Abstract
Horizontal cell (HC) to cone feedback helps establish the center-surround arrangement of visual receptive fields. It has been shown that HC activity influences cone synaptic output by altering the amplitude and voltage dependence of the calcium current (ICa) in cones. In this study, we obtained voltage-clamp recordings simultaneously from cones and HCs to directly control the membrane potential of HCs and thereby measure the influence of HC membrane potential changes on ICa in adjacent cones. Directly hyperpolarizing voltage clamped HCs produced a negative activation shift and increased the amplitude of ICa in cones. Both of these effects were abolished by enhancing extracellular pH buffering capacity with HEPES. In contrast, addition of the gap junction blocker, carbenoxolone, did not significantly alter the shifts or amplitude changes in cone produced by changes in HC ICa membrane potential. These results support the hypothesis that changes in the HC membrane potential alter the voltage dependence and amplitude of cone by altering extracellular pH levels at the ICa synapse.
INTRODUCTION
Center-surround antagonism of the receptive field is a fundamental feature of the visual system that improves edge detection (Hartline et al. 1956; Kuffler 1953). In the vertebrate retina, surround antagonism caused by inhibitory feedback from horizontal cells (HCs) is evident in cone photoreceptors (Baylor et al. 1971; Verweij et al. 2003). The mechanisms underlying HC to cone feedback have been controversial. Based on the presence of GABA in HCs (Lam et al. 1978) and GABA receptors in cones (Kaneko and Tachibana 1986), it was initially hypothesized that diminished GABA release accompanying light-evoked hyperpolarization of HCs produced a depolarizing postsynaptic feedback potential in cones. However, studies with GABA agonists and antagonists have failed to support this hypothesis (Tatsukawa et al. 2005; Thoreson and Burkhardt 1990; Verweij et al. 1996). Verweij et al. (1996) found that surround illumination produced a negative shift in activation of cone ICa; the resulting increase in intracellular calcium can depolarize cones by stimulating a calcium-activated chloride conductance (Thoreson and Burkhardt 1991; Verweij et al. 2003). Two principle hypotheses have emerged to explain the negative activation shift in cone ICa produced by HC hyperpolarization. 1) The ephaptic feedback hypothesis proposes that shifts in ICa activation arise from local extracellular voltage changes produced when current flows into the invaginating cone synapse during changes in HC membrane potential (Byzov and Shura-Bura 1986). It has been suggested that connexin 26 hemigap junctions at the tips of HC dendrites in the cone synapse produce the requisite current sink (Fahrenfort et al. 2004; Kamermans et al. 2001). 2) Hirasawa and Kaneko (2003) proposed that HC hyperpolarization alkalinizes the synaptic cleft, resulting in both a negative activation shift and increase in peak amplitude of ICa. Calcium imaging studies of zebrafish cone terminals also support this hypothesis (Vessey et al. 2005).
In studies on cone feedback, HC membrane potential is typically manipulated by light or the use of glutamate agonists and antagonists. However, light produces a concomitant hyperpolarization in the cones that can diminish the release of vesicular protons (DeVries 2001; Hosoi et al. 2005) and thereby alkalinize the synapse without involving HCs. Glutamatergic drugs can act directly on cones themselves (Brew and Attwell 1987; Tachibana and Kaneko 1988) and may have extracellular effects as a result of activating nearby bipolar cell dendrites that also invaginate the cone synapse. In this study, we manipulated the membrane potential of HCs directly by voltage clamp while simultaneously recording ICa in adjacent cones. We found that hyperpolarizing the HC membrane potential produced a negative activation shift and increased the amplitude of ICa recorded simultaneously in synaptically connected cones. Both of these effects were abolished by enhancing extracellular pH buffering with HEPES. In addition to blocking gap junctions, carbenoxolone can decrease cone sensitivity (Verweij et al. 2003), complicating analysis of its effects on feedback. Bypassing the need for light stimulation, we found that carbenoxolone did not significantly alter the changes in cone ICa produced by changes in HC membrane potential. These results show that HC membrane potential directly influences the voltage dependence and amplitude of ICa in adjacent cones and support the hypothesis that local pH changes are responsible for the modulation of cone ICa in center-surround antagonism.
METHODS
Experiments were performed on retinal slices from aquatic tiger salamanders (Ambystoma tigrinum) as described by Rabl et al. (2005). Animals were handled according to protocols approved by the UNMC Animal Care and Use Committee. Slices were superfused at ~1 ml/min with a solution containing (in mM) 101 NaCl, 22 NaHCO3, 2.5 KCl, 2 CaCl2, 0.5 MgCl2, 9 glucose, 0.001 strychnine, and 0.1 picrotoxin. The pH was determined to be 7.4 after bubbling with 95% O2-5% CO2. In some experiments, pH buffering capacity was increased by adding 10 mM HEPES without adjusting osmolarity. The pH of the HEPES-containing solution was adjusted to 7.4 after bubbling with 95% O2-5% CO2.
Whole cell recordings were obtained using 8− to 15-MΩ patch electrodes fabricated from borosilicate glass (1.2 mm OD, 0.95 mm ID, with internal filament; World Precision Instruments, Sarasota, FL) on a PP-830 micropipette puller (Narishige USA, East Meadow, NY). The pipette solution contained (in mM) 94 CsGluconate, 9.4 TEACl, 1.9 MgCl2, 9.4 MgATP, 0.5 GTP, 0.5 EGTA, and 32.9 HEPES (pH 7.2). Cones and HCs were voltage clamped simultaneously using a Multiclamp patch-clamp amplifier (Axon Instruments, Foster City, CA). Currents were acquired using a Digidata 1322 interface and pClamp 8.1 software (Axon Instruments).
Cones were identified by shape and HCs by their response characteristics (Thoreson et al. 1997). Cone input resistance averaged 430 ± 38 MΩ and charging curves were fit by single exponentials averaging 1.4 ± 0.1 ms (n = 25). Cone ICa was measured using a ramp voltage protocol (−90 to +60 mV, 0.5 mV/ms) applied from a steady holding potential of −70 mV. Passive cone membrane resistance measured between −80 and −60 mV was subtracted digitally, and ICa was fit with a Boltzmann function adjusted for driving force. With the adjacent HC held at −90 mV, the best fit Boltzmann function parameters for cone ICa averaged V50 −19.9 ± 1.1 mV; slope, −10.9 ± 0.7; Erev ±27 mV (n = 25).
HC input resistance averaged 353 ± 79 MΩ (n = 24). Access resistance averaged 29.0 ± 3.6 MΩ (n = 49), suggesting a steady-state voltage error in HC holding potential averaging 8%. Consistent with good space clamp of HC membrane potential, excitatory postsynaptic currents (EPSCs) evoked in HCs by voltage ramps applied to presynaptic cones reversed around 0 mV as expected for a glutamate-gated cation channel (n = 12).
The criterion for statistical significance was chosen to be P < 0.05 and evaluated with Student’s t-test using GraphPad Prism 4.0. Variability is reported as ± SE.
RESULTS
Changing the membrane potential of voltage-clamped HCs altered the amplitude and voltage dependence of ICa recorded simultaneously in adjacent cones. We recorded from 35 cone/HC pairs of sufficient stability and quality to measure cone ICa at HC holding potentials of −90, −70, −40, and 0 mV. In 25 of these pairs, progressive depolarization of the HC produced a decrease in amplitude and positive shift in the activation voltage of cone ICa (Fig. 1A). Synaptic connectivity between the cone and HC was established by the presence of EPSCs in the HC evoked by depolarizing steps in the cone (−70 to −10 mV). Almost all cell pairs that were synaptically connected (23/25) exhibited shifts in ICa in response to HC polarization. In contrast, 8/10 cell pairs with no EPSC showed no shift in ICa activation in response to changes in HC holding potential. There is thus a strong correlation between the presence of horizontal cell to cone feedback and feedforward synaptic contacts between cones and horizontal cells.
FIG. 1.
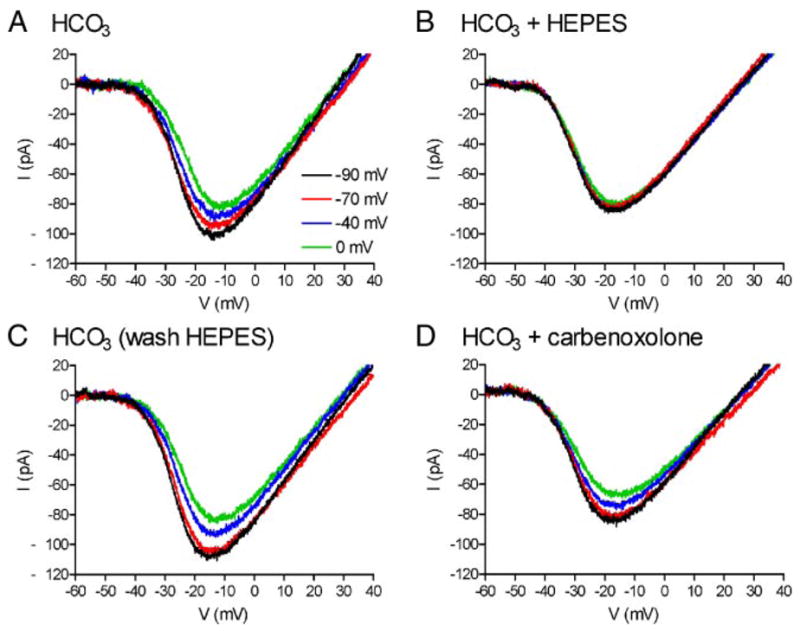
Changes in horizontal cell (HC) membrane potential alter the amplitude and voltage dependence of ICa in a simultaneously recorded cone; these effects are blocked by application of HEPES but not carbenoxolone. A: cone ICa is progressively diminished and shifted to more positive potentials by voltage clamping the HC at −90, −70, −40, and 0 mV. B: addition of HEPES (10 mM) abolished the voltage shift and amplitude changes produced by HC polarization. C: after removal of HEPES, cone ICa was once again progressively diminished and shifted to more positive potentials by voltage clamping the HC at −90, −70, −40, and 0 mV. D: voltage shift and amplitude changes produced by HC polarization persisted in the presence of carbenoxolone (0.2 mM). pH of all solutions was 7.4.
Depolarizing the HC from −90 to −70 mV shifted the ICa midpoint (V50) significantly more positive by +0.67 ± 0.11 mV (P < 0.0001, n = 25). Depolarizing the HC to −40 and then 0 mV shifted V50 further positive by +1.84 ± 0.20 and +2.43 ± 0.25 mV (P < 0.0001), respectively. Peak amplitude of ICa averaged −85.1 ± 10.2 pA (n = 25) when the HC holding potential was −90 mV. Depolarizing HCs to −70 mV significantly diminished the peak amplitude of cone ICa by 7.3 ± 1.7% (P < 0.0002). Depolarizing HCs to −40 and 0 mV further reduced ICa by 15.7 ± 2.6 and 24.5 ± 2.8% (P < 0.0001), respectively.
To test a role for synaptic cleft pH in the feedback regulation of ICa by HCs, we increased pH-buffering by adding HEPES (10 mM) to the HCO3-containing superfusate. Application of HEPES for 2–5 min blocked the changes in both amplitude and voltage dependence produced by HC polarization (Fig. 1B). These changes recovered after washout of HEPES (Fig. 1C). We tested whether the addition of HEPES may have distorted measurements of ICa made with the ramp (e.g., by reducing the possible impact of protons released from cones themselves; DeVries 2001; Hosoi et al. 2005). To do so, we compared ICa measured using ramps with ICa measured at the end of 100-ms steps after the impact of any protons released from the cone had subsided (DeVries 2001; Hosoi et al. 2005). There were no significant differences between the voltage dependence and peak amplitude of ICa measured with steps or ramps in bicarbonate medium (n = 5, data not shown) or after adding HEPES (n = 4). HEPES also did not significantly alter HC or cone Rin (HCs: ΔRin 61 ± 209 MΩ, P = 0.70, paired t-test, n = 18; cones: ΔRin 57 ± 80 MΩ, P = 0.07, paired t-test, n = 16).
Figure 2, A and B, shows the average changes in V50 and amplitude produced by HEPES application in 14 experiments compared with control measurements in the same cells. The shift in V50 is plotted relative to the V50 value obtained when the HC was held at −90 mV, and amplitude changes are plotted as a fraction of the peak amplitude measured when the HC was held at −90 mV. The shift in V50 and the amplitude changes in ICa produced by HC polarization were both significantly reduced by HEPES. In addition to blocking effects of HC polarization, HEPES produced a significant negative shift in V50 that averaged −4.0 + 1.0 mV (paired t-test, P = 0.0018, n = 14) and an insignificant increase in ICa amplitude that averaged 19 + 21% (paired t-test, P = 0.17) when the HC was held at −40 mV. The negative shift observed after adding HEPES is consistent with alkalinizing the synaptic cleft by 0.4 pH units (Barnes et al. 1993).
FIG. 2.
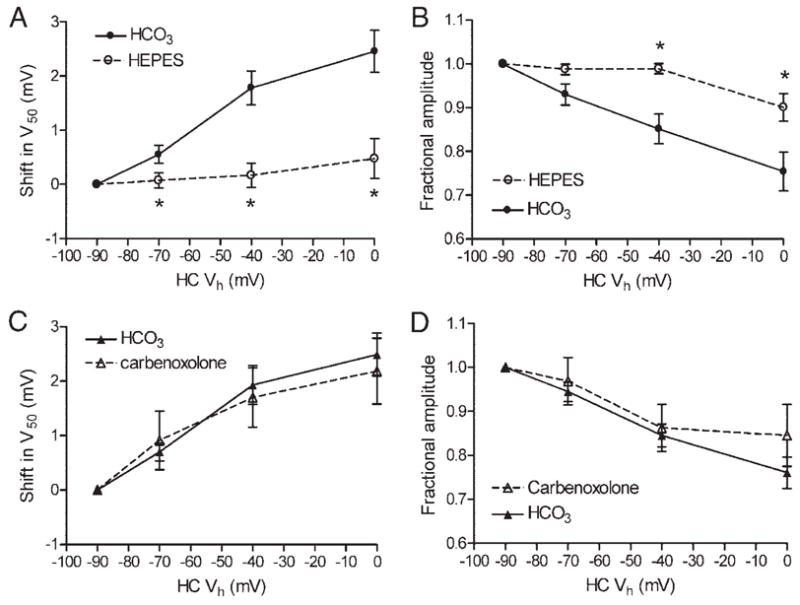
Changes in the activation midpoint (A and C) and peak amplitude (B and D) of cone ICa produced by progressive depolarization in simultaneously recorded HCs. Shift in (A and C) is V50 plotted relative to that obtained when the HC was held at −90 mV. Change in amplitude (B and D) is plotted as a fraction of amplitude measured when the HC was held at −90 mV. Application of HEPES (10 mM) significantly reduced changes in both V50 (A) and amplitude (B, ○) relative to paired control measurements (n = 14; 2– 4 measurements/cell) made before HEPES application in the same cones (●). Carbenoxolone (0.2 mM; ▵) did not significantly alter the shift (C) or amplitude (D) changes produced by HC polarization relative to paired controls (n = 7, ▴). *P < 0.05, paired t-test. pH of all solutions was 7.4.
To test for a contribution from hemigap junctions on HC to cone feedback, we applied the gap junction blocker, carbenoxolone. Consistent with a block of gap junctions in HCs, addition of carbenoxolone (0.2 mM) for 2–5 min significantly increased HC Rin by 86 ± 37 MΩ (P = 0.038, paired t-test, =14). Carbenoxolone did not significantly increase cone Rin (ΔRin 33 ± 76 MΩ, P = 0.34, paired t-test, n = 11). Application of carbenoxolone also did not significantly affect the changes in amplitude and voltage dependence of cone ICa produced by HC polarization (Figs. 1D and 2, C and D). With the HC held at −40 mV, carbenoxolone produced a shift in ICa midpoint that averaged −2.9 ± 1.0 mV (paired t-test, P = 0.02, n = 7) and a 13 ± 18% reduction in ICa amplitude that was not significant (P = 0.47, paired t-test).
DISCUSSION
Consistent with studies using light or glutamatergic drugs to manipulate HC membrane potential (Hirasawa and Kaneko 2003; Vessey et al. 2005; Verweij et al. 1996), this study shows that directly manipulating the membrane potential of a voltage-clamped HC can significantly influence the voltage dependence and amplitude of ICa in synaptically connected cones. Hyper-polarizing a single HC from −40 to −70 mV, similar to the membrane hyperpolarization evoked by a bright light flash, produced a −1.2-mV negative shift in the V50 value of ICa and increased the peak amplitude by 8%. These changes are smaller than those produced by diffuse illumination in newt cones (−2.55 mV and ~12%; Hirasawa and Kaneko 2003), presumably because unlike voltage-clamp experiments, light hyperpolarizes the entire syncytium of postsynaptic horizontal cells.
Carbenoxolone had no significant effect on the changes in voltage dependence and amplitude of ICa produced by HC polarization. Application of carbenoxolone for 2–5 min inhibits gap junctional communication between adjacent rods (Cadetti et al. 2005) and increased HC Rin, suggesting that it is likely to inhibit hemigap junctions at HC dendrites. We limited application time to minimize the direct inhibition of ICa that can be produced by carbenoxolone (Vessey et al. 2004), but it is conceivable that longer application of carbenoxolone might have had a stronger influence on the effects of HC polarization on cone ICa. With this caveat, these results do not support the hypothesis that HC to cone feedback derives from an ephaptic feedback mechanism involving hemigap junctions in HC dendrites (Fahrenfort et al. 2004; Kamermans et al. 2001).
Application of HEPES almost completely abolished the activation shifts and amplitude changes produced by membrane potential changes in voltage-clamped HCs. The efficacy of HEPES is consistent with previous studies (Hirasawa and Kaneko 2003; Vessey et al. 2005) suggesting that protons are responsible for the changes in ICa produced by HC to cone feedback. Extracellular alkalinization causes a negative activation shift and increases the amplitude of ICa (Barnes et al. 1993). The changes in ICa produced by HC hyperpolarization support the hypothesis that HC hyperpolarization alkalinizes the extracellular space in the synaptic cleft. The mechanism by which HC hyperpolarization produces such a pH change is unclear, although there is evidence for involvement of an amiloride-sensitive proton conductance in HCs (Vessey et al. 2005).
Acknowledgments
This study was supported by Fight for Sight, National Eye Institute Grant EY-10542 and Research to Prevent Blindness.
References
- Barnes S, Merchant V, Mahmud F. Modulation of transmission gain by protons at the photoreceptor output synapse. Proc Natl Acad Sci USA. 1993;90:10081–10085. doi: 10.1073/pnas.90.21.10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Fuortes MG, O’Bryan PM. Receptive fields of cones in the retina of the turtle. J Physiol. 1971;214:265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew H, Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature. 1987;327:707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Byzov AL, Shura-Bura TM. Electrical feedback mechanism in the processing of signals in the outer plexiform layer of the retina. Vision Res. 1986;26:33– 44. doi: 10.1016/0042-6989(86)90069-6. [DOI] [PubMed] [Google Scholar]
- Cadetti L, Tranchina D, Thoreson WB. A comparison of release kinetics and glutamate receptor properties in shaping rod-cone differences in EPSC kinetics. J Physiol. 2005;569:773–788. doi: 10.1113/jphysiol.2005.096545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron. 2001;32:1107–1117. doi: 10.1016/s0896-6273(01)00535-9. [DOI] [PubMed] [Google Scholar]
- Fahrenfort I, Sjoerdsma T, Ripps H, Kamermans M. Cobalt ions inhibit negative feedback in the outer retina by blocking hemichannels on horizontal cells. Vis Neurosci. 2004;21:501–511. doi: 10.1017/S095252380421402X. [DOI] [PubMed] [Google Scholar]
- Hartline HK, Wagner HF, Ratliff F. Inhibition in the eye of Limulus. J Gen Physiol. 1956;39:651– 673. doi: 10.1085/jgp.39.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol. 2003;122:657– 671. doi: 10.1085/jgp.200308863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi N, Arai I, Tachibana M. Group III metabotropic glutamate receptors and exocytosed protons inhibit L-type calcium currents in cones but not in rods. J Neurosci. 2005;25:4062–4072. doi: 10.1523/JNEUROSCI.2735-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- Kaneko A, Tachibana M. Effects of gamma-aminobutyric acid on isolated cone photoreceptors of the turtle retina. J Physiol. 1986;373:443– 461. doi: 10.1113/jphysiol.1986.sp016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953;16:37– 68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Lam DM, Lasater EM, Naka KI. gamma-Aminobutyric acid: a neuro-transmitter candidate for cone horizontal cells of the catfish retina. Proc Natl Acad Sci USA. 1978;75:6310–6313. doi: 10.1073/pnas.75.12.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabl K, Cadetti L, Thoreson WB. Kinetics of exocytosis is faster in cones than rods. J Neurosci. 2005;25:4633–4640. doi: 10.1523/JNEUROSCI.4298-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Kaneko A. L-glutamate-induced depolarization in solitary photoreceptors: a process that may contribute to the interaction between photoreceptors in situ. Proc Natl Acad Sci USA. 1988;85:5315–5319. doi: 10.1073/pnas.85.14.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsukawa T, Hirasawa H, Kaneko A, Kaneda M. GABA-mediated component in the feedback response of turtle retinal cones. Vis Neurosci. 2005;22:317–24. doi: 10.1017/S0952523805223076. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Burkhardt DA. Effects of synaptic blocking agents on the depolarizing responses of turtle cones evoked by surround illumination. Vis Neurosci. 1990;5:571–583. doi: 10.1017/s0952523800000730. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Burkhardt DA. Ionic influences on the prolonged depolarization of turtle cones in situ. J Neurophysiol. 1991;65:96–110. doi: 10.1152/jn.1991.65.1.96. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Nitzan R, Miller RF. Reducing extracellular Cl-suppress dihydropyridine-sensitive Ca2+ currents and synaptic transmission in amphibian photoreceptors. J Neurophysiol. 1997;77:2175–2190. doi: 10.1152/jn.1997.77.4.2175. [DOI] [PubMed] [Google Scholar]
- Verweij J, Hornstein EP, Schnapf JL. Surround antagonism in macaque cone photoreceptors. J Neurosci. 2003;23:10249–10257. doi: 10.1523/JNEUROSCI.23-32-10249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij J, Kamermans M, Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res. 1996;36:3943–5393. doi: 10.1016/s0042-6989(96)00142-3. [DOI] [PubMed] [Google Scholar]
- Vessey JP, Lalonde MR, Mizan HA, Welch NC, Kelly ME, Barnes S. Carbenoxolone inhibition of voltage-gated Ca channels and synaptic transmission in the retina. J Neurophysiol. 2004;92:1252–1256. doi: 10.1152/jn.00148.2004. [DOI] [PubMed] [Google Scholar]
- Vessey JP, Stratis AK, Daniels BA, Da Silva N, Jonz MG, Lalonde MR, Baldridge WH, Barnes S. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci. 2005;25:4108–4117. doi: 10.1523/JNEUROSCI.5253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


