liepert et al. 2005). It has also been suggested that motor thresholds in this period may have predictive value for eventual recovery (Heald et al. 1993; Catano et al. 1997b; Wittenberg et al. 2007): negative correlations were likewise observed in the present study (not shown) between active thresholds in the acute period and clinical scores at 6 months (P = 0.058), whereas the relationship was weaker for resting thresholds (P = 0.138). A relationship between raised thresholds and poor function has also been reported when assessed in the chronic stage, both for rMT (Werhahn et al. 2003) and aMT (Thickbroom et al. 2002), although patients have also been described with normal function despite raised thresholds (Byrnes et al. 2001). The present results suggest that the relationship may vary over time, becoming weaker by 6 months for rMT and to a lesser extent aMT. The difference between the clinical correlations observed for rMT and aMT in the chronic stage may be explained by increased spinal excitability (e.g., due to greater cortico-propriospinal drive—Mazevet et al. 2003), which may reduce rMT. This compensation would not affect aMT, however, which is tested when motor neurons are already near their firing potential—thus, aMT may more accurately reflect cortical excitability than rMT in the chronic stage (Talelli et al. 2006).
The overall reduction in motor thresholds with time was preceded by an early increase in aMT (10–17 days). Because aMTs reflect membrane excitability rather than synaptic activity, an early increase could reflect ongoing structural or metabolic consequences of the infarction, rather than altered input to M1, or other factors including decreasing membrane excitability at a spinal level due to immobility of the paretic limb.
Recruitment Curves
RC gradients changed differently with time in the 2 hemispheres, tending to decrease in the UH and increase in the AH. Gradients were smaller in the AH than in the UH during the acute period and at 1 month and were reduced compared with the healthy control group at all time points studied. Gradients in the AH correlated strongly with clinical scores during the acute period but less so at later time points.
Most previous work studying corticospinal excitability after stroke has tested MEP amplitudes at a fixed stimulus intensity relative to motor threshold, during either relaxation (Cicinelli et al. 1997; Traversa et al. 2000; Trompetto et al. 2000; Thickbroom et al. 2002) or active contraction (Cicinelli et al. 1997; D'Olhaberriague et al. 1997). This method is susceptible to errors resulting from small shifts in motor threshold. Furthermore, the shape of the RC may change following stroke, such that a single stimulus intensity may reflect different points on this curve in different patients. Here, we have measured the RC gradient, determining MEP amplitudes at 4 stimulus intensities and calculating the slope of the resulting curve. The range of intensities makes this method less vulnerable to these 2 sources of error.
Persistent corticospinal hypoexcitability in the AH is consistent with previous results showing reduced MEP amplitudes after 3 months (Traversa et al. 2000; Byrnes et al. 2001; Thickbroom et al. 2002; Delvaux et al. 2003) and at 6 months (Pennisi et al. 2002). A trend improvement with time is in keeping with a study during the subacute period by Cicinelli et al. (1997): however, other studies at more than 1 time point have reported no increase (Delvaux et al. 2003) or a significant increase (Traversa et al. 2000). It is difficult to say what may account for the differences between these studies, but it should be noted that they all recorded MEPs at a single intensity, whereas the present results represent MEP recruitment across a range of intensities. The early significant difference between the 2 hemispheres was resolved by 3 months in the present study, but a power analysis suggests that a difference between gradients in the 2 hemispheres may have been detected at 6 months with a larger patient group. The lack of difference observed here beyond 1 month may have been largely due to a reduction in excitability of the UH.
The relationship between clinical performance and RC gradient was log-linear, with test scores only dropping away when excitability became more severely impaired; this was also the case for motor thresholds. This suggests that function can be well maintained despite significant damage to the corticospinal tract, an idea that receives support from a case series of cerebral peduncle lesions in which 80% of the tract could be destroyed before finger movements were impaired (Jane et al. 1968). Recent work used fractional anisotropy to quantify structural disruption of the subcortical white matter tracts at the level of the internal capsules after stroke. In patients with poor motor cortex excitability to TMS, motor function dropped off rapidly with increasing corticospinal disruption (Stinear et al. 2007). This reinforces the idea that once a certain level of corticospinal disruption has been reached, no amount of upstream reorganization will be able to generate a useful motor output.
Correlations of MEP amplitudes with clinical scores have been described separately at various time points after stroke. These correlations have been significant at 1 month (Traversa et al. 2000), of trend significance at approximately 2 months (Cicinelli et al. 1997), and not significant for hand dexterity in the chronic period (Thickbroom et al. 2002). Although this suggests that the relationship may become less strong with time, it is arguably more suggestive to observe this pattern within a single patient group, as in the present results. It is important to consider what might drive the change in this relationship. In the acute period, 4 patients had gradients at or near 0 and these were the most impaired patients. Despite a similar range of relative impairments at 6 months, all patients except one now had easily measurable gradients. Conversely, the patient with the second best recovery at 6 months had a small gradient at this stage. So although this measure reflects function well in the first 3 weeks, the degree of subsequent increase appears not to reflect clinical improvement to such an extent. This is consistent with the idea that continuing behavioral improvement from 3 months relies less on recovery of excitability in the original corticospinal projection than on reorganization in alternative cortical networks. This would also be in keeping with the finding that the relationship between infarct volume and clinical deficit becomes less strong over this same time period.
Early corticospinal hyperexcitability of the UH has been previously reported, both with the target muscle at rest (Delvaux et al. 2003) and active (Cicinelli et al. 1997; Traversa et al. 1998). In fact, in the present study, RC gradients in the UH were never greater than in our control group. The difference may relate to the fact that Delvaux measured MEPs using maximum stimulator output, which may activate pyramidal cells directly rather than trans-synaptically, in which case the MEPs recorded may depend more on spinal excitability (increased following stroke) than cortical excitability. The UH hyperexcitability reported in that study may thus represent a different phenomenon to that observed here. Another possibility is that early UH hyperexcitability may have been more pronounced if MEPs had been elicited from active hand muscles. Reducing UH gradients with time could alternatively be explained by progressive pathological hypoexcitability, for example as a result of diaschisis. However, the absence of clinical correlations at any stage argues against either of these explanations.
Intracortical Excitability
No consistent effects of time were seen in SICI, ICF, or LICI in either hemisphere. Mean values calculated for each patient were raised in the AH, compared with the healthy group, for SICI and LICI: the larger conditioned MEPs imply that these 2 forms of inhibition were weaker than normal in the AH. Correlations with clinical scores were weak during the acute period (except for LICI in the AH), strong at 3 months, and then weak again at 6 months.
Affected Hemisphere
These measures (SICI, ICF, and LICI) all employ a conditioning-test design in which a CS activates inhibitory or excitatory influences on a later TS. The TS needs to evoke a consistent MEP, which in the AH may require a higher intensity than normal and may yield a smaller unconditioned MEP. It is reassuring to note that for the assessment of paired pulse measures, the amplitudes of unconditioned MEPs in the AH did not differ significantly from those in the UH at any time point. Moreover, at the 3-month time point (when strong clinical correlations were observed with intracortical measures) unconditioned MEP amplitudes did not covary with paired pulse measures in the AH. The choice of CS intensity (80% of aMT here) may also cause difficulties. This latter is usually expressed relative to motor threshold but after stroke, the relationship between the corticospinal threshold and the intracortical system being tested may be altered. A thorough study would therefore require testing all these parameters with a range of conditioning intensities (e.g., Butefisch et al. 2003), although this would prolong patient testing considerably.
Our finding of weak SICI in the AH is in agreement with 4 other studies in which SICI was measured within the first month after stroke (Liepert, Storch et al. 2000; Manganotti et al. 2002; Nardone and Tezzon 2002; Cicinelli et al. 2003). One study has reported the converse finding (Wittenberg et al. 2007), and the reason for this discrepancy is unclear. There is little information regarding SICI in the chronic stage—we found here that it was fairly stable with no overall effect of time. Normal ICF in the AH has been reported in the first 2 weeks (Liepert, Storch et al. 2000) and in the chronic period (Butefisch et al. 2003), in agreement with the present results. There are to our knowledge no prior studies of LICI following stroke. We found that LICI in the AH did not change with time and was significantly weaker than normal. Although LICI shares a dependence on GABAB receptors with the cortical silent period (Siebner et al. 1998; Werhahn et al. 1999; Chen 2004), which may be prolonged after stroke (Braune and Fritz 1995; Catano et al. 1997a; Liepert et al. 2005), recent work suggests that these 2 forms of inhibition may in fact reflect activity in differing neural populations (Inghilleri et al. 1996; McDonnell et al. 2006). Furthermore, LICI, unlike the silent period, does not depend on the ability of the cortex to sustain volitional input, which may be altered after stroke. The weak SICI and LICI observed here thus suggest increased net intracortical excitability, with reduced activity in local inhibitory circuits.
Unaffected Hemisphere
The stimulus intensity considerations relating to altered motor thresholds do not apply in the UH, where thresholds were normal. No group abnormalities were observed here in measures of intracortical excitability in the UH. However, the presence at 3 months of significant negative clinical correlations with excitability in all 3 measures strongly suggests net hyperexcitability in more impaired patients.
We did not observe an overall deficiency of SICI in the UH in the present study. Such a deficiency has been described in patients with cortical but not subcortical stroke (Liepert, Hamzei, and Weiller 2000; Liepert, Storch, et al. 2000; Liepert et al. 2005), although this distinction was not observed in some studies (Manganotti et al. 2002; Cicinelli et al. 2003). This may be explained by the clinical heterogeneity of the patient group in the present study, or alternatively, it is possible that a difference would have been observed if a range of CS intensities had been used (Butefisch et al. 2003). Our finding of normal ICF in the UH is in agreement with a previous study performed 2 weeks after stroke (Liepert, Hamzei and Weiller et al. 2000), but again the clinical correlation at 3 months implies that it is increased in some patients at this stage. LICI is previously undocumented, and although the group values were normal, the same observation applies regarding the situation at 3 months.
Clinical Correlations
In marked contrast to corticospinal excitability, intracortical excitability parameters did not correlate well with clinical performance in the acute period (except for LICI in the AH, see below) but showed marked correlations at 3 months. A stratified analysis (data not shown) suggests that this change may arise because disinhibition was initially present regardless of eventual outcome, normalizing only in patients who recovered well (although persisting or worsening in those who did not). This is in agreement with the study of Manganotti et al. (2002), who studied SICI in the UH at 2 time points within the first month and found that disinhibition resolved in patients with good recovery. Such a relationship between poor clinical status and increased net intracortical excitability was not observed when studied previously at a single time point within the first month (Butefisch et al. 2003) or in a group of patients at a wide range of time points after stroke (Shimizu et al. 2002). However, the present results suggest that the relationship between intracortical excitability and motor function is dynamic, depending crucially on the time since stroke onset. We argue below that this sequence reflects behavioral improvements shifting from reliance on restored corticospinal function to greater supporting input from intact (and maybe remote) cortical regions.
LICI in the AH differed from the other intracortical measures, correlating well both in the acute period and at 3 months. In the acute period, it was also a good predictor of clinical scores at 3 months (data not shown). It has been suggested (Orth and Rothwell 2004) that LICI may depend on activity in recurrent axon collaterals from the corticospinal discharge associated with the first (conditioning) stimulus. If LICI thus depends on the integrity of both corticospinal and intracortical populations, then its clinical correlations may be expected to reflect both aspects of this mechanism.
Relating Physiological Changes to Clinical Recovery
A major finding of the present study is that the corticospinal excitability measures correlated closely with clinical function in the acute period following stroke and more weakly at 3 and 6 months. Conversely, intracortical excitability measures correlated well at 3 months but not in the acute period (except for LICI in the AH); these correlations were no longer present at 6 months. Indeed, UH intracortical excitability parameters correlated strongly at 3 months despite normal corticospinal excitability (and no corticospinal–clinical correlations) in that hemisphere.
We interpret these findings as suggesting that in the acute period the patient is reliant on whatever remains of the pre stroke motor output system. Disinhibition is present, releasing connections to adjacent or distant neural populations for use (Jacobs and Donoghue 1991), but this has not yet been organized into a useful alternative system. With time and motor practice (e.g., during physiotherapy), synaptic strengthening may take place in these newly available networks so that as effective a motor output as possible may be generated. Thus, by 3 months, we suggest that such networks are not only available but also organized and therefore useful. Continued disinhibition is necessary to maintain access to these areas at this stage so that measures of intracortical excitability correlate with clinical performance.
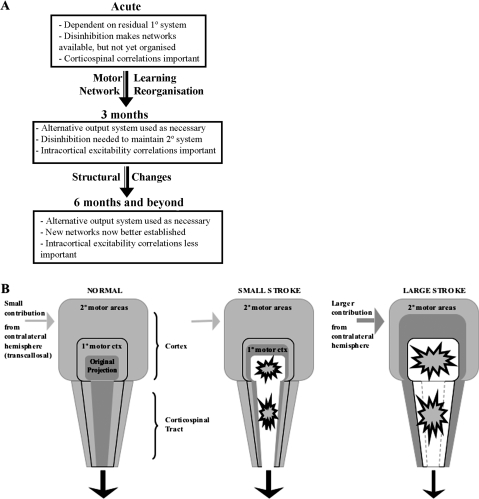
At 6 months, however, these correlations are greatly reduced. This may reflect decreased reliance on net intracortical disinhibition as training-induced synaptic strengthening becomes better established. It has also been previously suggested that early network reorganization may with time give rise to permanent structural changes, recently described 5 months after ischemic infarction in monkeys (Dancause et al. 2005). It is unclear whether such structural changes may contribute to the gradual “hard-wiring” of alternative networks. This proposed model of how the generation of a motor output changes after stroke is illustrated in Figure 6A.
Figure 6.
A) Proposed model for the relationship of physiological changes to recovery from the acute to the chronic stage, compatible with the present results. (B) A scheme depicting the possible roles in hand movement of primary and secondary motor areas of 1 hemisphere. The stroke scenarios are represented in the reorganized (chronic) state. Darker shading denotes greater involvement in movement of the affected hand. A stepwise recruitment of motor areas is depicted with increasing disruption of the corticospinal tract. Minor disruption (small stroke) results in the recruitment of perilesional M1, whereas more extensive disruption (large stroke) requires the use of secondary motor areas and even transcallosal inputs from the intact hemisphere. We propose that during the subacute stage after stroke a smaller or larger degree of intracortical disinhibition is necessary to maintain access to these additional networks, depending on the extent of disruption of the original corticospinal projection.
It is theoretically possible that the intracortical excitability correlations that we observed at 3 months may simply reflect more severe dysfunction of the wider motor system in more impaired patients, rather than representing an adaptive response as we have argued. It is difficult to separate these possible explanations on the basis of the current data, and to do this would require further work (e.g., testing the change in such parameters in response to a treatment intervention). However, it should be noted that between 3 and 6 months we observed a dissociation between clinical function (which did not significantly change) and the intracortical excitability correlations (which were completely abolished). This would be difficult to explain if such correlations were an epiphenomenon of dysfunction and would be more in keeping with the model we propose.
Post-stroke reorganization “outsources” the motor output to a number of nonprimary (including contralesional) motor areas. These newly recruited areas must still maintain some access to the spinal cord, however indirect, in order to assist in generating a motor output. This could be achieved by subcortical projections from these areas or alternatively via the remains of the original motor cortical projection. The second of these possibilities is perhaps the most likely, especially given the persistence of the corticospinal–clinical correlations into the chronic period. The role of the contralesional M1 in this context is unclear: TMS here does not prolong reaction times and exerts abnormally strong transcallosal inhibition during movement preparation (Werhahn et al. 2003; Murase et al. 2004). Functional roles for the dorsal premotor cortices (PMds), however, are well documented with ipsilesional PMd recruited in less affected patients and contralesional PMd in more affected patients (Johansen-Berg et al. 2002; Fridman et al. 2004). Furthermore, premotor regions take on M1-like properties in patients with greater corticospinal system damage (Ward et al. 2007). This suggests a stepwise recruitment of additional motor areas in the face of damage to the original output system (illustrated in Fig. 6B). The present results demonstrate that bilateral intracortical disinhibition similarly relates to motor impairment. We propose therefore that at 3 months, ongoing disinhibition is facilitating the continued use of these additional networks and that this disinhibition subsequently becomes less crucial as alternative networks become better established.
Clarification of this model will require larger correlation analyses but is likely to be worthwhile—as the number of novel interventions aiming to enhance recovery grows, it becomes increasingly important to define the physiological framework that underpins motor recovery after stroke.
Supplementary Material
Supplementary Figures 1–8 can be found at: http://www.cercor.oxfordjournals.org/.
Funding
The Wellington Hospital (to O.B.C.S.); The Patrick Berthoud Charitable Trust (to O.B.C.S.); The Medical Research Council (G0500258 to J.C.R.); The Wellcome Trust (GR071398MF2003 to N.S.W).
Supplementary Material
Acknowledgments
We would like to thank the patients involved with this study for their endless goodwill and patience. We would also like to thank Peter Asselman for his frequent assistance. Conflict of Interest: None declared.
References
- Braune HJ, Fritz C. Transcranial magnetic stimulation-evoked inhibition of voluntary muscle-activity (silent period) is impaired in patients with ischemic hemispheric lesion. Stroke. 1995;26:550–553. doi: 10.1161/01.str.26.4.550. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Netz J, Wessling M, Seitz RJ, Homberg V. Remote changes in cortical excitability after stroke. Brain. 2003;126:470–481. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- Byrnes ML, Thickbroom GW, Phillips BA, Mastaglia FL. Long-term changes in motor cortical organisation after recovery from subcortical stroke. Brain Res. 2001;889:278–287. doi: 10.1016/s0006-8993(00)03089-4. [DOI] [PubMed] [Google Scholar]
- Catano A, Houa M, Caroyer JM, Ducarne H, Noel P. Magnetic transcranial stimulation in acute stroke: early excitation threshold and functional prognosis. Electroencephalogr Clin Neurophysiol. 1996;101:233–239. doi: 10.1016/0924-980x(96)95656-8. [DOI] [PubMed] [Google Scholar]
- Catano A, Houa M, Noel P. Magnetic transcranial stimulation: clinical interest of the silent period in acute and chronic stages of stroke. Electroencephalogr Clin Neurophysiol. 1997a;105:290–296. doi: 10.1016/s0924-980x(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Catano A, Houa M, Noel P. Magnetic transcranial stimulation: dissociation of excitatory and inhibitory mechanisms in acute strokes. Electroencephalogr Clin Neurophysiol. 1997b;105:29–36. doi: 10.1016/s0924-980x(96)96515-7. [DOI] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Pasqualetti P, Zaccagnini M, Traversa R, Oliveri M, Rossini PM. Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage—a paired-pulse transcranial magnetic stimulation study. Stroke. 2003;34:2653–2658. doi: 10.1161/01.STR.0000092122.96722.72. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Traversa R, Rossini PM. Post-stroke reorganization of brain motor output to the hand: a 2–4 month follow-up with focal magnetic transcranial stimulation. Electroencephalogr Clin Neurophysiol. 1997;105:438–450. doi: 10.1016/s0924-980x(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, Nudo RJ. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvaux V, Alagona G, Gerard P, De Pasqua V, Pennisi G, de Noordhout AM. Post-stroke reorganization of hand motor area: a 1-year prospective follow-up with focal transcranial magnetic stimulation. Clin Neurophysiol. 2003;114:1217–1225. doi: 10.1016/s1388-2457(03)00070-1. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, Insola A, Profice P, Ranieri F, Capone F, et al. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006;96:1765–1771. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- D'Olhaberriague L, Gamissans JME, Marrugat J, Valls A, Ley CO, Seoane JL. Transcranial magnetic stimulation as a prognostic tool in stroke. J Neurol Sci. 1997;147:73–80. doi: 10.1016/s0022-510x(96)05312-9. [DOI] [PubMed] [Google Scholar]
- Eichhammer P, Langguth B, Wiegand R, Kharraz A, Frick U, Hajak G. Allelic variation in the serotonin transporter promoter affects neuromodulatory effects of a selective serotonin transporter reuptake inhibitor (SSRI) Psychopharmacology (Berl) 2003;166:294–297. doi: 10.1007/s00213-002-1370-1. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Heald A, Bates D, Cartlidge NE, French JM, Miller S. Longitudinal study of central motor conduction time following stroke. 2. Central motor conduction measured within 72 h after stroke as a predictor offunctional outcome at 12 months. Brain. 1993;116(Pt 6):1371–1385. doi: 10.1093/brain/116.6.1371. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Marchetti P, Manfredi M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp Brain Res. 1996;109:467–472. doi: 10.1007/BF00229631. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Jane JA, Yashon D, Becker DP, Beatty R, Sugar O. The effect of destruction of the corticospinal tract in the human cerebral peduncle upon motor function and involuntary movements. Report of 11 cases. J Neurosurg. 1968;29:581–585. doi: 10.3171/jns.1968.29.6.0581. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. J Neurosci. 1994;14:2140–2152. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve. 2000;23:1761–1763. doi: 10.1002/1097-4598(200011)23:11<1761::aid-mus14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Liepert J, Restemeyer C, Kucinski T, Zittel S, Weiller C. Motor strokes: the lesion location determines motor excitability changes. Stroke. 2005;36:2648–2653. doi: 10.1161/01.STR.0000189629.10603.02. [DOI] [PubMed] [Google Scholar]
- Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000;111:671–676. doi: 10.1016/s1388-2457(99)00312-0. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Patuzzo S, Cortese F, Palermo A, Smania N, Fiaschi A. Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clin Neurophysiol. 2002;113:936–943. doi: 10.1016/s1388-2457(02)00062-7. [DOI] [PubMed] [Google Scholar]
- Mazevet D, Meunier S, Pradat-Diehl P, Marchand-Pauvert V, Pierrot-Deseilligny E. Changes in propriospinally mediated excitation of upper limb motoneurons in stroke patients. Brain. 2003;126:988–1000. doi: 10.1093/brain/awg088. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;18(8):1909–1922. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nardone R, Tezzon F. Inhibitory and excitatory circuits of cerebral cortex after ischaemic stroke: prognostic value of the transcranial magnetic stimulation. Electromyogr Clin Neurophysiol. 2002;42:131–136. [PubMed] [Google Scholar]
- Newton JM, Ward NS, Parker GJ, Deichmann R, Alexander DC, Friston KJ. Non-invasive mapping of corticofugal fibres from multiple motor areas—relevance to stroke recovery. Brain. 2006;129:1844–1858. doi: 10.1093/brain/awl106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noskin O, Krakauer JW, Lazar RM, Festa JR, Handy C, O'brien KA, Marshall RS. Ipsilateral motor dysfunction from unilateral stroke: implications for the functional neuroanatomy of hemiparesis. J Neurol Neurosurg Psychiatry. 2007 Jul 17 doi: 10.1136/jnnp.2007.118463. doi:10.1136/jnnp.2007.118463. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Dafotakis M, Kust J, Karbe H, Fink GR. Dexterity is impaired at both hands following unilateral subcortical middle cerebral artery stroke. Eur J Neurosci. 2007;10:3173–3184. doi: 10.1111/j.1460-9568.2007.05551.x. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. Assessment and analysis of handedness—Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orth M, Rothwell JC. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol. 2004;115:1076–1082. doi: 10.1016/j.clinph.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Pennisi G, Alagona G, Rapisarda G, Nicoletti F, Costanzo E, Ferri R, Malaguarnera M, Bella R. Transcranial magnetic stimulation after pure motor stroke. Clin Neurophysiol. 2002;113:1536–1543. doi: 10.1016/s1388-2457(02)00255-9. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125(Pt 8):1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998;21:1209–1212. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Sunderland A. Recovery of ipsilateral dexterity after stroke. Stroke. 2000;31(2):430–433. doi: 10.1161/01.str.31.2.430. [DOI] [PubMed] [Google Scholar]
- Sunderland A, Bowers MP, Sluman SM, Wilcock DJ, Ardron ME. Impaired dexterity of the ipsilateral hand after stroke and the relationship to cognitive deficit. Stroke. 1999;30(5):949–955. doi: 10.1161/01.str.30.5.949. [DOI] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol. 2006;117(8):1641–1659. doi: 10.1016/j.clinph.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. Exploring theta burst stimulation as an intervention to improve motor recovery in chronic stroke. Clin Neurophysiol. 2007;118(2):333–342. doi: 10.1016/j.clinph.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Archer SA, Mastaglia FL. Motor outcome after subcortical stroke: MEPs correlate with hand strength but not dexterity. Clin Neurophysiol. 2002;113:2025–2029. doi: 10.1016/s1388-2457(02)00318-8. [DOI] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Oliveri M, Palmieri MG, Filippi MM, Pasqualetti P, Rossini PM. Neurophysiological follow-up of motor cortical output in stroke patients. Clin Neurophysiol. 2000;111:1695–1703. doi: 10.1016/s1388-2457(00)00373-4. [DOI] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Pasqualetti P, Filippi M, Rossini PM. Follow-up of interhemispheric differences of motor evoked potentials from the ‘affected’ and ‘unaffected’ hemispheres in human stroke. Brain Res. 1998;803:1–8. doi: 10.1016/s0006-8993(98)00505-8. [DOI] [PubMed] [Google Scholar]
- Trompetto C, Assini A, Buccolieri A, Marchese R, Abbruzzese G. Motor recovery following stroke: a transcranial magnetic stimulation study. Clin Neurophysiol. 2000;111:1860–1867. doi: 10.1016/s1388-2457(00)00419-3. [DOI] [PubMed] [Google Scholar]
- Twitchell TE. The prognosis of motor recovery in hemiplegia. Bull Tufts N Engl Med Cent. Jul–Sep. 1957;3(3):146–149. [PubMed] [Google Scholar]
- Wade DT. Measurement in neurological rehabilitation. Oxford: Oxford University Press; 1992. pp. 171–173. [Google Scholar]
- Wang JS, Devane CL. Pharmacokinetics and drug interactions of the sedative hypnotics. Psychopharmacol Bull. 2003;37:10–29. [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003a;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003b;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. The influence of time after stroke on brain activations during a motor task. Ann Neurol. 2004;55(6):829–834. doi: 10.1002/ana.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129(Pt 3):809–819. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur J Neurosci. 2007;25(6):1865–1873. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Conforto AB, Kadom N, Hallett M, Cohen LG. Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann Neurol. 2003;54:464–472. doi: 10.1002/ana.10686. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517(Pt 2):591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg GF, Bastings EP, Fowlkes AM, Morgan TM, Good DC, Pons TP. Dynamic course of intracortical tms paired-pulse responses during recovery of motor function after stroke. J Neurol Rehabil. 2007 May 23 doi: 10.1177/1545968307302438. doi: 10.1177/1545968307302438. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.