Abstract
Variation in endocrine pathways can be a major mechanism underlying life-history evolution. Yet it is unclear whether this insight, derived primarily from solitary species, explains the origins of complex life-history traits in highly social taxa. Thus, we here document and study variation in social life-history syndromes of female fecundity, behavior, and life span in selectively bred honeybee (Apis mellifera) strains. Associated variation in endocrine signaling was uncovered by RNA interference (RNAi) silencing of the juvenile hormone (JH) suppressor gene vitellogenin. High versus low endocrine reactivity in response to vitellogenin knockdown consistently correlated with rapid social behavioral ontogeny and short life span versus slow social behavioral ontogeny and long life span. Variation in JH reactivity, furthermore, was a function of variation in fecundity (ovary size and follicle development). A JH-mediated pleiotropy of female life-history traits, including fecundity, behavior, and life span, characterizes the distantly related solitary insect Drosophila. For the first time, we document a similar regulatory principle in a highly social species where most females are alloparental helpers (workers) that seldom reproduce. We conclude that variation in endocrine pathways of solitary origin can underlie variation and evolvability of complex social life-history traits.
Keywords: social evolution, juvenile hormone, vitellogenin, endocrine pleiotropy, insulin signaling, longevity
An explanation for the emergence of social phenotypes is that regulatory pathways present in solitary ancestors are substrates for social evolution (West-Eberhard 1987, 1996; Amdam et al. 2004). This proposition assumes that solitary physiological and behavioral traits under coordinated regulatory control are co-opted during evolutionary time to produce the fundamental features of social life histories. This hypothesis predicts considerable pleiotropy between physiological and behavioral traits in social species that mirror trait associations in solitary ancestors. The prediction appears to hold true for the honeybee, where the caste of facultatively sterile worker females shows an ancestral linkage between reproductive physiology and pollen- (protein source) versus nectar- (carbohydrate source) foraging behavior (reviewed by Page and Amdam [2007]).
Explicitly, worker honeybees disruptively selected for pollen versus nectar collection and storage (high- vs. low-pollen-hoarding strains) are characterized by expression of highly divergent social life-history syndromes that are equally found within populations of unselected workers (wild-type bees) and as racial differences between Apis mellifera subspecies (Page and Erber 2002; Pankiw 2003; Amdam et al. 2004, 2006a). The bias toward pollen hoarding—a maternal brood-provisioning behavior in many solitary insects—correlates with large ovary size (ovariole number), enhanced synthesis of the yolk precursor protein vitellogenin in nest bees (young adults that perform social tasks within the nest), and rapid behavioral ontogeny (subsequent transition from social nest tasks to foraging and provisioning tasks in the field). Bias toward nectar collection is associated with the opposite of all of these, and nectar feeding is also characteristic of solitary insect females that are not reproductively active and not provisioning brood (reviewed by Page and Amdam [2007]).
The yolk precursor protein vitellogenin, interestingly, affects central aspects of honeybee social behavior. High circulating levels during the nest stage prime bees to collect pollen as foragers (Nelson et al. 2007). The transition from nest tasks to foraging, furthermore, is preceded by a drop in the vitellogenin titer (Engels and Fahrenhorst 1974). This drop provides a mechanism that can drive an increase in the juvenile hormone (JH) level (Amdam and Omholt 2003; Guidugli et al. 2005). The important systemic hormone JH causes further suppression of vitellogenin synthesis (Pinto et al. 2000), and the resulting endocrine feedback loop can lock workers into the forager state (Rutz et al. 1974; Robinson 1987; Amdam and Omholt 2003; Toth and Robinson 2005). Worker ontogeny is also sensitive to pheromonal inhibition (nest bees may delay the shift to foraging tasks in response to forager-produced ethyl oleate; Leoncini et al. 2004), which may act through the feedback loop of vitellogenin and JH (Amdam and Omholt 2003). In sum, these insights indicate that endocrine pleiotropy is at least partly responsible for physiological and behavioral trait associations that characterize the life history of worker honeybees.
Life-history evolution is suggested to emerge through exploitation of variation in endocrine mechanisms (West-Eberhard 2003; Flatt et al. 2005; Giray et al. 2005). We ask whether social life-history syndromes, as exemplified by the complex pleiotropic trait associations of worker honeybees, likewise can be understood as the coordinated outcomes of variation in endocrine regulation. In resolving this question, we use workers from high- and low-pollen-hoarding strains, the exemplars of the diverging life-history syndromes of honeybees.
Methods
Honeybee Colony Setups
To verify physiological and behavioral traits of bees in different pollen-hoarding strains and to obtain data on relative longevity, newly emerged bees (0–12 h old) from four cofostered high- and low-strain sources were uniquely tagged. Thirty bees per strain were not tagged but were set aside for quantification of ovariole number. Three hundred tagged high-strain bees and 300 tagged low-strain bees were introduced into each of two observation hives, together with approximately 5,000 wild-type bees, brood, and a queen. A subsample of 10 bees per strain was collected as 5-day-olds for quantification of vitellogenin levels (see “Vitellogenin Protein Quantification”). Colonies were surveyed two or three times per week during nonforaging hours to establish survivorship. Each colony was observed during peak foraging hours for 40 min daily, as described previously (Nelson et al. 2007), and all tagged foragers were recorded. Foraging onset was the adult age when a bee was first seen returning from foraging. Time of death was estimated as the day after the last day a bee was observed alive, as described previously (Nelson et al. 2007).
To obtain detailed data on vitellogenin dynamics (titer rates of change) during the behavioral ontogeny of high-and low-pollen-hoarding-strain bees, pharate adults and newly emerged bees from four cofostered high- and low-strain sources were collected for analyses, and in addition, 600 newly emerged bees of each strain were marked on the thorax with paint to indicate age and strain. Marked workers were added in equal numbers to three unrelated “host” colonies (five frame nucleus hives) and later collected for sampling.
RNA Interference (RNAi)
To test the endocrine reactivity of bees from different pollen-hoarding strains, newly emerged workers from four cofostered high- and low-strain sources were divided into three groups marked with separate colors of paint. One group per strain was set aside as noninjected control, one group was injected with double-stranded RNA (dsRNA) derived from green fluorescent protein, and the last group was injected with dsRNA for vitellogenin (AJ517411), as described previously (Amdam et al. 2003, 2006b; 10 µg dsRNA in nuclease-free water; injection volume was 2 µL). Twenty bees of each treatment group and strain were transferred to each of two cages. Cages had two compartments divided by a wire screen. Treated bees were kept in one compartment, and about 150 wild-type bees of mixed age were kept in the other, allowing social contact between treated workers and older bees (Huang and Robinson 1992). Cages received 30% sucrose solution and pollen dough ad lib. until bees were collected after 7 days. This and similar protocols reduce vitellogenin messenger RNA (mRNA) and corresponding protein level in knockdowns relative to controls, and this vitellogenin knockdown affects the endocrine activity of worker bees (Amdam et al. 2003, 2006b; Guidugli et al. 2005; Nelson et al. 2007). Mortality was < 1%.
Sample Collection and Quantification of Ovarian Physiology
Hemolymph was retrieved with Drummond micropipettes (1 ± 0.001 µL). One microliter was dissolved in 10 µL Tris buffer (Amdam et al. 2006b) for vitellogenin quantification, and 1–3 µL was dissolved in acetonitrile for JH radioimmunoassay. The abdomen was dissected in phosphate-buffered saline, and ovariole number was determined. Ovarian activation was scored on a relative scale (Amdam et al. 2006a): 1 = nonactivated ovary, 2= previtellgenic activated ovary, 3 = vitellogenic ovary with developing oocytes, and 4 = mature ovary with at least one egg. Fat body tissue (site of vitellogenin synthesis) was transferred to 1 mL TRIzol (Invitrogen).
Vitellogenin Protein Quantification
Samples were separated by 7.5% SDS-PAGE (Amdam et al. 2006b). Gels were stained with Commassie Brilliant Blue (Sigma-Aldrich). Gel-to-gel variation in staining intensity was controlled by background correction and a β-galactosidase standard loaded in equal-dilution series on each gel (Amdam et al. 2006b). Densitometrical quantification was performed with Quantity One imaging software (Bio-Rad).
Vitellogenin mRNA Quantification
Vitellogenin and actin (LOC406122, a housekeeping gene) mRNA in fat body was quantified with real-time reverse transcription polymerase chain reaction (RT-PCR) using QuantiTect SYBR Green RT-PCR kit (Qiagen) and ABI Prism 7500 (Applied Biosystems) as before (Amdam et al. 2004). Individual vitellogenin mRNA levels were determined by the comparative cycle time method as a relative quantity based on triplicate readings.
JH Radioimmunoassay
The protocol established for honeybee hemolymph (Huang et al. 1994) was used to extract JH. The antiserum was developed by Goodman et al. (1990), and the protocol (Goodman et al. 1995) was validated for bees (Guidugli et al. 2005). The assays used the tritiated [10-3H(N)]-JH III (specific activity 19.4 Ci/mmol; NEN Life Science Products) and JH III (Fluka) as the nonradioactive ligand. The JH titers of samples were calculated by log-linear regression analysis of standard curve doses (log pg JH III) on logit binding values.
Statistics
Ovariole number in newly emerged bees was analyzed by two-way ANOVA with strain and replicate as categorical factors (no effect of replicate was detected; P = .33). Vi-tellogenin titers of 5-day-old high- and low-strain bees were analyzed with two-way ANOVA using strain and replicate as categorical factors (no effect of replicate was detected; P = .52). Age at foraging onset and survival were analyzed with Cox’s proportional hazard regression stratified by colony (Nelson et al. 2007). Rates of change were analyzed by calculating mean vitellogenin levels of pharate adults, emerging bees, and 5-, 10-, and 15-day-old workers (n = 16–20 for each age and strain). Next, average rates of change were estimated within colony and strain by subtracting each mean from the average vitellogenin level detected at the prior time point. Nonparametric Friedman ANOVA was used to test for time effects on average rates of change, and a Mann-Whitney U-test was used for post hoc comparisons. Factorial ANOVA and Fisher least significant difference (LSD) post hoc tests were used to verify vitellogenin knockdown and to analyze effects on JH titer; no effects of replicates were detected (P > .45). The numbers of mature high- and low-strain bees that never foraged were compared to the numbers of confirmed foragers (see “Honeybee Colony Setups”) with a χ2 test. Correlation of ovariole number and JH titer was determined by polynomial regression using one second-order term. Ovariole number and ovarian activation were examined using two-way ANOVA with replicate as a second categorical factor (no effect of replicate was detected; P = .25) and a χ2 test.
Results and Discussion
Pleiotropic Associations between Social Life-History Traits
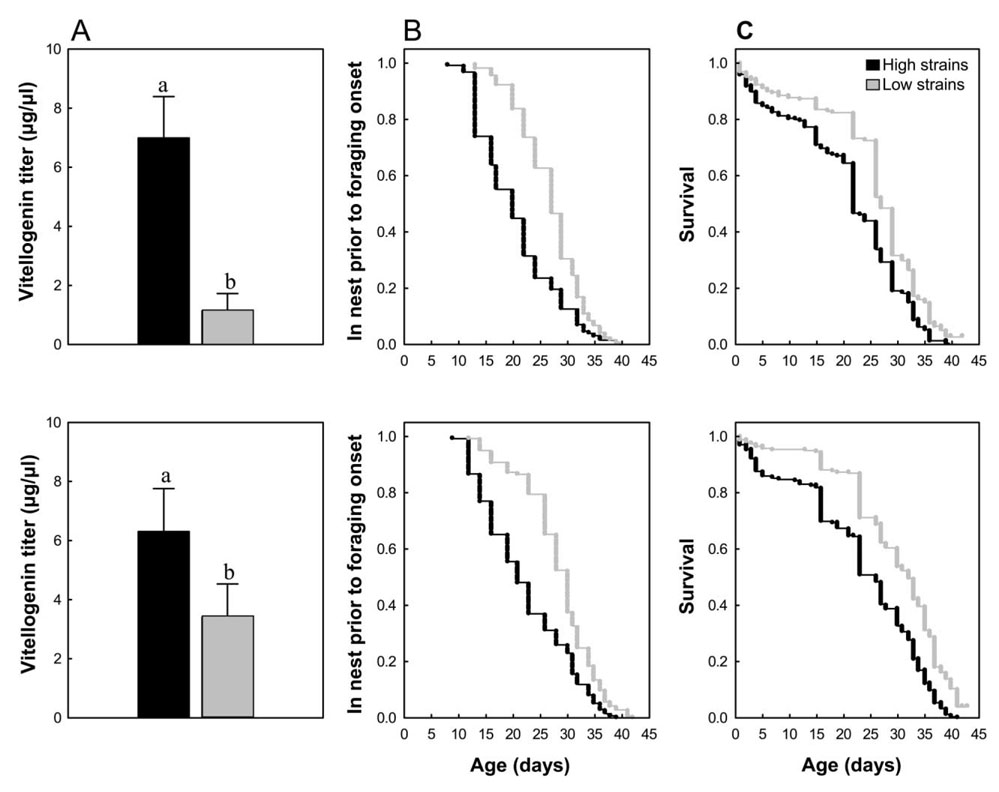
Because pollen-hoarding strains are neither isogenic nor inbred lines, we initially checked to what extent the trait associations described before (Page and Amdam 2007) persisted in our current stocks. We confirmed that high-pollen-hoarding strains are characterized by a higher number of ovarioles (mean for both ovaries ± SE = 8.28 ± 0.55 n = 30) than low-strain bees (5.90 ± 0.57 n = 30; two-way ANOVA: F = 8.98, df = 1, 59, P < .005) and hemolymph samples drawn 5 days after adult emergence also verified that high strains have elevated vitellogenin levels during the nest stage (fig. 1A; two-way ANOVA: F = 13.08, df = 1, 17, P < .01; Amdam et al. 2004). Worker bees from high strains predictably forage earlier in life (fig. 1B; Cox’s regression: χ2 = 45.29, P < .000001; Pankiw and Page 2001), and we further show, for the first time, that they have shorter life spans than low-strain bees (fig. 1C; Cox’s regression: χ2 = 64.72, P < .00005; see figure legend for sample sizes).
Figure 1.
Pleiotropic trait associations in honeybee workers of selected high- and low-pollen-hoarding strains. A, Mean vitellogenin titer of 5-day-old bees in two replicate colony setups (upper and lower panels). Error bars indicate standard errors; lowercase letters indicate significant differences between strains (two-way ANOVA: P < .05 n = 10). B, Daily cumulative probability of workers staying in the hive to perform nest tasks before the onset of foraging behavior (n = 262 and 259 for high and low strains, respectively). Only data from bees that actually foraged are included. C, Cumulative survival of strains, including bees that died without having foraged (n = 467 and 522 for high and low strains, respectively).
Regulatory Convergence between Physiology, Behavioral Ontogeny, and Life Span
As a first step toward understanding the regulatory origins of honeybee social life histories, we compared the confirmed syndromes of high- and low-pollen-hoarding strains with footprints of known regulators of behavioral specialization, behavioral ontogeny, and life span. The yolk precursor protein vitellogenin (fig. 1) is one such pleiotropic regulator: it primes worker honeybees for pollen foraging, suppresses behavioral ontogeny (Amdam and Omholt 2003; Nelson et al. 2007), and can also prolong life span by its capacity to scavenge free radicals (Seehuus et al. 2006). While performing nest tasks, high-pollen-hoarding-strain bees have higher vitellogenin levels than low-strain bees (fig. 1A; Amdam et al. 2004), consistent with their preference for collecting pollen as foragers (Pankiw and Page 2001). However, for the high-pollen-hoarding phenotype to also be consistent with the roles of vitellogenin in regulation of foraging onset and longevity, the vitellogenin titers of high strains must drop at younger ages than those in low strains (fig. 1B; Page and Amdam 2007). Foraging behavior is initiated, at the earliest, when high-strain bees are 12–13 days old (fig. 1B). Thus, to clarify whether pollen-hoarding syndromes conform to signatures of an established life-history regulator, we next quantified changes in the vitellogenin level until selected bees of the pollen-hoarding strains were 15 days old.
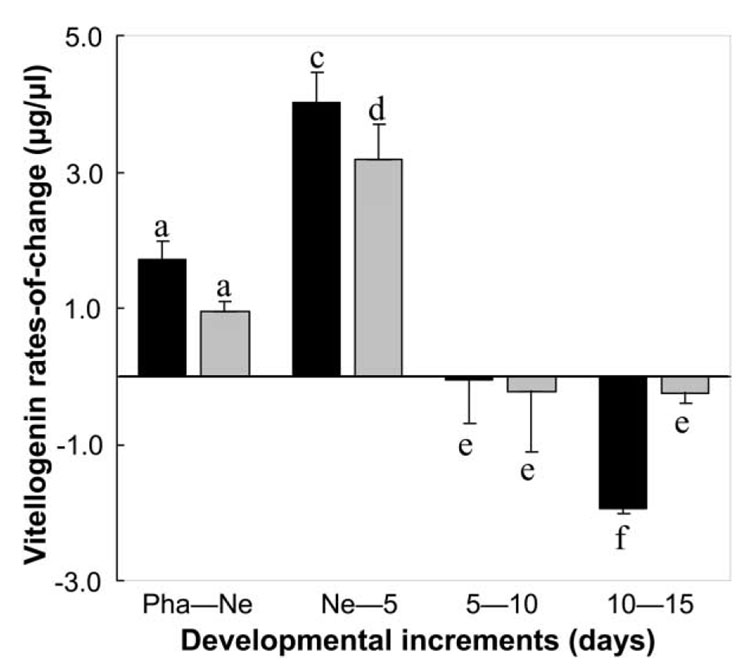
We found that vitellogenin increases more rapidly in young workers of high-pollen-hoarding strains (Amdam et al. 2004) but subsequently also declines more rapidly than in low-strain bees (fig. 2; Friedman ANOVA: χ2 = 15.00, P < = .01). The drop occurred between days 10 and 15 of adult life, an age at or just before the foraging onset of many high-strain bees (fig. 1B). Few bees of this age from low strains initiated foraging (fig. 1B), and their vitellogenin levels were constant (fig. 2). These findings show that although high-strain bees are characterized by higher circulating titers of vitellogenin as nest bees, their titers drop at younger ages, consistent with their early onset of provisioning behavior and short life spans compared to low-strain bees. We conclude that linkage of behavioral ontogeny and longevity in pollen-hoarding strains is consistent with previously documented pleiotropic effects of vitellogenin (Amdam and Omholt 2003; Guidugli et al. 2005; Nelson et al. 2007). Comparable covariation between amplitude of yolk protein levels and timing of life-history transitions has been documented in the grasshopper Romalea microptera (Hatle et al. 2004).
Figure 2.
Vitellogenin rates of change in selected pollen-hoarding strains measured from onset of synthesis in pharate adults (Pha) and until workers were 15 days old. For each successive life stage, the plot quantifies how much the vitllogenin level changes relative to the prior stage. An increase, therefore, is shown as a positive rate of change, a decline as a negative rate of change, and a constant titer as no (0) rate of change. Mean rates of change were calculated from individual hemolymph titers (n = 16–20 for each age and strain) obtained from three replicate colony setups. Error bars indicate standard errors; lowercase letters indicate significant difference between high and low strains (Mann-Whitney U-test: P < .05).
Genotypic Differences in Endocrine Feedback-Loop Sensitivity and Social Behavior
To confirm that endocrine mechanisms underlie the life-history differences of high and low strains, we used the association between vitellogenin and the pollen-hoarding syndromes as a starting point. One unifying explanation for differences in yolk protein expression and life-cycle progression is variation in JH signaling, as observed in Drosophila (Flatt et al. 2005). Yet prior evidence does not suggest that JH alone underlies genotypic variation in the physiological and behavioral ontogeny of honeybees. Twelve-day-old high- and low-pollen-hoarding-strain bees have similar JH levels (Schulz et al. 2004). Variation in foraging onset between selected (Giray et al. 1999; Elekonich et al. 2003) and wild-type (Robinson et al. 1987) bees also is not strictly associated with variation in JH. The alternative hypothesis is that ontogeny is influenced by regulatory sensitivity to vitellogenin or JH (Giray et al. 1999; Guidugli et al. 2005). We thus examined the endocrine coupling strength of vitellogenin and JH in selected strains by RNAi knockdown of vitellogenin activity (Amdam et al. 2003). In wild-type bees, vitellogenin knockdown triggers a significant but variable increase in the systemic JH level that is easily detected in 7-day-old bees (Guidugli et al. 2005). Likewise, it triggers a significant but variable decrease in age at foraging onset and longevity (Seehuus et al. 2006; Nelson et al. 2007).
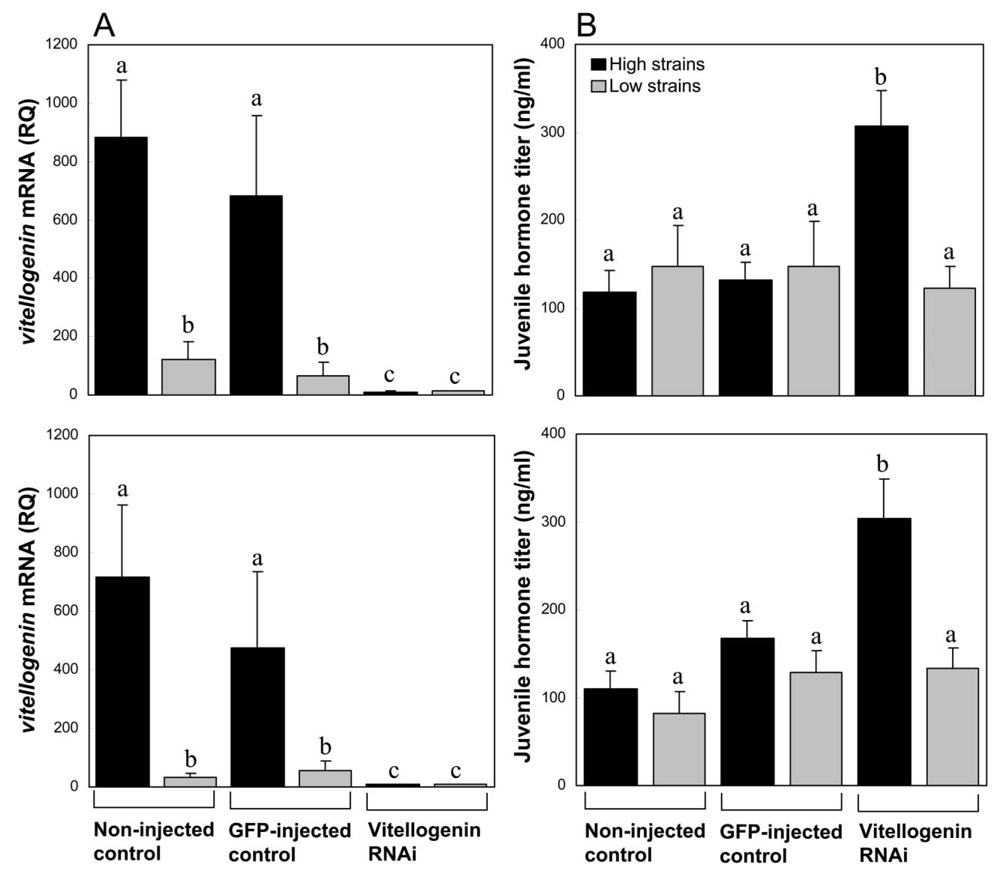
Young bees (7 days old) of high-pollen-hoarding strains are characterized by a higher vitellogenin mRNA level than workers of low strains (fig. 3A; factorial ANOVA: F = 20.87, df = 1,60, P < .0001), in accordance with their higher vitellogenin protein level (fig. 1A). The RNAi treatment effect (factorial ANOVA: F = 8.38, df = 2,60 P < .001) eliminated this genotypic difference between strains and produced a uniform knockdown phenotype (LSD post hoc test: P = .78). As reported for wild-type bees, we found that vitellogenin RNAi caused a significant change in the systemic JH level (factorial ANOVA: F = 11.64, df = 2, 135, P < .0001), but the effect emerged solely from a dramatic increase in high-pollen-hoarding-strain bees (fig. 3B; LSD post hoc test: P < .00001). Low-strain knockdowns had low JH titers that were equal to those of controls (fig. 3B; LSD post hoc test: P > .35). Our results suggest that the sensitivity, or endocrine reactivity, of the positive feedback loop between vitellogenin and JH (Amdam and Omholt 2003) is stronger in high-pollen-hoarding strains than in low strains. This conclusion is independently supported by the finding that Africanized honeybees (Apis mellifera scutellata hybrids) show a stronger JH dose response to vitellogenin knockdown than European honeybees (A. mellifera carnica; Guidugli et al. 2005). High- and low-pollen-hoarding strains, therefore, mimic the endocrine regulatory circuitry of Africanized and European bees, respectively; and, interestingly, they also mimic their racial life-history syndromes (Robinson et al. 1987; Pankiw 2003).
Figure 3.
Juvenile hormone (JH) reactivity in response to vitellogenin RNA interference (RNAi) in 7-day-old workers of selected pollen-hoarding strains. A, Mean vitellogenin messenger RNA level in noninjected controls, controls injected with double-stranded RNA derived from green florescent protein (GFP), and vitellogenin gene knockdowns in two replicate cage setups (upper and lower panels; n = 25 21, and 22 for vitellogenin RNAi, GFP control, and noninjected control, respectively). B, Mean JH response to vitellogenin downregulation for each replicate in the corresponding panel of A (n = 63 38, and 40 for vitellogenin RNAi, GFP control, noninjected control, respectively). Error bars indicate standard errors; lowercase letters indicate significant difference between strains (Fisher least significant difference post hoc tests: P < .05).
Numeric Simulation Modeling of Endocrine Feedback-Loop Sensitivity
We next examined implications of variation in the endocrine response to low vitellogenin levels with the double-repressor model of Amdam and Omholt (2003). This model describes the feedback loop between vitellogenin and JH and its effect on worker physiology and behavior in mathematical terms. It assumes that vitellogenin or a derivative competes for binding to a repressor receptor in the allatoregulatory neuroendocrine axis. The second competitor ligand is a derivative of pheromonal cues that affect worker behavioral ontogeny (Huang and Robinson 1992, 1996; Leoncini et al. 2004). Synthesis of JH is conceptually modeled as inversely proportional to the fractional ligand occupation of repressor receptor molecules. Since dynamic properties of this simple model can account for an array of physiological and behavioral data (Amdam and Omholt 2003; Toth and Robinson 2005), we modified the basic algorithm to simulate variable feedback-loop sensitivity as variation in the downstream JH dose response to any given fractional ligand occupation.
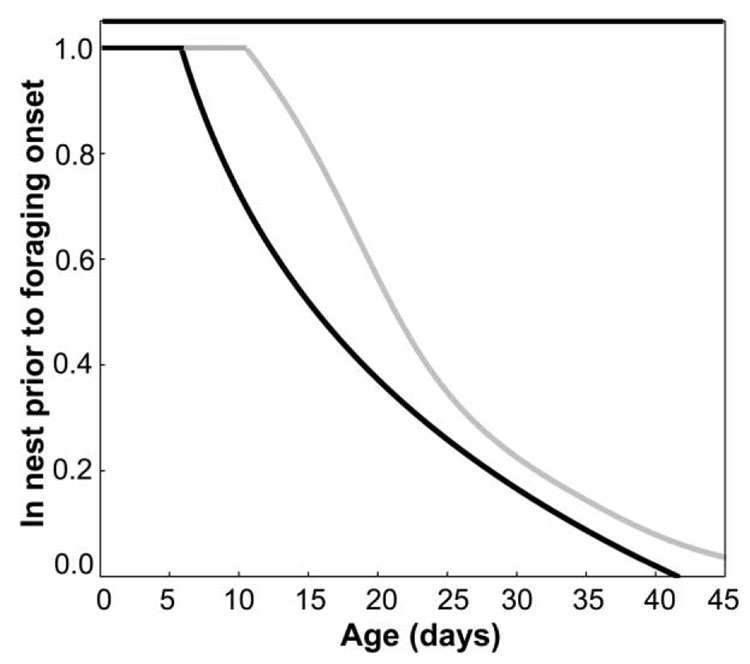
Our simulations show that variation in the endocrine coupling strength of vitellogenin and JH translates into variation in foraging onset: bees with reduced feedback-loop sensitivity are predicted to forage later in life (fig. 4). The mathematical model also predicts that some individuals with low JH reactivity to reduced vitellogenin levels are unlikely to initiate foraging before they die (fig. 4). Our records of behavior and life span (fig. 1B, 1C) confirm this predicted pattern. Workers that never initiated foraging were more common among low-strain bees (χ2 test:. χ2 = 4.16, P < .05, n = 205 for the high strain, n = 263 for the low strain). Life-history implications of variation in endocrine reactivity are suggested also by the finding that vitellogenin RNAi triggers a dramatic reduction in age at foraging onset in high-pollen-hoarding-strain bees, while low-strain bees remain nest workers (K. E. Ihle, R. E. Page, and G. V. Amdam, unpublished data), exactly paralleling the JH response of the strains (fig. 3). These theoretical and empirical insights link variation in social ontogeny to a plausible endocrine mechanism.
Figure 4.
Simulation output from the double-repressor model of Amdam and Omholt (2003). The model was parameterized for high endocrine feedback-loop sensitivity by a 5 % increase in the parameter β (originally set to 500), which determines the theoretical rate of change of juvenile hormone (JH) in response to any fractional occupation of available repressor receptor molecules in the allatoregulatory neuroendocrine axis. Low feedback-loop sensitivity was modeled as 5% decrease in β. The model output shows that variation in the coupling strength of vitellogenin and JH produces variation in foraging onset and that some individuals with low feedback-loop sensitivity have a low probability of initiating foraging behavior before they die (intersection between X-axis and grayline, lower right-hand corner).
Pleiotropy of Endocrine Feedback-Loop Sensitivity and Reproductive Physiology
Our report supports the hypothesis that variation in endocrine mechanisms underlies variation in honeybee life histories. Yet our findings so far have not identified the regulatory architecture or evolutionary origin of the complex syndromes. It has been proposed that vitellogenin and JH interact at foraging onset because a reproductive regulatory pathway was co-opted during social evolution to control the behavior of honeybees (Amdam et al. 2004;Guidugli et al. 2005). Under this reproductive-ground plan hypothesis (see also the ovarian-ground plan framework by West-Eberhard [1987, 1996]), pleiotropy of social ontogeny and reproductive physiology is predicted (Amdam et al. 2004). Endocrine feedback-loop sensitivity should, if it is an element of this architecture, be associated with variation in reproductive traits (Flatt et al. 2005). Thus, we next examined whether variation in the coupling strength of vitellogenin and JH is indeed linked to variation in the reproductive system of worker bees.
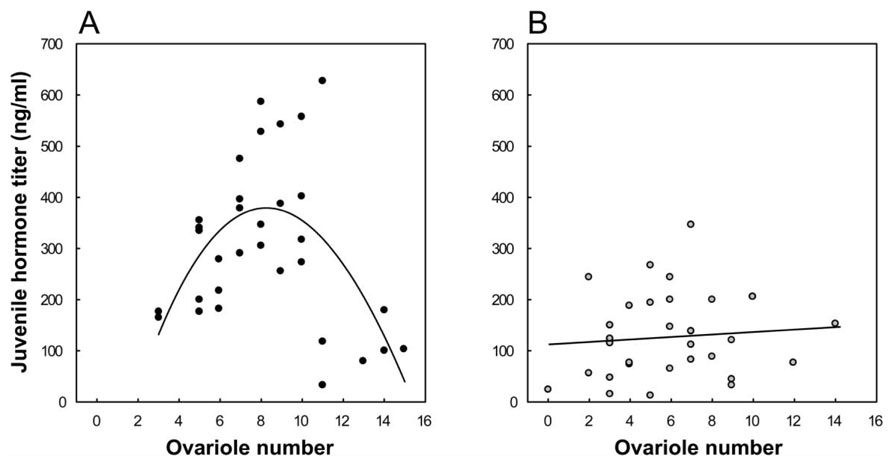
Ovariole number is determined during honeybee larval development and is positively affected by JH signaling (Schmidt-Capella and Hartfelder 1998). High-pollen-hoarding-strain bees are characterized by more ovarioles than low-strain bees (see “Pleiotropic Associations between Social Life-History Traits”), but in addition, they are characterized by a remarkable correlation between ovariole number and endocrine feedback-loop sensitivity, measured as the increase in JH after vitellogenin knock-down (fig. 5A). The relationship is positive and linear in the 2–10 ovariole range but plunges dramatically in bees with particularly large ovaries (11–15 ovarioles; fig. 5A; see next subsection for more on these outliers). The correlation is best explained by a nonlinear model (polynomial regression: r = 0.59, F = 7.78, df = 2, 29, P < .005) and is absent in low-strain bees (fig. 5B; r = 0.11, P = .53).
Figure 5.
Relationship between endocrine feedback-loop sensitivity and reproductive physiology in worker honeybees of selected pollen-hoarding strains. Juvenile hormone (JH) titers of vitellogenin knockdowns from the replicate setups in figure 3 are plotted as functions of individual ovariole numbers for the high- (A) and low- (B) pollen-hoarding strains (n = 32 and 30, respectively). The second- and first-order regressions of the JH titer on ovariole number are shown in A and B, respectively.
Pleiotropy of reproductive physiology and ontogeny, as shown here for worker bees, is in solitary invertebrates the outcome of reproductive regulatory activity, signaling during both development and adult life (Dillin et al. 2002; Tu and Tatar 2003). Elevated endocrine reactivity in honey-bees, accordingly, would contribute to differentiation of an enlarged ovary in larvae (Schmidt-Capella and Hartfelder 1998) and also link this condition to an early adult foraging onset and a short life span in high-pollen-hoarding-strain bees. This inference is consistent with the phenotypes of wild-type bees, which show the same respective linkage of high ovariole number, foraging preference for pollen, and early foraging onset (Amdam et al. 2006a); of early foraging onset and high JH sensitivity (Giray et al. 1999); and of early foraging onset and reduced longevity (Neukirch 1982).
Ovarian Activation and Behavioral Regulation
Ovariole number and JH reactivity to vitellogenin knock-down show a nonlinear relationship in high-pollen-hoarding-strain bees (fig. 5A). In the absence of fecund queens, some honeybee workers become active reproductives, and their level of previtellogenic and vitellogenic follicle development is linked genetically to their ovariole number (Amdam et al. 2006a; Makert et al. 2006). Our test of endocrine feedback-loop sensitivity separated workers from queens (see “Methods”), and under these conditions high-pollen-hoarding-strain bees show higher propensity for ovarian activation (Amdam et al. 2006a). Oogenesis is linked to changes in the neuroendocrine axis of bees (Sasaki and Nagao 2002) and of insects in general (Simonet et al. 2004). Thus, a plunge in the endocrine feedback-loop sensitivity of workers with more than 10 ovarioles (fig. 5A) could signify a shift to an oogenic neuroendocrine state. To address this hypothesis, we studied levels of ovarian activation in a subset of the workers.
Our data confirmed that there is a significant positive association between ovariole number and oocyte maturation (two-way ANOVA:F = 5.86, df = 12,46 P < .00001). Activated ovaries were found almost exclusively in high-pollen-hoarding-strain bees with 10 ovarioles or more (χ2 test: χ2 = 18.38, P < .00001, n = 60) and included previtellogenic activation in high-strain bees with vitellogenin knockdown phenotype (n = 2 of 10).We propose, therefore, that the feedback loop of vitellogenin and JH can be affected by the changing state of reproductive maturation, as suggested by recent findings in Diploptera punctata (Elliott et al. 2006). A prediction from this hypothesis is that queenless worker bees that are active re-productives behave as queens: they do not forage. A low propensity for foraging labor in egg-laying workers is suggested by prior observations (Martin et al. 2002), and this relationship between physiology and behavior is widespread in social insects in general (West-Eberhard 1987; Hölldobler and Wilson 1990).
Conclusion
Hormones are major coordinators of reproduction, behavioral ontogeny, and life span, and hormonal pleiotropy can explain correlations between life-history traits and variation in life-cycle organization within and between solitary species (Finch and Rose 1995). We have shown that complex life-history syndromes of worker honeybees can be explained by variation in an endocrine signaling pathway. This pathway appears to affect ovary differentiation in larvae as well as age at foraging onset and life span in adults. Correlations between life-history traits in a highly social insect, therefore, resemble pleiotropic trait associations in the distantly related solitary insect Drosophila (Flatt et al. 2005). In Drosophila, JH signaling during larval life has a positive effect on ovary size (Tu and Tatar 2003). In addition, JH has a positive effect on oocyte maturation and reproductive behavior and a negative effect on longevity in adult female flies (Tatar 2004; Flatt et al. 2005). Reduced JH signaling produces Drosophila phenotypes with arrested oogenesis, suppressed reproductive behavior, and enhanced survival (Flatt et al. 2005). Recent findings further suggest not only that these trait associations are characteristic of individuals with varying rates of JH synthesis but also that they emerge in response to variation in sensitivity to JH (Tu et al. 2006). These and other observations have led to the proposition that hormonal pleiotropy is a major director of Drosophila life history (Flatt et al. 2005). Our findings suggest that similar mechanisms underlie life-history variation in honeybee workers.
The evidence presented here demonstrates that solitary regulatory architectures can be clearly recognized as pleiotropic signatures in social descendants. The data support the view that solitary regulatory pathways provide a ground plan from which complex social phenotypes can be assembled (West-Eberhard 1987,1996; Amdam et al. 2004). Our work, therefore, broadens the understanding of the mechanistic and evolutionary origins of life-history syndromes in highly social species.
Acknowledgments
We thank A. Siegel and J. Tsuruda for assistance, R. E. Page for honeybee strains and spirited discussions, and C. Brent, T. Flatt, S. Pratt, and two anonymous reviewers for comments. The JH antiserum was kindly provided by W. G. Goodman. G.V.A. and K.-A.N. were supported by Norwegian Research Council grants 171958 and 175413, National Science Foundation grant 0615502, and National Institute on Aging grant PO1 AG22500, and K.H. was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo grants 2004/10836-0 and 2005/03926-5.
Literature Cited
- Amdam GV, Omholt SW. The hive bee to forager transition in honeybee colonies: the double repressor hypothesis. Journal of Theoretical Biology. 2003;223:451–464. doi: 10.1016/s0022-5193(03)00121-8. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Simões ZLP, Guidugli KR, Norberg K, Omholt SW. Disruption of vitellogenin gene function in adult honeybees by intra-abdominal injection of double-stranded RNA. BMC Biotechnology. 2003;3:1. doi: 10.1186/1472-6750-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Fondrk MK, Page RE., Jr Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proceedings of the National Academy of Sciences of the USA. 2004;101:11350–11355. doi: 10.1073/pnas.0403073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Csondes A, Fondrk MK, Page RE., Jr Complex social behaviour derived from maternal reproductive traits. Nature. 2006a;439:76–78. doi: 10.1038/nature04340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Page RE, Jr, Erber J, Scheiner R. Downregulation of vitellogenin gene activity increases the gustatory responsiveness of honey bee workers (Apis mellifera) Behavioural Brain Research. 2006b;169:201–205. doi: 10.1016/j.bbr.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- Elekonich MM, Jez K, Ross AJ, Robinson GE. Larval juvenile hormone treatment affects pre-adult development, but not adult age at onset of foraging in worker honey bees (Apis mellifera) Journal of Insect Physiology. 2003;49:359–366. doi: 10.1016/s0022-1910(03)00020-9. [DOI] [PubMed] [Google Scholar]
- Elliott KL, Woodhead AP, Stay B. A stage-specific ovarian factor with stable stimulation of juvenile hormone synthesis in corpora allata of the cockroach Diploptera punctata. Journal of Insect Physiology. 2006;52:929–935. doi: 10.1016/j.jinsphys.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Engels W, Fahrenhorst H. Alters- und kastenspezifische Veräbderungen der Haemolymph-Protein-Spektren bei Apis mellificia. Roux’s Archives of Developmental Biology. 1974;174:285–296. doi: 10.1007/BF00573233. [DOI] [PubMed] [Google Scholar]
- Finch CE, Rose MR. Hormones and the physiological architecture of life history evolution. Quarterly Review of Biology. 1995;70:1–52. doi: 10.1086/418864. [DOI] [PubMed] [Google Scholar]
- Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- Giray T, Huang ZY, Guzman-Novoa E, Robinson GE. Physiological correlates of genetic variation for rate of behavioral development in the honeybee, Apis mellifera. Behavioral Ecology and Sociobiology. 1999;47:17–28. [Google Scholar]
- Giray TM, Giovanetti M, West-Eberhard MJ. Juvenile hormone, reproduction, and worker behavior in the Neotropical social wasp Polistes canadensis. Proceedings of the National Academy of Sciences of the USA. 2005;102:3330–3335. doi: 10.1073/pnas.0409560102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WG, Coy DC, Baker FC, Xu L, Toong YC. Development and application of a radioimmunoassay for the juvenile hormones. Insect Biochemistry. 1990;20:357–364. [Google Scholar]
- Goodman WG, Orth AP, Toong YC, Ebersohl R, Hiruma K, Granger NA. Recent advances in radioimmunoassay technology for the juvenile hormones. Archives of Insect Biochemistry and Physiology. 1995;30:295–306. doi: 10.1002/arch.940300215. [DOI] [PubMed] [Google Scholar]
- Guidugli KR, Nascimento AM, Amdam GV, Barchuk AR, Omholt SW, Simões ZLP, Hartfelder K. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Letters. 2005;579:4961–4965. doi: 10.1016/j.febslet.2005.07.085. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Andrews AL, Crowley MC, Juliano SA. Interpopulation variation in developmental titers of vitellogenin, but not storage proteins, in lubber grasshoppers. Physiological and Biochemical Zoology. 2004;77:631–640. doi: 10.1086/420946. [DOI] [PubMed] [Google Scholar]
- Hölldobler B, Wilson EO. The ants. Cambridge MA: Belknap; 1990. [Google Scholar]
- Huang Z-Y, Robinson GE. Honeybee colony integration: worker-worker interactions mediate hormonally regulated plasticity in division of labor. Proceedings of the National Academy of Sciences of the USA. 1992;89:11726–11729. doi: 10.1073/pnas.89.24.11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z-Y, Robinson GE. Regulation of honey bee division of labor by colony age demography. Behavioral Ecology and Sociobiology. 1996;39:147–158. [Google Scholar]
- Huang Z-Y, Robinson GE, Borst DW. Physiological correlates of division of labor among similarly aged honey bees. Journal of Comparative Physiology A. 1994;174:731–739. doi: 10.1007/BF00192722. [DOI] [PubMed] [Google Scholar]
- Leoncini I, Le Conte Y, Costagliola G, Plettner E, Toth AL, Wang MW, Huang Z, et al. Regulation of behavioral maturation by a primer pheromone produced by adult worker honey bees. Proceedings of the National Academy of Sciences of the USA. 2004;101:17559–17564. doi: 10.1073/pnas.0407652101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makert GR, Paxton RJ, Hartfelder K. Ovariole number: a predictor for differential reproductive success among worker subfamilies in queenless honey bee (Apis mellifera L.) colonies. Behavioral Ecology and Sociobiology. 2006;60:815–825. [Google Scholar]
- Martin S, Wossler T, Kryger P. Usurpation of African Apis mellifera scutellata colonies by parasitic Apis mellifera capensis workers. Apidologie. 2002;33:215–231. [Google Scholar]
- Nelson CM, Ihle KE, Fondrk MK, Page RE, Jr, Amdam GV. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biology. 2007;5:e62. doi: 10.1371/journal.pbio.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neukirch A. Dependence of the life span of the honeybee (Apis mellifera) upon flight performance and energy consumption. Journal of Comparative Physiology B. 1982;146:35–40. [Google Scholar]
- Page RE, Jr, Amdam GV. The making of a social insect: developmental architectures of social design. Bioessays. 2007;29:334–343. doi: 10.1002/bies.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RE, Jr, Erber J. Levels of behavioral organization and the evolution of division of labor. Naturwissenschaften. 2002;89:91–106. doi: 10.1007/s00114-002-0299-x. [DOI] [PubMed] [Google Scholar]
- Pankiw T. Directional change in a suite of foraging behaviors in tropical and temperate honey bees (Apis mellifera L.) Behavioral Ecology and Sociobiology. 2003;54:458–464. [Google Scholar]
- Pankiw T, Page RE., Jr Genotype and colony environment affect honeybee (Apis mellifera L.) development and foraging behavior. Behavioral Ecology and Sociobiology. 2001;51:87–94. [Google Scholar]
- Pinto LZ, Bitondi MMG, Simões ZLP. Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. Journal of Insect Physiology. 2000;46:153–160. doi: 10.1016/s0022-1910(99)00111-0. [DOI] [PubMed] [Google Scholar]
- Robinson GE. Regulation of honey bee age polyethism by juvenile hormone. Behavioral Ecology and Sociobiology. 1987;20:329–338. [Google Scholar]
- Robinson GE, Strambi A, Strambi C, Simões ZLP, Tozetto SO, Negraes-Barbosa JM. Juvenile hormone titers in European and Africanized honey bees in Brazil. General and Comparative Endocrinology. 1987;66:457–459. doi: 10.1016/0016-6480(87)90258-9. [DOI] [PubMed] [Google Scholar]
- Rutz W, Gerig L, Wille H, Lüscher M. A bioassay for juvenile hormone (JH) effects of insect growth regulators (IGR) on adult worker honeybees. Bulletin de la Société Entomologique Suisse. 1974;47:307–313. [Google Scholar]
- Sasaki K, Nagao T. Brain tyramine and reproductive states of workers in honeybees. Journal of Insect Physiology. 2002;48:1075–1085. doi: 10.1016/s0022-1910(02)00200-7. [DOI] [PubMed] [Google Scholar]
- Schmidt-Capella IC, Hartfelder K. Juvenile hormone effect on DNA synthesis and apoptosis in caste-specific differentiation of the larval honey bee (Apis mellifera L.) ovary. Journal of Insect Physiology. 1998;44:385–391. doi: 10.1016/s0022-1910(98)00027-4. [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Pankiw T, Fondrk MK, Robinson GE, Page RE., Jr Comparisons of juvenile hormone hemolymph and octopamine brain titers in honey bees (Hymenoptera: Apidae) selected for high and low pollen hoarding. Annals of the Entomological Society of America. 2004;97:1313–1319. [Google Scholar]
- Seehuus SC, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects sterile honey bee workers from oxidative stress. Proceedings of the National Academy of Sciences of the USA. 2006;103:962–967. doi: 10.1073/pnas.0502681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet G, Poels J, Claeys I, Van Loy T, Franssens V, De Loof A, Vanden Broeck J. Neuroendocrinological and molecular aspects of insect reproduction. Journal of Neuroendocrinology. 2004;16:649–659. doi: 10.1111/j.1365-2826.2004.01222.x. [DOI] [PubMed] [Google Scholar]
- Tatar M. The neuroendocrine regulation of Drosophila aging. Experimental Gerontology. 2004;39:1745–1750. doi: 10.1016/j.exger.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Toth AL, Robinson GE. Worker nutrition and division of labour in honeybees. Animal Behaviour. 2005;69:427–435. [Google Scholar]
- Tu M-P, Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Tu M-P, Flatt T, Tatar M. Juvenile and steroid hormones in Drosophila melanogaster longevity. In: Masoro EJ, Austad SN, editors. Handbook of the biology of aging. San Diego, CA: Academic Press; 2006. pp. 415–448. [Google Scholar]
- West-Eberhard MJ. Flexible strategy and social evolution. In: Itô Y, Brown JL, Kikkawa J, editors. Animal societies: theories and fact. Tokyo: Japan Scientific Societies Press; 1987. pp. 35–51. [Google Scholar]
- West-Eberhard MJ. Wasp societies as microcosms for the study of development and evolution. In: Turillazzi S, West-Eberhard MJ, editors. Natural history and evolution of paper-wasps. New York: Oxford University Press; 1996. pp. 290–317. [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and evolution. New York: Oxford University Press; 2003. [Google Scholar]