Abstract
Klotho is a mammalian senescence-suppression protein that has homology with glycosidases. The extracellular domain of Klotho is secreted into urine and blood and may function as a humoral factor. Klotho-deficient mice have accelerated aging and imbalance of ion homeostasis. Klotho treatment increases cell-surface abundance of the renal epithelial Ca2+ channel TRPV5 by modifying its N-linked glycans. However, the precise sugar substrate and mechanism for regulation by Klotho is not known. Here, we report that the extracellular domain of Klotho activates plasma-membrane resident TRPV5 through removing terminal sialic acids from their glycan chains. Removal of sialic acids exposes underlying disaccharide galactose-N-acetylglucosamine, a ligand for a ubiquitous galactoside-binding lectin galectin-1. Binding to galectin-1 lattice at the extracellular surface leads to accumulation of functional TRPV5 on the plasma membrane. Knockdown of β-galactoside α2,6-sialyltransferase (ST6Gal-1) by RNA interference, but not other sialyltransferases, in a human cell line prevents the regulation by Klotho. Moreover, the regulation by Klotho is absent in a hamster cell line that lacks endogenous ST6Gal-1, but is restored by forced expression of recombinant ST6Gal-1. Thus, Klotho participates in specific removal of α2,6-linked sialic acids and regulates cell surface retention of TRPV5 through this activity. This action of Klotho represents a novel mechanism for regulation of the activity of cell-surface glycoproteins and likely contributes to maintenance of calcium balance by Klotho.
The Klotho gene was identified from a mouse strain in which mutation of the gene causes multiple phenotypes closely resembling human aging, including shortened life span, infertility, muscle and skin atrophy, pulmonary emphysema, osteopenia, hyperphosphatemia, and vascular and soft tissue calcification (1). The encoded protein, Klotho, is a single-pass transmembrane protein with a large extracellular domain (952 aa in human), a membrane-spanning segment, and a short (11 aa) intracellular carboxyl terminus (1). Overexpression of Klotho extends life span in mice, supporting that Klotho is an aging-suppression molecule (2). The role of Klotho in human aging is suggested by reports that polymorphisms of KLOTHO are correlated with life span, osteoporosis, and coronary artery disease in humans (3–5).
The biological function of Klotho and how deficiency of Klotho contributes to aging-associated phenotypes remains elusive. Klotho is predominantly expressed in distal renal tubules, parathyroid glands, and epithelial cells of the choroids plexus (1, 2, 6). The extracellular domain of Klotho is shed extracellularly (in urine, blood, and cerebrospinal fluid) and elicits biological effects on target cells (2, 7, 8), suggesting that it functions as a circulating hormone. With respect to the mechanism of hyperphosphatemia, recent studies report that both full-length Klotho and Klotho ectodomain bind to multiple FGF receptors and increase their affinity for FGF23 (9, 10). FGF23 is a circulating hormone that decreases serum phosphate levels by suppressing renal phosphate reabsorption. These findings underscore that Klotho−/− mice and Fgf 23−/− mice share many phenotypes, including hyperphosphatemia (11). To support an important role of Klotho in the regulation of mineral metabolism in humans, Ichikawa et al. (12) recently reported that a mutation in KLOTHO causes severe tumoral calcinosis in a young girl.
The extracellular domain of Klotho is composed of two internal repeats, KL1 and KL2, each sharing amino acid sequence homology to family 1 glycosidases (1, 13). Recently, Chang et al. (14) reported that Klotho treatment increases cell-surface abundance of the renal epithelial Ca2+ channel TRPV5 by modifying its N-linked glycans. However, the precise sugar substrate for Klotho and how modification of glycans by Klotho increases surface expression of TRPV5 proteins are not known. Here, we report that Klotho ectodomain increases surface abundance of TRPV5 through mediating and removing terminal sialic acids from their glycan chains. Removal of sialic acids exposes underlying disaccharide N-acetyllactosamine (LacNAc), a ligand for galectin-1. Binding to galectin-1 at the extracellular surface leads to accumulation of functional TRPV5 on the plasma membrane. This action of Klotho represents a novel mechanism for regulation of the activity of cell-surface glycoproteins and likely contributes to the maintenance of calcium homeostasis by Klotho.
Results
The Extracellular Domain of Klotho Participates in Removal of Sialic Acids from N-Glycans of TRPV5, Causing Its Retention at the Cell Surface.
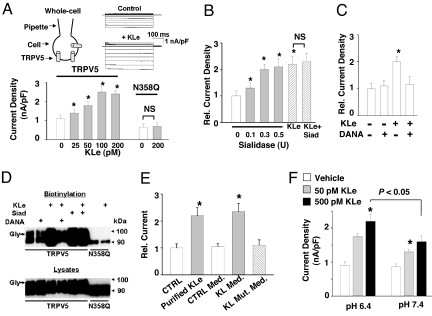
Sialic acid (N-acetylneuraminic acid) residues often cap the terminal galactose residues of glycoproteins (15). We tested the hypothesis that Klotho increases surface abundance of TRPV5 by hydrolyzing terminal sialic acid residues of the glycan chains. The abundance of functional TRPV5 channels at the cell surface was examined by measuring whole-cell current density in transfected HEK cells (16) (Fig. 1A). The extracellular domain of Klotho (KLe) likely functions as a humoral factor (2, 7, 8). Incubation of TRPV5-transfected cells with purified recombinant murine KLe for ≈16–24 h increased TRPV5 current density dose-dependently (Fig. 1A). The median effective concentration (EC50) for KLe is ≈50 pM. Mutation of a single N-linked glycosylation site, Asn-358, to glutamine (N358Q) prevented the regulation of TRPV5 by Klotho (Fig. 1A), supporting the notion that Klotho modifies an N-glycan of TRPV5 (14). A sialidase from Clostridium perfringens had similar effects on TRPV5 (Fig. 1B). The effect of bacterial sialidase was not additive to that of Klotho. A specific inhibitor of sialidase, 2-deoxy-2,3-dehyro-N-acetylneuraminic acid (DANA) (17), prevented the effect of Klotho (Fig. 1C) and bacterial sialidase (data not shown).
Fig. 1.
Klotho and bacterial sialidase increase surface abundance of TRPV5. (A) (Upper) Klotho increases whole-cell current density of WT TRPV5 but not of N358Q mutant. HEK cells were transiently transfected with GFP-tagged WT or mutant TRPV5. Twenty-four hours after transfection, cells were incubated with purified KLe for 16–24 h before whole-cell recordings. Whole-cell current density (currents normalized to the cell surface area) was measured by voltage steps from −150 to +100 mV. Voltage-current (I–V) relation curves show characteristic inwardly rectifying currents in TRPV5-transfected cells treated with KLe (+KLe; 100 pM) or without KLe (control). No TRPV5 currents were detected in mock-transfected cells (16). (Lower) Current density (nA/pF; at −100 mV) is shown. *, P < 0.05 vs. vehicle (no KLe; open bar). NS, not significant. (B) Effect of bacterial sialidase on TRPV5. The effect of sialidase (Siad) is not additive to that of KLe (200 pM). Current density (nA/pF; at −100 mV) relative to vehicle (no KLe or Siad; open bar, designated 1) is shown. *, P < 0.05 vs. vehicle. NS, not significant. (C) Effect of DANA on Klotho-induced increase of TRPV5. DANA (0.1 μM) was added to cells simultaneously with KLe (200 pM). *, P < 0.05 vs. vehicle (no KLe or DANA). (D) Effects of KLe and bacterial sialidase on surface abundance of TRPV5. Gly indicates glycosylated TRPV5. (E) Mutations of the putative active site abolished the effect of Klotho on TRPV5. Cells were incubated with purified Klotho (200 pM) or conditioned media (Med) harvested from control HEK cells or cells expressing the extracellular domain of WT Klotho or mutant Klotho carrying NEE → AAA triple mutations (200 pM each). *, P < 0.05 vs. control (CTRL). (F) Effect of medium pH on regulation of TRPV5 by Klotho. HEK cells expressing GFP-TRPV5 were incubated with vehicle or KLe (at indicated concentrations) in a physiological salt solution at pH 6.4 or 7.4 for 15 min. See Fig. 2C for effectiveness of short incubation with Klotho. After extensive washing, cells were further incubated in the cell culture medium (at pH 7.4) for 16 h before recording. The short incubation in acidic pH avoids untoward effects on cells. *, P < 0.05 vs. vehicle (−KLe; open bar).
The effects of Klotho and bacterial sialidase on surface abundance of TRPV5 were confirmed by biotinylation assays. Cell-surface biotinylated TRPV5 proteins consist of glycosylated (indicated by “Gly”) and underglycosylated forms (14) (Fig. 1D), likely because TRPV5 exists as a tetramer in the plasma membrane (18) and glycosylation of subunits is heterogeneous. Consistent with the results of whole-cell current density, Klotho and the bacterial sialidase each increased the surface abundance of WT TRPV5 but not of N358Q mutant (Fig. 1D). DANA prevented this effect. No detectable shift in migration for KLe- or Siad-treated TRPV5 was observed (in low-exposure films; data not shown), which is consistent with the idea that only limited number of sugar residues (such as terminal sialic acids) are removed by treatment with KLe or sialidase. Together, these results are consistent with the hypothesis that Klotho controls surface expression of TRPV5 via removal of sialic acids from glycan chains. Mutations including Asn-241, Glu-416, and Glu-691 in the putative active sites of mouse Klotho (13, 19) abolished the activity that increases TRPV5 currents (Fig. 1E). Thus, one or more of these residues are essential for regulating the channels. We further examined the effect of Klotho in the physiological urinary pH (≈6.4) and found that the regulation of TRPV5 by Klotho was enhanced in extracellular pH 6.4 relative to that in pH 7.4 (Fig. 1F). The critical role of Klotho in regulating TRPV5 was further confirmed by using neutralizing antibodies against Klotho (20) [see supporting information (SI) Text and Fig. S1].
Klotho Increases Surface Abundance of TRPV5 by Decreasing Its Endocytosis.
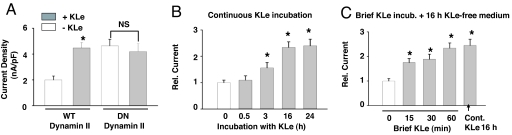
Next, we investigated the mechanism for increase of the surface abundance of TRPV5 by Klotho. We recently found that TRPV5 undergoes constitutive dynamin-dependent endocytosis (unpublished results). Interference of endocytosis by a dominant-negative dynamin (K44A rat dynamin II) increased baseline TRPV5 current and prevented an increase of current by Klotho (Fig. 2A). These results suggest that Klotho decreases dynamin-dependent retrieval of TRPV5 channels, allowing them to accumulate at the cell surface. Consistent with its effect on surface abundance of the channel, we found that Klotho did not affect single-channel open probability or single-channel conductance of TRPV5 (data not shown). Moreover, Klotho did not increase TRPV5 currents within 30 min (Fig. 2B). The increase in TRPV5 currents, however, could be detected after ≥3 h of incubation with Klotho (Fig. 2B). To exclude that the delayed increase in currents is caused by a slow activity of Klotho, TRPV5-transfected cells were incubated with Klotho for 15–60 min, followed by extensive washing to remove Klotho in the medium and further incubation in a Klotho-free medium for 16–24 h. We found that incubation with Klotho for 15 min was sufficient to cause an increase of TRPV5 current measured at ≥16 h later (Fig. 2C). These results are consistent with the idea that Klotho increases surface abundance of TRPV5 by decreasing endocytosis of the channels. The delay in increase of TRPV5 after treatment by Klotho may be related to the rate of endocytosis of TRPV5 and/or the interaction between TRPV5 and galectin-1 (see below).
Fig. 2.
Klotho decreases dynamin-dependent internalization of TRPV5. (A) Effect of dominant-negative (DN) dynamin on TRPV5 currents with or without treatment by Klotho (KLe). WT dynamin was used as control. *, P < 0.05 vs. vehicle (−KLe; open bar). NS, not significant. (B) Time course of increase in TRPV5 currents with continuous Klotho incubation. TRPV5-transfected cells were incubated with KLe (200 pM) for 0.5, 3, 16, and 24 h. Currents were recorded at the end of each period of incubation (i.e., at 0.5, 3, 16, or 24 h, respectively). (C) Time course of increase in TRPV5 currents with brief incubation of Klotho. TRPV5-transfected cells were first incubated with KLe (200 pM) for 15, 30, or 60 min, washed extensively to remove KLe, and further incubated in a KLe-free medium for 16–24 h. Currents were recorded at the end of 16–24 h of incubation in the KLe-free medium. For comparison, some cells were incubated with KLe continuously for 16 h and recorded at end of 16 h of incubation (Cont. KLe 16 h).
Removal of Terminal Sialic Acids from N-Glycans of TRPV5 Exposes Underlying LacNAc for Binding to Galectin-1.
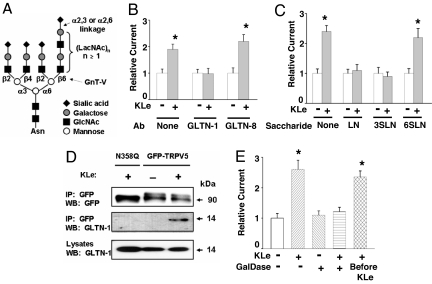
Removal of sialic acids from typical complex-type asparagine-linked glycans exposes terminal LacNAc (15) (Fig. 3A), which is a ligand for galectins. Galectins are a family of galactoside-binding animal lectins (21). Many galectins are secreted and interact with proteins at the extracellular surface. We examined the role of galectin binding in mediating the effect of Klotho on ion channels. HEK cells express multiple galectins, including galectin-1, galectin-8, and galectin-9 (Fig. S2A). Galectin-1 is ubiquitously expressed (21). Knockdown of galectin-1, but not of galectin-8 and galectin-9, prevented the increase of TRPV5 by Klotho (Fig. S2B). Extracellular application of an antibody against galectin-1, but not galectin-8, after treatment with Klotho prevented the increase of TRPV5 (Fig. 3B).
Fig. 3.
Galectin-1 is critical for the increase of surface abundance of channels by Klotho. (A) Typical complex-type tetra-antennary N-glycan present in mammalian cell surface glycoproteins (27, 37–40). The Golgi enzyme GnT-V catalyzes the addition of GlcNAc to the α6-mannose, initiating the GlcNAcβ(1,6) branch. The polymeric form of LacNAcn (n > 1) is frequently present in the GlcNAcβ(1,6) branch. See Fig. 4E legend for further details. (B) Antibody against galectin-1 (GLTN-1), but not against galectin-8 (GLTN-8), prevents the increase of TRPV5 current by Klotho. Transfected cells were incubated with KLe for 30 min, washed off KLe, and incubated with the indicated antibody (15 nM) in a KLe-free medium for 16–24 h before recording. (C) LacNAc (LN), α2,3-sialylated LacNAc (3SLN), but not α2,6-sialylated LacNAc (6SLN), prevents the increase of TRPV5 current by Klotho. Cells were incubated with KLe for 30 min, washed off KLe, and incubated with the indicated compounds (10 mM) (37, 38) in a KLe-free medium for 16–24 h before recording. (D) Galectin-1 (GLTN-1) coimmunoprecipitates with TRPV5 after treatment by Klotho. Cells were transfected with WT GFP-TRPV5 or N358Q mutant, incubated with or without Klotho, and cross-linked by DTSSP before lysis and immunoprecipitated by anti-GFP antibodies. Cross-linked proteins were reduced before separation by gel electrophoresis. (E) Terminal galactose residues are required for the increase of TRPV5 current by Klotho. Cells were incubated with KLe and/or galactosidase (GalDase; 0.5 units/ml) for 1 h, washed off, and further incubated in a KLe-free medium for 16–24 h before recording. In the experiment labeled Before KL, cells were treated with GalDase for 1 h, washed off with GalDase, incubated with KLe for 1 h, washed off withKLe, and incubated in a KLe-free medium for 16–24 h before recording. *, P < 0.05 vs. vehicle control (open bars).
Galectin-1 binds LacNAc and α2,3-sialylated LacNAc, but not α2,6-sialylated LacNAc (22). Extracellular application of LacNAc and α2,3-sialylated LacNAc, but not α2,6-sialylated LacNAc, blocked the effects of Klotho (Fig. 3C). Coimmunoprecipitation experiments show that galectin-1 interacted with TRPV5 only after treatment with Klotho (Fig. 3D). As controls, galectin-1 did not interact with a glycosylation-defective TRPV5 mutant, N358Q. Thus, binding with galectin-1 is N-glycan-mediated and is essential for the Klotho-induced cell-surface retention of ion channels. The increase in TRPV5 currents was prevented by removing terminal galactose by application of β-galactosidase (an exo-galactosidase) simultaneously with Klotho, but not before Klotho (Fig. 3E). These results show that Klotho-mediated removal of terminal sialic acids and exposure of underlying galactose is essential for galectin-1 binding to TRPV5.
α2,6-Linked Sialic Acid Is the Target for Klotho.
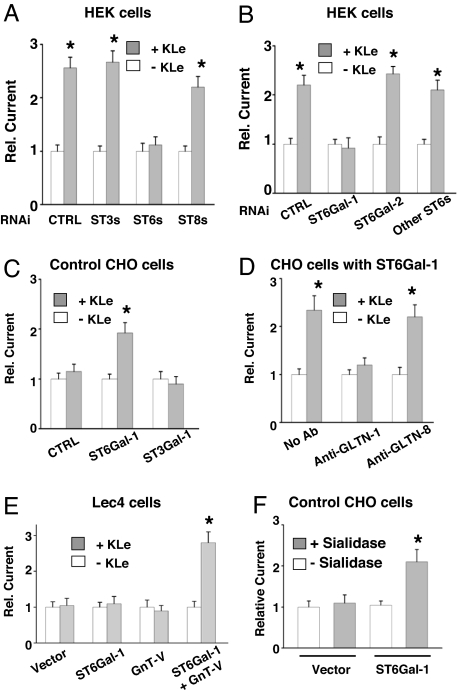
Sialyltransferases catalyze the transfer of sialic acid residues from the activated donor cytidine monophosphate-sialic acid to acceptor glycoproteins or glycolipids via either α2,3, α2,6 or α2,8 glycosidic linkage (named ST3, ST6, and ST8, respectively) (23, 24). To identify which sialic acid linkage is the target for Klotho, we knocked down expression of individual sialyltransferase by RNA interference. Because interference of N-glycan modification may affect forward trafficking of cell membrane proteins (25), we analyzed the relative increase of currents by Klotho in each RNAi experiment. Knockdown of all five ST6s by pooled RNAi, but not of all six ST3s or all five ST8s, prevented the increase of TRPV5 by Klotho (Fig. 4A). There are five members of human ST6s, each with unique oligosaccharide substrate specificity (23, 24). Among these ST6s, ST6Gal-1 is ubiquitously expressed and catalyzes the transfer of sialic acids via α2–6-linkage to galactose residues of galactoseβ1–4N-acetylglucosamine (24). Knockdown of ST6Gal-1, but not the other ST6s, prevented the effect of Klotho on TRPV5 (Fig. 4B), indicating that ST6Gal-1 is responsible for the synthesis of sialic acid substrate for Klotho. Additionally, we confirmed that Klotho had no effects on TRPV5 in CHO cells that contain endogenous ST3s but not ST6s (26) (Fig. 4C). Forced expression of recombinant ST6Gal-1, but not of ST3Gal-1, conferred the regulation by Klotho in CHO cells (Fig. 4C). The effect of Klotho on TRPV5 in ST6Gal-1-expressing CHO cells was also prevented by extracellular application of anti-galectin-1 antibodies (Fig. 4D). Thus, Klotho regulates TRPV5 channels only when they are sialylated via α2,6-linkage.
Fig. 4.
Sialyltransferase ST6Gal-1 is responsible for the synthesis of sialic acid substrate for Klotho. (A) Knockdown of ST6s, but not ST3s and ST8s, in HEK cells prevents the increase of TRPV5 current by Klotho. Cells were transfected with GFP-TRPV5 mixed with control oligonucleotide or pooled RNAi oligonucleotides for ST3s, ST6s, or ST8s. (B) Knockdown of ST6Gal-1, but not ST6Gal-2 and other ST6s, in HEK cells prevents the increase of TRPV5 current by Klotho. Cells were transfected with GFP-TRPV5 mixed with control oligonucleotide or RNAi oligonucleotides for ST6Gal-1, ST6Gal-2, or pooled RNAi oligonucleotides for other ST6s. (C) Klotho increases TRPV5 currents in ST6Gal-1-transfected but not control or ST3Gal-1-transfected CHO cells. (D) Anti-galectin-1 antibody prevents the increase of TRPV5 current by Klotho in ST6Gal-1-transfected CHO cells. (E) Klotho increased TRPV5 current density in Lec4 cells expressing both ST6Gal-1 and GnT-V, but not in Lec4 cells expressing only ST6Gal-1 or only GnT-V. In A–E, *, P < 0.05 vs. vehicle control (−KLe; open bar). (F) Effect of bacterial sialidase on TRPV5 in CHO cells. Cells were cotransfected with GFP-TRPV5 plus ST6Gal-1 or an empty vector and incubated with bacterial sialidase (0.5 units/ml) for 16 h. *, P < 0.05 vs. vehicle (−sialidase; open bar).
The Golgi enzyme N-acetylglucosaminyltransferase V (GnT-V) catalyzes the addition of β1,6-N-acetylglucosamine [GlcNAcβ(1,6)] to the α6-mannose producing the tri(2,2,6) and tetra(2,4,2,6) antenarry glycans (27) (Fig. 3A). The GlcNAcβ(1,6) branch is preferred substrate for addition of poly LacNAc. The affinity of polymeric LacNAc for galectin-1 is much higher than that of LacNAc monomer (22). Lec4 is a mutant CHO cell line that lacks GnT-V thus containing no cell surface N-linked GlcNAcβ (1, 6) branches (28). We found that Klotho increased current density of TRPV5 in Lec4 cells cotransfected with ST6Gal-1 and GnT-V, but not in mock-transfected Lec4 cells, Lec4 cells transfected with ST6Gal-1 alone or with GnT-V alone (Fig. 4E). Thus, LacNAc or more likely poly LacNAc in the (2,2,6)tri- and/or (2,4,2,6)tetra-antenary N-gycans is involved in the enhancement of TRPV5 at the cell surface by Klotho.
Although the sialidase from C. perfringens hydrolyzes α2,3-linked sialic acids more efficiently, it also hydrolyzes α2,6-linked sialic acids at higher concentrations (29). We asked whether the bacterial sialidase can regulate TRPV5 in CHO cells lacking ST6Gal-1. The bacterial sialidase did not increase TRPV5 in CHO cells (Fig. 4F). Expression of ST6Gal-1 conferred stimulation by the bacterial sialidase even though it prefers the α2,3-linkage. Thus, like Klotho, the bacterial sialidase stimulated TRPV5 channels only when they contained α2,6-sialic acids. The concentration of the bacterial sialidase required for increasing TRPV5 currents, however, is 0.1 unit/ml (≈0.2 μM), which is >1,000-fold higher than that of Klotho (see Fig. 1 A and B).
Klotho Treatment Removes α2,6-Linked, But Not α2,3-Linked, Sialic Acids from TRPV5 Analyzed Using Epitope-Specific Plant Agglutinins.
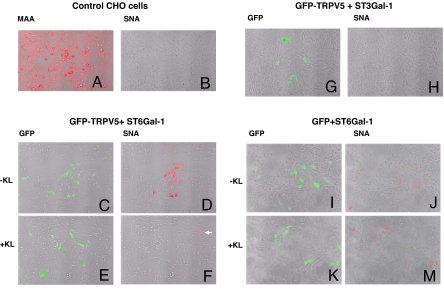
Plant lectins Sambucus nigra agglutinin (SNA) and Maackia amurensis agglutinin (MAA) specifically bind α2,6-sialylated and α2,3-sialylated glycoconjugates, respectively (30). We used SNA labeling to examine α2,6-sialic acids on TRPV5 and the effect of Klotho to remove these residues. CHO cells were transfected with GFP-TRPV5 or GFP along with ST6Gal-1 or ST3Gal-1 and labeled by fluorescent-attached SNA or MAA. Control untransfected CHO cells were labeled by fluorescent-attached MAA (Fig. 5A), but not by fluorescent-attached SNA (Fig. 5B), confirming that they express ST3s but not ST6s, endogenously. In CHO cells cotransfected with GFP-TRPV5 and ST6Gal-1 (Fig. 5 C–F), SNA labeling (Fig. 5D, red) was detected in almost every transfected cell (Fig. 5C, green) but not in untransfected cells, supporting that expression of ST6Gal-1 produces α2,6-sialic acids in CHO cells. Treatment with Klotho markedly reduced SNA labeling in cells cotransfected with GFP-TRPV5 and ST6Gal-1 (Fig. 5 F vs. D; see Fig. S3 for additional images). The specificity of SNA for α2,6-sialic acid was further confirmed by the result that transfection with ST3Gal-1 did not give rise to SNA labeling (Fig. 5 G and H). In contrast, Klotho treatment did not affect SNA labeling in cells cotransfected with GFP and ST6Gal-1 (Fig. 5 I, J, K and M and Fig. S3). Note that SNA labeling was less in cells expressing ST6Gal-1 alone (Fig. 5 J) than in cells expressing ST6Gal-1 and channels (Fig. 5D), likely because, in the absence of ST6Gal-1, its potential substrates are capped by other enzymes (31). Only transfected ion channels and a fraction of endogenous glycoproteins synthesized during the expression of ST6Gal-1 would be sialylated via α2,6-linkage. Klotho treatment did not affect MAA labeling in control CHO cells (data not shown). Thus, Klotho treatment removes α2,6-sialic acids from glycan chains of TRPV5, but not from the majority resident surface membrane proteins in CHO cells. For comparison, we found that neither Klotho nor sialidase treatment increases the current density of TRPC6, another TRP channel also present in kidney (data not shown). Our results, however, do not exclude the possibility that Klotho can remove α2,6-sialic acids from a subset of GlcNAc α(1,6) branch-containing glycoproteins besides TRPV5.
Fig. 5.
Treatment by Klotho prevents binding of SNA to TRPV5. (A and B) Control CHO cells were incubated with biotinylated MAA (A) or SNA (B), followed by Alexa 594-labeled streptavidin. (C–F) CHO cells were transfected with GFP-TRPV5 and ST6Gal-1 and treated with Klotho (500 pM) (E and F) or without Klotho (C and D) for 1 h before staining by SNA. In each experimental condition, fluorescent images were acquired by gating for GFP fluorescence (C and E) and Alexa 594 fluorescence (D and F) to detect transfected cells and labeling by SNA, respectively. White arrow in F indicates that cells with relatively less TRPV5 expression are less sensitive to Klotho, which is consistent with the notion that Klotho is specific for α2,6-sialic acids from glycan chains of TRPV5, but not from the majority resident surface membrane proteins in CHO cells (see I–M). (G and H) CHO cells were cotransfected with GFP and ST3Gal-1 and stained by SNA. (I–M) CHO cells were cotransfected with GFP and ST6Gal-1 and treated with (K and M) or without Klotho (I and J) for 1 h before staining by SNA.
Discussion
Despite the sequence homology to glycosidases, the physiological role of Klotho as a glycosidase and its endogenous substrate remain elusive. Chang et al. (14) reported that Klotho treatment increases cell-surface abundance of TRPV5 by modifying its N-linked glycans. However, the precise sugar substrates on TRPV5 and how its modification alters the cell-surface abundance are not known. Our present study shows that Klotho participates in removal of α2,6-, but not α2,3-linked sialic acids, of TRPV5 in intact cells. Removal of terminal sialic acids from N-glycans exposes underlying LacNAc for binding with galectin-1 to enhance retention of TRPV5 at the cell surface (see Fig. 6). The interaction of TRPV5 with galectin-1 is likely strengthened by the multiplicity of N-glycans from tetrameric structure of the channel and the presence of polymeric LacNAc, a high-affinity ligand for galectin-1. These results provide an example of glycosidase activity of Klotho acting through a physiologically relevant sugar substrate.
Fig. 6.
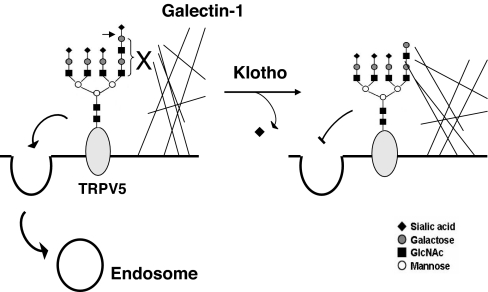
Model for increase in cell-surface abundance of TRPV5 by Klotho. Klotho removes terminal sialic acid from the GlcNAcβ(1,6) branch of (tri-antenary or) tetra-antenary N-glycan of TRPV5. Removal of sialic acids exposes underlying LacNAc, a ligand for galectin-1. Binding to galectin-1 lattice at the extracellular surface leads to accumulation of functional TRPV5 on the plasma membrane.
It has been reported by Tohyama et al. (32) that Klotho exhibits weak β-glucuronidase activity in vitro. Based on the finding that purified bovine liver β-glucuronidase also increases TRPV5 activity, Chang et al. (14) concluded that the effect of Klotho on TRPV5 is through hydrolysis of glucuronic acids from TRPV5. However, although glucuronic acids may be sugar moieties of cell-membrane proteins in the form of heparin sulfate proteoglycans (33), they are not common moieties of N-glycans of mammalian cell-surface glycoproteins (27). To understand the study of Chang et al., we confirmed their finding that purified bovine β-glucuronidase increases surface abundance of TRPV5 in HEK cells (data not shown). However, the EC50 for β-glucuronidase regulation of TRPV5 in HEK cells reported by Chang et al. (14) (and in our experiments as well) is ≈0.1 μM, which is ≈2,000-fold higher than that for Klotho (50 pM) observed in this study. The concentration of Klotho in human urine (from where Klotho is expected to regulate TRPV5 localized in the apical membrane of distal renal tubules) (34) is estimated ≈20–200 pM (unpublished results). We found that β-glucuronidase (0.1–1 μM), like Klotho and bacterial sialidase, increased TRPV5 currents only in CHO cells that expressed recombinant ST6Gal-1, but not in control CHO cells (Fig. S4). Moreover, we found that knockdown of endogenous ST6Gal-1 by siRNA prevented the increase of TRPV5 currents by β-glucuronidase in HEK cells (data not shown). Thus, pharmacological concentrations of β-glucuronidase target α2,6-linked sialic acids of TRPV5. Indeed, Tohyama et al. (32) have shown that high concentrations of β-glucuronidase have off-target enzymatic activities toward other sugar substrates besides glucuronic acids.
Renal Ca2+ reabsorption is decreased in Klotho-deficient mice when compared with WT counterparts (35), primarily because of decreased Ca2+ entry across the apical membrane of connecting tubules (35). TRPV5 is the main channel responsible for apical Ca2+ entry in the distal nephron (34). Thus, the effect to increase cell-surface retention of TRPV5 is important for the regulation of renal Ca2+ transport by Klotho. Klotho also regulates calcium homeostasis through other mechanisms. Klotho inhibits the expression of 25-hydroxyvitamin D 1α-hydroxylase (36), the key enzyme for synthesis of 1,25-dihydroxyvitamin D. Accordingly, Klotho-deficient mice have higher circulating levels of 1,25-dihydroxyvitamin D and hypercalcemia from increased gastrointestinal absorption of calcium (1, 36). Furthermore, Klotho promotes the release of parathyroid hormone from parathyroid glands in response to decreases in the extracellular Ca2+ levels (6). Overall, the effects of Klotho to increase cell-surface retention of TRPV5 and release of parathyroid hormone will enhance renal Ca2+ reabsorption and counteract the effect of decrease in the synthesis of 1,25-dihydroxyvitamin D on calcium metabolism.
The regulation of TRPV5 by Klotho represents a novel mechanism for regulation of the activity of cell-surface glycoproteins. Increasing evidence indicates that N-glycan-mediated binding with galectins is important for regulating the residence time of cell surface glycoproteins (37–40). Up- and down-regulation of N-acetylglucosaminyltransferases (GnT-IV or GnT-V; also known as Mgat4 and Mgat5, respectively) in the Golgi increases and decreases LacNAc content of cytokine receptors and Glut-2 transporters, respectively (37, 38). These effects underscore the enhanced responses of tumor cells to growth-promoting factors and the reduced surface abundance of Glut-2 in pancreatic β-cells caused by high-fat diets, respectively (37, 38). Additionally, Lau et al. (39) recently reports that an increase in intracellular concentration of N-acetylglucosamine stimulates N-glycan processing in the Golgi and enhances association of receptors with galectins. In contrast to the regulation of synthesis and branching of N-glycans occurring at the Golgi, Klotho functions from the outside of cell as a humoral factor and by modifying N-glycans of mature cell surface glycoproteins at the plasma membrane. Whether Klotho regulates other potential targets related to aging phenotypes via the same mechanism remains to be determined.
Materials and Methods
GFP-tagged TRPV5 have been described (16). cDNAs for human ST6Gal-1 and ST3Gal-1 were cloned into pEF1 expression vector. Sequences for RNAi oligonucleotides are in Table S1. Preparation of purified KLe has been described (2). Cells were transiently transfected with cDNAs encoding GFP-TRPV5 with or without additional constructs. Whole-cell currents were recorded by using an Axopatch 200B amplifier as described (16). For immunoprecipitation of GFP-TRPV5 with galectins-1, cell surface proteins were cross-linked by using a membrane-impermeable dithio-bis-suffosuccinimydyl propionate (DTSSP) and lysed, and supernatants were immunoprecipitated by a monoclonal anti-GFP antibody. Immunoprecipitates were reduced by DTT, separated by SDS/PAGE, and analyzed for galectin-1 by using a polyclonal anti-galectin-1 antibody. For staining by SNA and MAA, fixed CHO cells were incubated with biotinylated SNA or MAA followed by Alexa 594-conjugated streptavidin. Fluorescent images were obtained with a Nikon Eclipse TE2000-U fluorescent microscope and overlaid with differential interference contrast images as described (41). In each experiment, gain for fluorescence detection was adjusted equally by using untransfected cells as a control.
Supplementary Material
Acknowledgments.
We thank Drs. Pamela Stanley (Albert Einstein College of Medicine, New York, NY) and Mike Pierce (University of Georgia, Athens, GA) for Lec4 cells and cDNA for GnT-V, Drs. James Paulson and Ola Blixt (The Scripps Research Institute, La Jolla, CA), and the Consortium for Functional Glycomics (National Institutes of Health Grant GM62116) for glycan compounds, and Drs. Mark Lehrman, Carolyn Bertozzi, Michel Baum, and Orson Moe for discussions and comments. This work was supported by National Institutes of Health Grants DK20543 and DK59530 (to C.-L.H.) and AG19712 and AG25326 (to M.K.-o. and K.P.R.), American Heart Association Grant 0440019N (to C.-L.H.), the Eisai Research Fund (M.K.-o., the Ellison Medical Foundation (M.K.-o.), and the Ted Nash Long Life Foundation (M.K.-o.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803223105/DCSupplemental.
References
- 1.Kuro-o M, et al. Mutation of the mouse klotho gene leads to a syndrome resembling aging. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Kurosu H, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arking DE, et al. Association of human aging with a functional variant of Klotho. Proc Natl Acad Sci USA. 2002;99:856–861. doi: 10.1073/pnas.022484299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418. doi: 10.1161/01.RES.0000157171.04054.30. [DOI] [PubMed] [Google Scholar]
- 5.Ogata N, et al. Association of klotho gene polymorphism with bone density and spondylosis of the lumbar spine in postmenopausal women. Bone. 2002;31:37–41. doi: 10.1016/s8756-3282(02)00786-x. [DOI] [PubMed] [Google Scholar]
- 6.Imura A, et al. Alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 7.Imura A, et al. Secreted Klotho protein in sera and CSF: Implication for posttranslational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 8.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurosu H, et al. Regulation of fibroblast growth factor-23 signaling by Klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urakawa I, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 11.Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichikawa S, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito S, Fujimori T, Hayashizaki Y, Nabeshima Y. Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim Biophys Acta. 2002;1576:341–345. doi: 10.1016/s0167-4781(02)00281-6. [DOI] [PubMed] [Google Scholar]
- 14.Chang Q, et al. The β-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 15.Schauer R. Biosynthesis and function of N- and O-substituted sialic acids. Glycobiology. 1991;1:449–452. doi: 10.1093/glycob/1.5.449. [DOI] [PubMed] [Google Scholar]
- 16.Yeh BI, Kim YK, Jabbar W, Huang CL. Conformational changes of pore helix coupled to gating of TRPV5 by protons. EMBO J. 2005;24:3224–3234. doi: 10.1038/sj.emboj.7600795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Usuki S, Hoops P, Sweeley CC. Growth control of human foreskin fibroblasts and inhibition of extracellular sialidase activity by 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. J Biol Chem. 1988;263:10595–10599. [PubMed] [Google Scholar]
- 18.Hoenderop JG, et al. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J. 2003;22:1–10. doi: 10.1093/emboj/cdg080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rye CS, Withers SG. Glycosidase mechanisms. Curr Opin Chem Biol. 2000;4:573–580. doi: 10.1016/s1367-5931(00)00135-6. [DOI] [PubMed] [Google Scholar]
- 20.Kato Y, et al. Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun. 2000;267:597–602. doi: 10.1006/bbrc.1999.2009. [DOI] [PubMed] [Google Scholar]
- 21.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins: Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–20810. [PubMed] [Google Scholar]
- 22.Leppanen A, Stowell S, Blixt O, Cummings RD. Dimeric galectin-1 binds with high affinity to α2,3-sialylated and nonsialylated terminal N-acetyllactosamine units on surface-bound extended glycans. J Biol Chem. 2005;280:5549–5562. doi: 10.1074/jbc.M412019200. [DOI] [PubMed] [Google Scholar]
- 23.Patel RY, Balaji PV. Identification of linkage-specific sequence motifs in sialyltransferases. Glycobiology. 2006;16:108–116. doi: 10.1093/glycob/cwj046. [DOI] [PubMed] [Google Scholar]
- 24.Yasukawa Z, Sato C, Kitajima K. Inflammation-dependent changes in α2,3-, α2,6-, and α2,8-sialic acid glycotopes on serum glycoproteins in mice. Glycobiology. 2005;15:827–837. doi: 10.1093/glycob/cwi068. [DOI] [PubMed] [Google Scholar]
- 25.Vagin O, Turdikulova S, Sachs G. The H,K-ATPase β subunit as a model to study the role of N-glycosylation in membrane trafficking and apical sorting. J Biol Chem. 2004;279:39026–39034. doi: 10.1074/jbc.M405453200. [DOI] [PubMed] [Google Scholar]
- 26.Fukuta K, Yokomatsu T, Abe R, Asanagi M, Makino T. Genetic engineering of CHO cells producing human interferon-γ by transfection of sialyltransferases. Glycoconj J. 2000;17:895–904. doi: 10.1023/a:1010977431061. [DOI] [PubMed] [Google Scholar]
- 27.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 28.Chaney W, Sundaram S, Friedman N, Stanley P. The Lec4A CHO glycosylation mutant arises from miscompartmentalization of a Golgi glycosyltransferase. J Cell Biol. 1989;109:2089–2096. doi: 10.1083/jcb.109.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corfield AP, Higa H, Paulson JC, Schauer R. The specificity of viral and bacterial sialidases for α2–3- and α2–6-linked sialic acids in glycoproteins. Biochim Biophys Acta. 1983;744:121–126. doi: 10.1016/0167-4838(83)90080-8. [DOI] [PubMed] [Google Scholar]
- 30.Brinkman-Van der Linden EC, Sonnenburg JL, Varki A. Effects of sialic acid substitutions on recognition by Sambucus nigra agglutinin and Maackia amurensis hemagglutinin. Anal Biochem. 2002;303:98–104. doi: 10.1006/abio.2001.5539. [DOI] [PubMed] [Google Scholar]
- 31.Martin LT, Marth JD, Varki A, Varki NM. Genetically altered mice with different sialyltransferase deficiencies show tissue-specific alterations in sialylation and sialic acid 9-O-acetylation. J Biol Chem. 2002;277:32930–32938. doi: 10.1074/jbc.M203362200. [DOI] [PubMed] [Google Scholar]
- 32.Tohyama O, et al. Klotho is a novel β-glucuronidase capable of hydrolyzing steroid β-glucuronides. J Biol Chem. 2004;279:9777–9784. doi: 10.1074/jbc.M312392200. [DOI] [PubMed] [Google Scholar]
- 33.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1047. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 34.Hoenderop JG, Nilius B, Bindels RJ. Molecular mechanism of active Ca2+ reabsorption in the distal nephron. Annu Rev Physiol. 2002;64:529–549. doi: 10.1146/annurev.physiol.64.081501.155921. [DOI] [PubMed] [Google Scholar]
- 35.Tsuruoka S, et al. Nephrol Dial Transplant. 2006;21:2762–2767. doi: 10.1093/ndt/gfl335. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida T, Fujimori T, Nabeshima Y. Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1α-hydroxylase gene. Endocrinology. 2002;143:683–689. doi: 10.1210/endo.143.2.8657. [DOI] [PubMed] [Google Scholar]
- 37.Partridge EA, et al. Regulation of cytokine receptors by Golgi N-glycans processing and endocytosis. Science. 2004;306:120–124. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- 38.Ohtsubo K, et al. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell. 2005;123:1307–1321. doi: 10.1016/j.cell.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 39.Lau KS, et al. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 2007;129:123–134. doi: 10.1016/j.cell.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 40.Stanley P. A method to the madness of N-glycan complexity? Cell. 2007;129:27–29. doi: 10.1016/j.cell.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Zeng WZ, et al. Evidence for endocytosis of ROMK potassium channels via clathrin-coated vesicles. Am J Physiol. 2002;283:F630–F639. doi: 10.1152/ajprenal.00378.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.