Abstract
Subjects with unilateral vestibular loss exhibit motor control impairments as shown by body and limb deviation during gait. Biofeedback devices have been shown to improve stance postural control, especially when sensory information is limited by environmental conditions or pathologies such as unilateral vestibular loss. However, the extent to which biofeedback could improve motor performance or learning while practicing a dynamic task such as narrow gait is still unknown. In this cross-over design study, 9 unilateral vestibular loss subjects practiced narrow gait with and without wearing a trunk-tilt, biofeedback device in 2 practice sessions. The biofeedback device informed the subjects of their medial-lateral angular tilt and tilt velocity during gait via vibration of the trunk. From motion analysis and tilt data, the performance of the subjects practicing tandem gait were compared over time with and without biofeedback.
By practicing tandem gait, subjects reduced their trunk-tilt, center of mass displacement, medial-lateral feet distance, and frequency of stepping error. In both groups, use of tactile biofeedback consistently increased postural stability during tandem gait, beyond the effects of practice alone. However, one single session of practice with biofeedback did not result in conclusive short-term after-effects consistent with short-term retention of motor performance without this additional biofeedback. Results from this study support the hypothesis that tactile biofeedback acts similar to natural sensory feedback to improve dynamic motor performance but does not facilitate a recalibration of motor performance to improve function after short-term use.
Keywords: Tactile biofeedback, unilateral vestibular loss, motor learning, sensory integration, tandem gait
1. Introduction
Integration of vestibular, visual, and somatosensory information is fundamental to maintain balance and to perform motor tasks [15]. When sensory information is missing, as for example in subjects with unilateral vestibular loss (UVL), postural control is impaired and subjects show an increased reliance on visual and somatosensory information [16]. UVL is often a consequence of unilateral vestibular neurectomy to remove an acoustic neuroma [19]. After the neuroma removal, subjects undergo a period during which the central nervous system relearns how to cope with mismatching sensory information from vestibular, proprioceptive, and visual senses [23]. Although subjects show improvement of balance after surgery [23], most UVL subjects, even years afterwards, continue to show balance disorders such as 1) inability to stand with eyes closed on a sway-referenced surface [31], 2) body and limb deviation toward the unaffected side with eyes closed [16,20], and 3) difficulty balancing with eyes closed a) on one foot, b) in tandem stance, and c) in tandem gait [29].
Sensory information can be augmented by using a biofeedback (BF) system [28]. BF systems have been suggested to be beneficial when aimed to improve daily living tasks such as gait [12,21]. However, most of the published studies to date have investigated the use of BF systems only during quiet and perturbed stance [7, 11,26,32,34].
BF systems for postural control aim to encode some crucial kinematic or kinetic information not normally accessible to subjects into information useful for nervous system control of the task [28]. For example, during gait, information about trunk movement in the medial-lateral plane is crucial for postural stability [37]. More specifically, tandem gait with a narrow base of support limits crucial BF information for postural instability to medial lateral trunk excursions.
Visual, acoustic, and tactile BF systems have been used successfully to improve stance balance in subjects lacking vestibular, visual, and somatosensory information [10,34,39], respectively. However, use of visual and acoustic BF systems, could partly interfere with the ability to deal with visual and acoustic information important for daily living. Thus, tactile BF may be more suitable than other BF for providing additional feedback to improve balance during daily living activities [35].
Improvements in specific motor tasks after practice with BF have been reported in many studies [9,12, 17]. However, practice of a specific motor task itself stimulates brain plasticity and improves motor performance [16]. Thus, unless a control group is used to determine the extent of spontaneous learning, the effects of BF on retention of motor performance remain inconclusive [38]. Knowing the extent to which BF practice facilitates retention of postural performance improvements could help determine if BF intervention should be temporary (used only during exercise sessions) or permanent (used as a prosthesis device).
In this study, the effect of augmented medial-lateral trunk tilt information via tactile BF during repetition of a tandem gait task was assessed in subjects with UVL. A cross-over design was used to limit order effects when examining short term retention after practicing tandem gait with and without trunk tilt BF.
2. Materials and methods
2.1. Subjects
Nine UVL subjects (5 males and 4 females, age: 49 ± 11yrs, height: 172 ± 10 cm, and weight: 89 ± 21kg) participated in this experiment after signing an informed consent. This informed consent was approved by the academic, ethics committee to guarantee the subjects' rights according to the Declaration of Helsinki. All subjects were free from orthopedic and neurological diseases or disorders, except for the total vestibular loss on either the left (6 subjects) or the right (3 subjects) side. All subjects presented a complete unilateral loss of vestibular function based on operative procedures, reports, or 100% asymmetries on recent caloric tests. VOR rotation test data were obtained in 7 of the 9 subjects. Six of the 7 subjects with VOR data had extensive tests including standard clinical rotation tests of horizontal VOR consisting of sinusoidal rotations at 0.05, 0.2, and 0.8 Hz with 60•/s peak velocity. The results from the 0.05 Hz sinusoidal rotation test are most informative about the status of unilateral vestibular loss since previous studies have shown that an abnormal VOR phase advance at low frequencies is correlated with a large partial or total unilateral loss [14]. All six subjects showed greater than normal phase leads at 0.05 Hz (mean phase lead = 21.1 ± 3.7•; normal phase 10.5 ± 4.8• SD [24]). None of the VOR bias (average slow phase eye velocity) or VOR gain asymmetry (comparison of VOR gains during rotations to the right and left) measures for these 6 subjects were outside of normal limits at 0.05 Hz. The seventh subject had only experimental VOR rotation tests, but these tests included eye movement recordings made in the dark with no motion. There was no significant spontaneous nystagmus recorded at rest in the dark consistent with good compensation for unilateral vestibular loss. Subjects' demographics and pathologies are shown in Table 1.
Table 1.
Subject characteristics
| Subject ID | Age | Sex | Years post-surgery | Pathology | Side affected | Group |
|---|---|---|---|---|---|---|
| 1 | 60 | F | 12 | Ac. Neuroma | Right | 1 |
| 2 | 26 | M | 10 | Temporal bone fracture | Left | 1 |
| 3 | 43 | F | 8 | Ac. Neuroma | Left | 1 |
| 4 | 46 | F | 8 | Ac. Neuroma | Right | 1 |
| 5 | 53 | M | 4 | Ac. Neuroma | Right | 1 |
| 6 | 56 | M | 5 | Ac. Neuroma | Left | 2 |
| 7 | 63 | F | 7 | Ac. Neuroma | Left | 2 |
| 8 | 42 | M | 7 | Ac. Neuroma | Right | 2 |
| 9 | 49 | M | 5 | Ac. Neuroma | Right | 2 |
As part of the cross-over experimental design, the subjects were divided into two groups such that the differences between averaged ages, heights, and weights in the two groups were not statistically significant (p< 0.05) when compared with a 2-tailed t-test.
2.2. Apparatus
During the experiments, the subjects were asked to tandem-walk heel to toe, on a firm surface while a commercial metronome was set to “beep” at 30 beats per minute (0.5 Hz). Since gait velocity can affect postural stability, subjects were asked to take one step for each beep to assure consistent cadence. Kinematics measures were acquired using a Motion Analysis system with 8 Falcon cameras (Santa Rosa, CA). A symmetric set of 20 markers was used (Fig. 1). The markers were fixed on the following anthropometric landmark: ectoorbitale, tragion, acromion, radiale, stylion, great trochanter, tibiale, lateral malleolus, fifth metatarsal, and hallux of each side of the subject. During all trials, the subjects were wearing a vibrotactile BF system [35] consisting of a vest with 4 columns of tactors (3 tactors per column; Audiological Engineering, Somerville, MA, USA) and a one-axis tilt sensor unit (Draper Laboratory, Cambridge MA, USA). The vest was placed around the trunk of the subject with an elastic girdle so that 2 columns of tactors were in contact with the left side of the subject's trunk and the other 2 columns in contact with the right side of the subject's trunk. The sensor unit was aligned so that it could sense the subject's medial-lateral (ML), trunk tilt. The sensor unit consisted of a rate gyroscope and a linear accelerometer. A specially developed algorithm combined these two inputs to produce an estimate of the subject's orientation to the vertical that was accurate to within 0.2 degree. In particular, the angular velocity sensed by the gyroscope was high-pass filtered, integrated, and then summed to the low-pass filtered acceleration sensed by the accelerometer. High pass and low pass filtering used a 0.03 Hz cut-off frequency and were implemented with matching digital filters so that the phase relationships were preserved [36]. Before each experimental trial, the software allowed the experimenter to “zero” the instrumentation while the subject stood quietly in a vertical position. The sensor unit was mounted on the right side of the subjects at L5 level using a Velcro™ belt. This position was preferred because it is close to the center of mass (COM) and minimally affected by artifacts such as breathing and heart beat. A computer (Macintosh Powerbook G3) was used to activate the tactors on the vest depending on the subject's ML, trunk tilt detected by the sensor unit. The tactors on each side were activated in pairs using a step-wise scheme depending on a combination of angular tilt and angular tilt velocity [25]. Under static conditions (zero velocity), the lowest pair was activated when the sum of the measured tilt and one half of the measured tilt velocity exceeded a 2 degrees “dead-zone”, switching to the middle pair at 7 degrees and to the highest pair when exceeding 12 degrees (Fig. 2). Under non-static conditions (non-zero velocity) the same thresholds were used to activate the tactors depending on the combination of tilt and velocity [25]. During the experiment, all subjects wore exactly the same type of polyester T-shirt so that the intensity of the vibration was as similar as possible for each subject. Data were acquired with a sampling frequency of 120-Hz from the tilt sensor and 60-Hz from the Motion Analysis system.
Fig. 1.
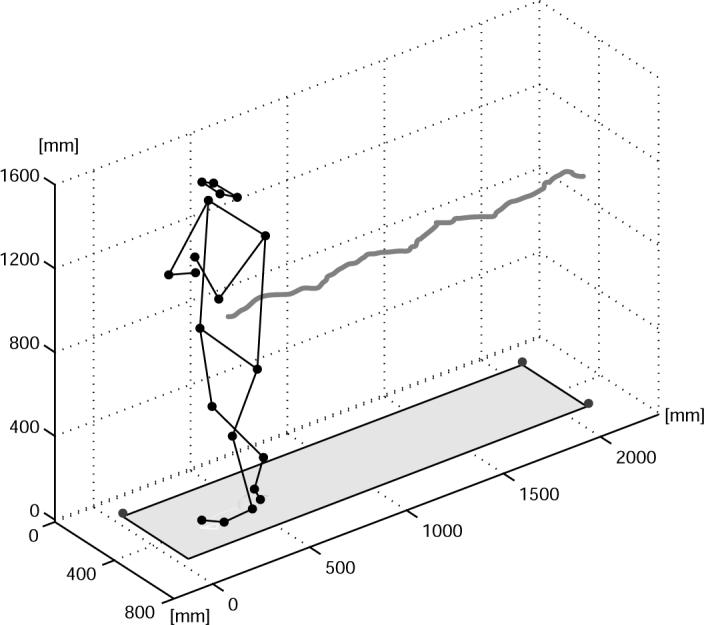
3D reconstruction of the experimental set-up. Markers from Motion Analysis are represented as black spheres. Trace of the center-of-mass, calculated from the Motion Analysis data and the subject's anthropometric measures, is also represented.
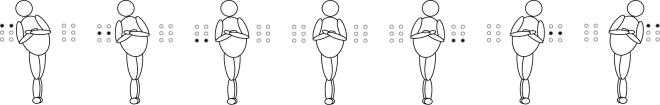
Fig. 2.
Qualitative effect of trunk tilt on tactors activation in static (zero velocity) conditions. Circles on the left and right side of the human figure indicate the tactors. Dark-filled circles indicate tactors on, empty circles indicate tactors off.
2.3. Procedure
Tandem gait task was chosen because it was difficult enough to measure an effect of learning on postural stability across many trials in UVL subjects. The nature of this task, with a narrow base of support, also allowed us to limit the biofeedback to the medial-lateral plane. Also, subjects were asked to keep their arms crossed so that stability was maintained with trunk and leg motions. Crossing the arms also prevented subjects from obscuring the reflective hip markers for kinematics measurement of the body COM. In addition, this tandem gait task is relevant and practical as it is difficult, but not impossible for patients with unilateral vestibular loss. In fact, it is quite common for vestibular rehabilitation to include tandem walking with eyes open and then with eyes closed, if possible, to help improve balance in patients with unilateral vestibular loss [13].
Before starting the data collection, all UVL subjects learned how to perform tandem gait safely and correctly during a 5- to 10-min-long training period. During this training, all UVL subjects gradually learned how to take one step for each beat from the metronome while keeping their eyes closed and arms crossed. Specifically, subjects started practicing with eyes open then with eyes closed without the metronome, and finally with eyes closed and the metronome. During the training, the experimenter provided verbal cues to help subjects understand the goals of the task. All subjects, at first, were very skeptical about their ability to tandem walk with eyes closed. However, after this very short training, all subjects were able to successfully complete all trials. At the very beginning of the training period, subjects had difficulty maintaining balance and made large lateral trunk movements. Subjects attempted to compensate by using wider lateral foot placements during gait. This effect was controlled by monitoring the ML deviation of the foot placement across the steps and providing verbal feedback to correct performance. Once subjects gained more confidence with narrow stance, they tended to walk as fast as possible so that their own body inertia helped to maintain balance. This effect was controlled by monitoring the actual frequency of step. Data collection started once the subjects understood the task and they demonstrated that they were able to perform such a challenging task.
Following training, each subject performed a test session of 30 trials of tandem walking barefoot with eyes closed and arms crossed, taking one step for each beep of the metronome. A second identical test session of the experiment was performed two weeks after the first one. We refer to these two test sessions as “practice sessions” because we hypothesized that motor learning would occur during each session and that results from the second session would be influenced by practicing tandem gait in the first session.
In both sessions, the first 3 and the last 3 trials were performed with the tactile-BF device turned off. A cross-over design was used for the remaining 24 middle trials. Group 1 (5 subjects) performed the 24 middle trials of the first session with the tactile-BF device turned off and the 24 middle trials of the second session (two weeks later) with the device turned on. For Group 2 (4 subjects) this order was reversed.
Each walking trial was 2.5 meters long so that the subjects could take at least 5 complete steps. During the experimental session, a safety spotter from our laboratory walked on one side of the subjects to catch them in case they lost balance.
2.4. Data- and statistical-analysis
From the kinematics data and the anthropometric measures of each subject, the 3D-coordinates of the COM during each trial were calculated according to standard methods [2,6,33]. Trunk tilt and COM data were synchronized via recording of trigger signals from the Motion Analysis system. Steps were recognized from the position in time of the 6 markers on the feet. The first and the last stance phases of stepping were neglected for the calculation of the following parameters.
The ML standard deviation (SD) of the COM was calculated for each trial and used as an indicator of subjects' ML postural stability. The SD of ML trunk tilt from the BF system sensor unit was calculated for each trial and used as an indicator of subjects' ability to use the BF to increase trunk stability. In addition, the mean frequency error (i.e. the difference between the subjects' actual frequency of stepping and 0.5 Hz) was calculated for each trial as well as the mean across steps of the feet ML distances between the feet (step width) during the double-stance phases. This mean ML step width and the mean frequency error were used as an indicator of the accuracy of the subjects' performance of tandem-walking. All parameters were averaged across the two cross-over groups to minimize the influence of possible order effect.
Linear regressions were used to determine the statistical significance of changes in the across-practice trials. Simple paired t-tests were also used to determine any significant short-term retention effects in terms of the percentage change of the parameters before and after each practice sessions (with and without BF). The parameters were considered independent for statistical purposes since they were obtained from independent measures, as a consequence Bonferroni correction was used only when paired t-test were repeatedly applied to the same set of parameters. T-tests also verified that the percentage change of each parameter across the two sessions of the experiment was not statistically significantly different between the two groups; in other words, that the change in parameters across the first and last trials were not significantly different across subjects. Finally, ANCOVA was used to verify whether the different parameters trends over time were significantly different between trials with and without BF.
3. Results
3.1. Initial tandem gait training
Training was necessary for subjects to learn how to perform tandem gait. At the very beginning of the training period subjects: 1) had difficulty maintaining balance and made large trunk movements flexing profusely at the hips, 2) were not able to take more than one step without support from the attendant, 3) tried to compensate their inability of narrowing their base of support by rotating their feet inward. In the rest of the training, subjects gradually learned to 1) reduce the flexion at the hip, 2) not rely on the attendant's support, and 3) place the feet in tandem position without feet rotation.
3.2. Beginning of tandem gait experiment
Even after training, tandem gait proved to be a very challenging task for our UVL subjects' stability. In fact, in the first trial of the experiment, just after the preparatory training, subjects showed 1) a large medial-lateral trunk movements (average COM SD was 68.8 mm and trunk tilt SD was 5.5 degrees), 2) a large base of support (average ML feet distance was 67.1 mm), and 3) a large variability in stepping time (average frequency error was 0.22 Hz). However, no significant difference (p < 0.05) was found between the side of the vestibular lesion and the contralateral side in terms of lateral trunk movement or foot-stepping and -placement.
3.3. Immediate effects of BF
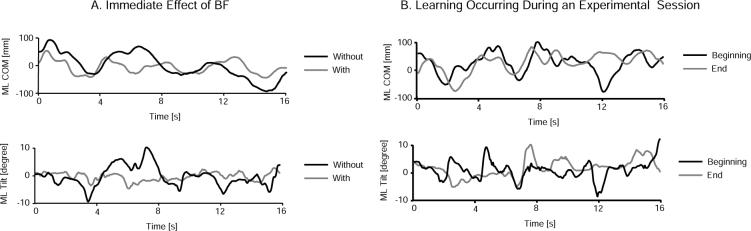
All subjects in both groups improved their stability as soon as the BF device was turned on. Specifically, in the first three trials with BF, COM SD was significantly reduced by 3.8%, trunk tilt SD by 17.8%, and ML step width by 20% compared to the previous three trials without BF. Frequency error was the only parameter that increased (by 34.5%) when BF was turned on. Figure 3 shows raw data of COM displacement and trunk tilt from one representative subject with and without BF. Note the decrease in variability and amplitude of COM displacement and trunk tilt occurring with BF.
Fig. 3.
Panel A shows the immediate effect of BF on lateral trunk tilt and COM displacement from one representative subject. Panel B shows the effect of learning during one experimental session on lateral trunk tilt and COM displacement from one representative subject without BF.
3.4. Effects of practicing tandem gait
During the experiment, all subjects improved their stability with repetition of tandem gait trials; COM SD, trunk tilt SD, ML step width, and frequency error were significantly lower for all subjects in the three trials recorded at the end of the two practice sessions compared to the three trials recorded before the two practice sessions (average values are reported in Table 2). Figure 3B shows some raw data of COM displacement and trunk tilt from one representative subject in the first and last trial of the first experimental sessions. Note the decrease in variability and amplitude of trunk tilt and COM displacement occurring with practice.
Table 2.
Parameter at the very beginning (before the first practice session) and at the very end (after the second practice session). Each value corresponds to the average of three trials (1−3 and 28−30 for Beginning and End, respectively)
| COM SD [mm] |
Tilt SD [degree] |
ML Feet Distance [mm] |
Freq. Error [Hz] |
|||||
|---|---|---|---|---|---|---|---|---|
| Subject # | Beginning | End | Beginning | End | Beginning | End | Beginning | End |
| 1 | 44.89 | 39.65 | 4.85 | 3.38 | 64.71 | 34.70 | 0.38 | 0.01 |
| 2 | 57.01 | 53.48 | 5.73 | 4.25 | 67.46 | 53.52 | 0.13 | 0.07 |
| 3 | 83.06 | 35.85 | 6.92 | 5.36 | 71.59 | 68.90 | 0.16 | 0.01 |
| 4 | 51.45 | 28.31 | 6.47 | 2.82 | 76.14 | 33.71 | 0.09 | 0.01 |
| 5 | 65.67 | 27.90 | 4.18 | 2.85 | 79.71 | 48.26 | 0.33 | 0.09 |
| 6 | 73.03 | 62.25 | 3.84 | 3.32 | 59.91 | 66.98 | 0.32 | 0.19 |
| 7 | 27.59 | 27.23 | 2.63 | 2.24 | 48.45 | 39.05 | 0.08 | 0.02 |
| 8 | 47.66 | 25.17 | 2.78 | 1.75 | 37.27 | 36.05 | 0.08 | 0.14 |
| 9 | 49.27 | 41.09 | 3.25 | 2.32 | 70.39 | 43.47 | 0.24 | 0.05 |
| Mean (SD) | 55.5 (16.5) | 37.9 (12.9) | 4.52 (1.58) | 3.14 (1.11) | 64.0 (13.6) | 47.1 (13.4) | 0.20 (0.12) | 0.07 (0.07) |
3.5. Effects of practicing tandem gait in the session without BF
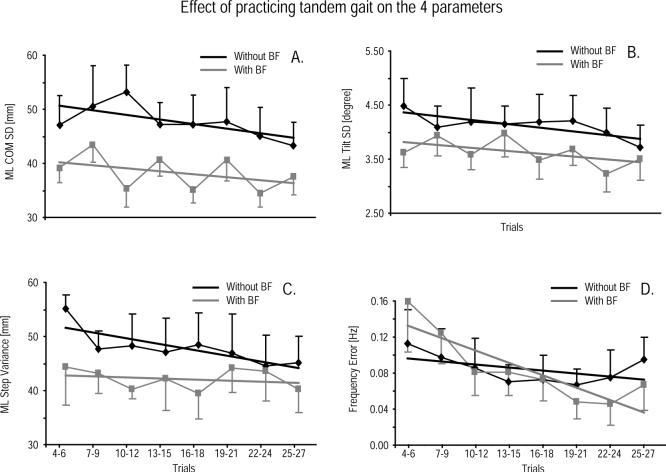
The subjects' COM SD significantly decreased over the course of the session without BF (Fig. 4A). The regression slope was negative and differed significantly from zero (p < 0.05), and the linear regression accounted for 70% of the variance. The subjects' SD of trunk tilt also showed significant reduction while practicing without BF (Fig. 4B). The slope of the linear regression of the trunk tilt SD values across trials was negative and was significantly different from zero (p < 0.05), and the linear regression accounted for 60% of the total variation. Subjects' ML step width (Fig. 4C) also significantly decreased over time while practicing tandem gait without BF, with 60% of the variance accounted for by the linear regression. The subjects' stepping frequency error, however, did not significantly change across trials by practicing tandem gait without BF (p > 0.05; Fig. 4D).
Fig. 4.
Effect of practicing tandem gait across trials. Each value represents the average among the subjects of three consecutive trials. Error bars represent standard errors.
3.6. Effects of practicing tandem gait in the session with BF
During the session with BF, subjects received vibration 65% of the time. During this time, the first level of vibration (2-degree threshold) was reached 80% of the time, the second (7-degree threshold) 15% of the time, and the third (12-degree) threshold 5% of the time.
During the session with BF, subjects consistently exhibited a smaller trunk COM SD, trunk tilt SD, and ML step width than in the session without BF (p < 0.05; Fig. 4A–C). Although the linear regression slope was negative for these three parameters, the statistical analysis showed that the regression slope was not significantly different from zero. The variance accounted for by the linear regression was 17% for COM SD, 30% for trunk tilt SD, and 6% for ML step width. In contrast, the step frequency error did improve with practice (regression slope negative and significantly different from zero, p < 0.01). The variance accounted for by the linear regression was 75% for step frequency error. ANCOVA verified that regression lines with and without BF were not significantly different for trunk tilt SD, COM SD and ML step width but were significantly different for frequency error. Even if a significant difference could always be found for at least one of the parameters (Fig. 4) between trials with and without biofeedback, both practice with and without BF reduced the tilt and COM SD. Thus, after 21 practice trials without BF, the lateral tilt SD was not statistically different from the first trials with BF and after 24 practice trials without BF, the lateral COM SD was not significantly different from the first trials with BF.
3.7. Short-term retention effect of practicing tandem gait
Short-term retention, i.e. the difference between the performances at the beginning and at the end of each session, was higher after practicing without BF than after practicing with BF. Table 3 reports the percentage changes between the averages of the first and last three trials of each session for each parameter. Practicing without BF significantly reduced trunk COM SD, trunk tilt SD, ML step width, and step frequency error in performing tandem gait (Table 3). Practicing with BF, only frequency error showed significantly further improvements (Table 3). COM displacement SD decreased for most of the subjects after practicing with BF, however this change was not significant (p = 0.08; Table 3).
Table 3.
Short-term retention after practicing tandem gait without and with BF. Each value represents the percentage change occurred between before and after each practice session (i.e. the percentage difference between trials 1−3 and 28−30)
| COM SD % |
Tilt SD % |
ML Feet Distance % |
Freq. Error % |
|||||
|---|---|---|---|---|---|---|---|---|
| Subject # | Without | With | Without | With | Without | With | Without | With |
| 1 | • 4.40 | 13.61 | • 37.34 | • 7.98 | • 44.67 | • 13.74 | • 86.94 | • 78.89 |
| 2 | • 18.65 | 11.14 | • 22.31 | 67.03 | • 25.12 | 1.76 | • 71.53 | 29.26 |
| 3 | 2.40 | • 9.06 | • 44.51 | • 25.83 | • 64.48 | 14.40 | • 91.70 | • 89.00 |
| 4 | • 32.99 | • 23.08 | • 61.07 | • 26.22 | • 41.76 | 14.86 | • 64.40 | • 81.27 |
| 5 | • 16.40 | • 39.05 | • 44.65 | 8.88 | • 61.57 | • 11.40 | • 98.06 | 38.00 |
| 6 | • 7.24 | • 19.86 | • 31.83 | 15.83 | • 23.38 | 4.07 | • 49.50 | • 13.57 |
| 7 | • 15.21 | • 1.27 | • 22.85 | 5.34 | • 11.57 | • 6.32 | • 84.96 | • 65.50 |
| 8 | • 4.03 | • 14.20 | • 23.70 | • 1.19 | • 25.86 | 3.68 | 87.60 | • 29.42 |
| 9 | • 2.31 | • 9.80 | • 12.02 | • 31.44 | • 3.27 | 8.30 | • 41.43 | • 69.82 |
| Mean (SD) | • 10.9 (10.9)• | • 10.2 (16.6) | • 33.4 (15.0)• | 0.5 (30.1) | • 33.5 (21.1)• | 1.7 (10.4) | • 55.7 (57.0)• | • 40.0 (48.5)• |
Indicate statistical significance of the overall mean percentage change.
4. Discussion
4.1. Motor learning during tandem gait practice
After practicing tandem gait, all UVL subjects improved their performance in terms of postural stability and stepping accuracy. These improvements included 1) an increased ML stability, shown by the reduction of the trunk tilt and COM SD; and 2) a higher accuracy in maintaining the tandem position of the feet while walking, shown by the reduction of the mean ML step width, as well as a higher accuracy in stepping to the metronome rhythm. These results suggest that with practice, subjects with UVL can learn to better control their posture during a complex task such as tandem gait. In fact, the lower mean step width represents reduced lateral stepping deviation, which is a typical clinical syndrome of ataxic UVL subjects [5,29]. This improved tandem stepping performance may be due to reduced vestibular-somatosensory conflict and/or increased gain of the proprioceptive postural loop [1] or to improved feedforward control of the complex multi-segmental task [4].
4.2. Practice sessions with and without BF
The cross-over design adopted for this experiment cancelled the potential effects of session order by averaging across sessions (with and without BF). In other words, the results reported in Fig. 4 were not influenced by the order effect of trials with and without BF so that the effect of any learning, that occurred with task repetition, was equally divided between the 2 sessions. A few previous studies of the effects of BF on postural control did not control for such a practice effect and attributed all of the improvement in performance to effects of BF [11,32].
During trials with BF, all subjects consistently achieved better performance than in practice trials without BF. Specifically, trunk stability and stepping accuracy were consistently better in trials with BF than in trials without BF. These results suggest that UVL subjects were able to effectively use BF to improve their performance during tandem gait, consistent with previous studies with other, less dynamic tasks such as stance [18,34]. Furthermore, this improved performance occurred at the start of the very first trials with the BF device and did not require a period of practice to be effective. This immediate improvement of postural control with BF is consistent with our previous studies of effects of audio-biofeedback on stance posture in subjects with bilateral vestibular loss and controls [7–9]. The different improvement time frames (immediate with BF and slower without BF) suggest that both BF and training affect motor performance via different, complimentary mechanisms.
During the practice trials in the session with BF, UVL subjects did not increase their relative stability as much as during the practice trials without BF. This result was probably due to the significantly greater stability level induced in the first trial with BF, leaving a smaller potential for additional improvement (i.e. a floor effect). However, the trend of improved stability over time was not significantly different between sessions with and without BF. Further, BF consistently improved the accuracy of the tandem gait performance across practice trials. Specifically, the frequency error was initially larger in trials with BF than in trials without BF although, in the end, the error was significantly lower (Fig. 4D). The higher error in frequency of stepping shown at the beginning of the session with BF may be due to the subjects' initial inability to pay enough attention to the metronome and the BF at the same time. Over time, however, all subjects could decrease this error to the point that they achieved the best performance, in terms of frequency error, in the trials with BF. This particular result suggests that the use of BF becomes more automatic (i.e. requires less attention) with practice [22].
4.3. Short-term retention of motor learning
Immediately after practice, subjects retained their performance improvements achieved by practicing tandem gait without BF in terms of trunk stability and accuracy of foot placement, as shown by the four parameters analyzed in Table 3. This result is further evidence of the extensive potential for motor learning in UVL subjects [3,30]. Only limited short-term retention effects were evident after practice in the session with BF. Only one out of four parameters, the frequency error, was found to retain significant improvements without BF, after practicing with BF (Table 3). This result may suggest that, immediately after turning the BF device off, subjects retained a higher level of cognitive attention; attention that they then focused upon the only remaining external cue, the metronome beat. As a consequence, they more accurately controlled the frequency of stepping.
Three factors may have limited short-term retention of performance in the other three parameters (COM SD, trunk tilt SD, and ML step width) after practice with BF: 1) the short duration (about 10 minutes) of the practice; 2) the greater number of trials performed without BF (30) than with BF (24) due to the fact that tandem gait without BF was both the task for practicing and for verifying retention of performance; and 3) the experimental protocol was not purposely designed to facilitate transfer and retention of postural performance. To be more effective for retention, the protocol could have alternated trials with BF and without BF so that, at the beginning, trials with BF were more frequent, and then, over time, trials with BF were gradually diminished [27]. Future studies are needed to examine the long-term effects of BF on consolidation and long-term retention of improved balance and gait performance.
4.4. Conclusions
UVL subjects can integrate vibrotactile BF information in their postural control to effectively improve stability and performance accuracy during tandem gait. This improvement occurs as soon as the BF device is turned on and does not require a period of practice. However, this integration of augmented sensory information becomes more automatic with practice over trials. Thus, vibrotactile BF acts similarly to natural sensory feedback in improving dynamic motor performance and not as a method to recalibrate motor performance to improve function after short-term use.
Acknowledgements
We would like to thank Edward King and Dr. Charles Russell for technical support, Martina Mancini and Tara Phillips for assistance during the experiments, Triana Nagel-Nelson for recruitment of subjects, and all of our subjects for donating their time. This study was supported by grants from the NIH: DC01849, DC04082, and DC06201and by the European Commission through the FP6 project SENSACTION-AAL, INFSO-IST-045622.
References
- 1.Bronstein AM, Hood JD, Gresty MA, Panagi C. Visual control of balance in cerebellar and parkinsonian syndromes. Brain. 1990;113(Pt 3):767–779. doi: 10.1093/brain/113.3.767. [DOI] [PubMed] [Google Scholar]
- 2.Chandler RF, Clauser CE, McConville JT, Reynolds JT, Young HM. Investigation of inertial properties of the human body. Aerospace Medical Research Laboratory; Washington D.C.: 1975. [Google Scholar]
- 3.Cohen H. Vestibular rehabilitation improves daily life function. Am J Occup Ther. 1994;48:919–925. doi: 10.5014/ajot.48.10.919. [DOI] [PubMed] [Google Scholar]
- 4.Corna S, Nardone A, Prestinari A, Galante M, Grasso M, Schieppati M. Comparison of Cawthorne-Cooksey exercises and sinusoidal support surface translations to improve balance in patients with unilateral vestibular deficit. Arch Phys Med Rehabil. 2003;84:1173–1184. doi: 10.1016/s0003-9993(03)00130-8. [DOI] [PubMed] [Google Scholar]
- 5.Curthoys IS, Halmagyi GM. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J Vestib Res. 1995 Mar–Apr;:67–107. [PubMed] [Google Scholar]
- 6.Dempster WT. Space Requirements of the Seated Operator. In: John Wiley & Sons, editor. Biomechanics and Motor Control of Human Movement. 1955. [Google Scholar]
- 7.Dozza M, Chiari L, Chan B, Rocchi L, Horak FB, Cappello A. Influence of a portable audio-biofeedback device on structural properties of postural sway. J Neuroengineering Rehabil. 2005;2:13. doi: 10.1186/1743-0003-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dozza M, Horak F, Chiari L. Auditory Biofeedback Substitutes for Loss of Sensory Information in Maintaining Stance. Exp Brain Res. 2006 doi: 10.1007/s00221-006-0709-y. [DOI] [PubMed] [Google Scholar]
- 9.Dozza M, Horak FB, Chiari L. Auditory biofeedback substitutes for loss of sensory information in maintaining stance. Exp Brain Res. 2007;178:37–48. doi: 10.1007/s00221-006-0709-y. [DOI] [PubMed] [Google Scholar]
- 10.Easton RD, Greene AJ, DiZio P, Lackner JR. Auditory cues for orientation and postural control in sighted and congenitally blind people. Exp Brain Res. 1998;118:541–550. doi: 10.1007/s002210050310. [DOI] [PubMed] [Google Scholar]
- 11.Hamann KF, Krausen C. Clinical applications of posturography – Body tracking and biofeedback training. In: Brandt T, Paulus W, Bles W, editors. Disorders of Posture and Gait. Georg Thieme Verlag Stuttgart; New York: 1990. pp. 295–298. [Google Scholar]
- 12.Hegeman J, Honegger F, Kupper M, Allum JH. The balance control of bilateral peripheral vestibular loss subjects and its improvement with auditory prosthetic feedback. J Vestib Res. 2005;15:109–117. [PubMed] [Google Scholar]
- 13.Herdman SJ, Hall CD, Schubert MC, Das VE, Tusa RJ. Recovery of dynamic visual acuity in bilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2007;133:383–389. doi: 10.1001/archotol.133.4.383. [DOI] [PubMed] [Google Scholar]
- 14.Honrubia V, Jenkins HA, Minser K, Baloh RW, Yee RD. Vestibulo-ocular reflexes in peripheral labyrinthine lesions: II. Caloric testing. Am J Otolaryngol. 1984;5:93–98. doi: 10.1016/s0196-0709(84)80027-7. [DOI] [PubMed] [Google Scholar]
- 15.Horak FB, Macpherson JM. Postural equilibrium and orientation. In: Rowell RB, Shepherd JT, editors. Handbook of Physiology. Published for the American Physiology Society by Oxford University Press; New York: 1996. pp. 255–292. [Google Scholar]
- 16.Horak FB, Shupert CL. Role of the vestibular system in postural control. In: Herdman SJ, editor. Vestibular Rehabilitation. F. A. Davis Company; Philadelphia: 1994. pp. 22–46. [Google Scholar]
- 17.Huang H, Wolf SL, He J. Recent developments in biofeedback for neuromotor rehabilitation. J Neuroengineering Rehabil. 2006;3(11):11. doi: 10.1186/1743-0003-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kentala E, Vivas J, Wall C., III Reduction of postural sway by use of a vibrotactile balance prosthesis prototype in subjects with vestibular deficits. Ann Otol Rhinol Laryngol. 2003;112:404–409. doi: 10.1177/000348940311200503. [DOI] [PubMed] [Google Scholar]
- 19.Lacour M, Barthelemy J, Borel L, Magnan J, Xerri C, Chays A, Ouaknine M. Sensory strategies in human postural control before and after unilateral vestibular neurotomy. Exp Brain Res. 1997;115:300–310. doi: 10.1007/pl00005698. [DOI] [PubMed] [Google Scholar]
- 20.Mbongo F, Patko T, Vidal PP, Vibert N, Tran Ba HP, de WC. Postural control in patients with unilateral vestibular lesions is more impaired in the roll than in the pitch plane: a static and dynamic posturography study. Audiol Neurootol. 2005;10:291–302. doi: 10.1159/000086081. [DOI] [PubMed] [Google Scholar]
- 21.Moore ST, Woollacott M. The use of biofeedback to improve postural stability. Phys Ther Practice. 1993;2:1–19. [Google Scholar]
- 22.Mulder T, Zijlstra W, Geurts A. Assessment of motor recovery and decline. Gait Posture. 2002;16:198–210. doi: 10.1016/s0966-6362(01)00157-6. [DOI] [PubMed] [Google Scholar]
- 23.Parietti-Winkler C, Gauchard GC, Simon C, Perrin PP. Sensorimotor postural rearrangement after unilateral vestibular deafferentation in patients with acoustic neuroma. Neurosci Res. 2006;55:171–181. doi: 10.1016/j.neures.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Peterka RJ, Black FO, Schoenhoff MB. Age-related changes in human vestibulo-ocular reflexes: sinusoidal rotation and caloric tests. J Vestib Res. 1990;1:49–59. [PubMed] [Google Scholar]
- 25.Peterka RJ, Wall C, III, Kentala E. Determining the effectiveness of a vibrotactile balance prosthesis. J Vestib Res. 2006;16:45–56. [PubMed] [Google Scholar]
- 26.Petersen H, Magnusson M, Johansson R, Fransson PA. Auditory feedback regulation of perturbed stance in stroke patients. Scand J Rehabil Med. 1996;28:217–223. [PubMed] [Google Scholar]
- 27.Schmidt RA. Motor Control and Leaning: A Behaviral Emphasis. Human Kinetic Pub; Champaign IL: 1982. [Google Scholar]
- 28.Schwarz M, Olson PR. A hystorical perspective on the field of biofeedback and applied psychophysiology. In: Schwarz M, Andrasik F, editors. Biofeedback: A Practitioner's Guide. Guilford; New York: 2003. pp. 3–19. [Google Scholar]
- 29.Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction of balance. Suggestion from the field. Phys Ther. 1986;66:1548–1550. doi: 10.1093/ptj/66.10.1548. [DOI] [PubMed] [Google Scholar]
- 30.Shumway-Cook A, Horak FB. Rehabilitation strategies for patients with vestibular deficits. Neurol Clin. 1990;8:441–457. [PubMed] [Google Scholar]
- 31.Shupert CL, Horak FB, Black FO. Hip sway associated with vestibulopathy. J Vestib Res. 1994;4:231–244. [PubMed] [Google Scholar]
- 32.Tyler M, Danilov Y, Bach-y-Rita P. Closing an open-loop control system: vestibular substitution through the tongue. J Integr Neurosci. 2003;2:159–164. doi: 10.1142/s0219635203000263. [DOI] [PubMed] [Google Scholar]
- 33.Vaughan CL, Davis BL, O'Connor JC. Dynamics of Human Gait. Kiboho; 1999. [Google Scholar]
- 34.Wall C, III, Kentala E. Control of sway using vibrotactile feedback of body tilt in patients with moderate and severe postural control deficits. J Vestib Res. 2005;15:313–325. [PubMed] [Google Scholar]
- 35.Wall C, III, Weinberg MS, Schmidt PB, Krebs DE. Balance prosthesis based on micromechanical sensors using vibrotactile feedback of tilt. IEEE Trans Biomed Eng. 2001;48:1153–1161. doi: 10.1109/10.951518. [DOI] [PubMed] [Google Scholar]
- 36.Weinberg MS, Wall C, Robertsson J, O'neil E, Sienko K, Fields R. Tilt Determination in MEMS Inertial Vestibular Prosthesis. J Biomech Eng. 2006;128:943–956. doi: 10.1115/1.2378922. [DOI] [PubMed] [Google Scholar]
- 37.Winter DA, MacKinnon CD, Ruder GK, Wieman C. An integrated EMG/biomechanical model of upper body balance and posture during human gait. Prog Brain Res. 1993;97:359–367. doi: 10.1016/s0079-6123(08)62295-5. [DOI] [PubMed] [Google Scholar]
- 38.Wolf SL. Electromyographic biofeedback applications to stroke patients. A critical review. Phys Ther. 1983;63:1448–1459. doi: 10.1093/ptj/63.9.1448. [DOI] [PubMed] [Google Scholar]
- 39.Wu G. Real-time feedback of body center of gravity for postural training of elderly patients with peripheral neuropathy. IEEE Trans Rehabil Eng. 1997;5:399–402. doi: 10.1109/86.650298. [DOI] [PubMed] [Google Scholar]