Abstract
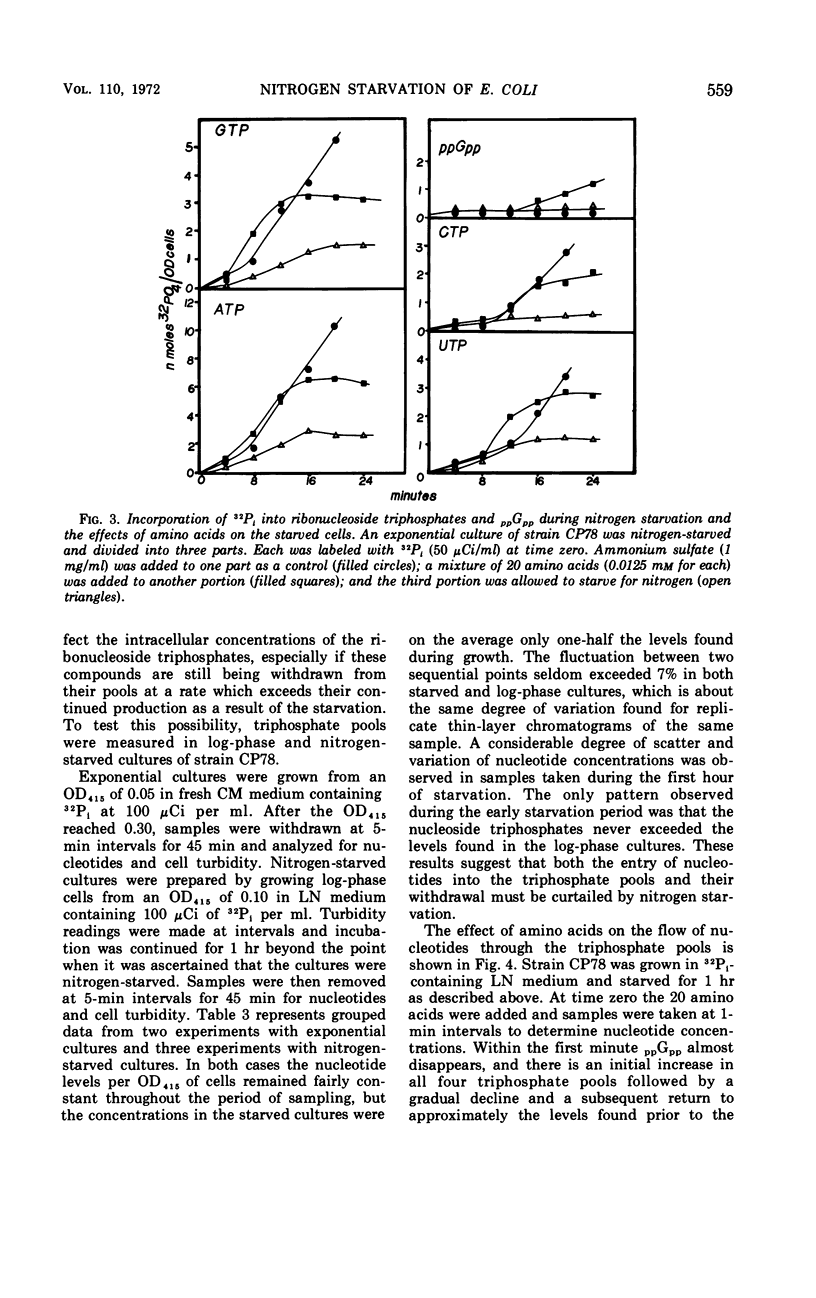
Ribosomal ribonucleic acid (RNA) synthesis and ribonucleoside triphosphate metabolism were studied in cultures of Escherichia coli subjected to starvation for inorganic nitrogen. In a strain that was under stringent control, a 50-fold reduction in the formation of both 16S and 23S RNA was accompanied by a severe restriction on nucleotide biosynthesis. These inhibitions were relieved in part by incubating the starved cells with amino acids. This result suggests that regulation by the functional RNA control (RC) gene is involved in the effect. This suggestion was confirmed by showing that the effector of the stringent response, guanosine-5′-diphosphate-2′- or 3′-diphosphate (ppGpp), accumulated at the onset of starvation and disappeared immediately when the amino acids were added. Ribosomal RNA synthesis was severely restricted and the same nucleotide, ppGpp, accumulated at the onset of nitrogen starvation of a relaxed mutant too. These findings suggest that a control mechanism other than the one provided by the functional rel gene might operate to regulate RNA synthesis and that this mechanism is expressed through the synthesis of ppGpp.
Full text
PDF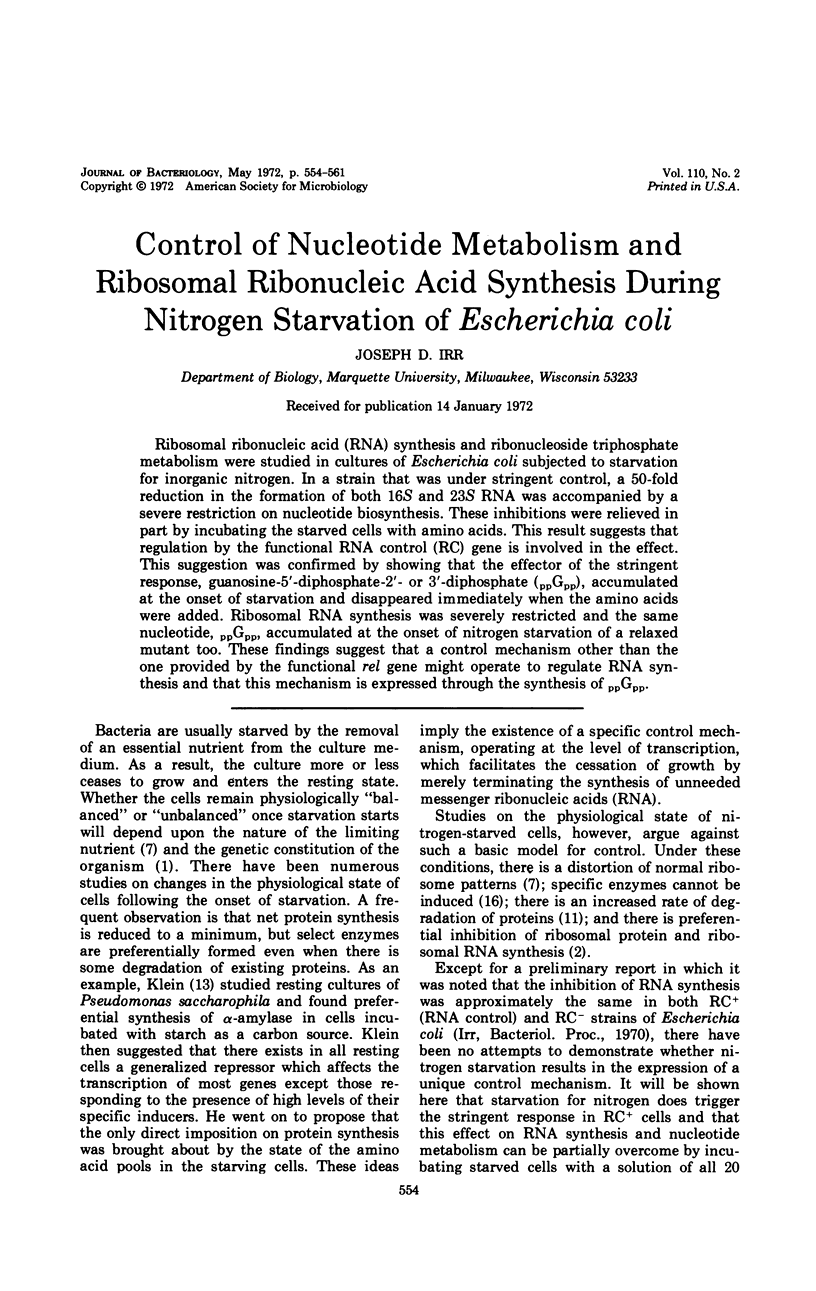





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALFOELDI L., STENT G. S., HOOGS M., HILL R. PHYSIOLOGICAL EFFECTS OF THE RNA CONTROL (RC) GENE IN E. COLI. Z Vererbungsl. 1963 Nov 21;94:285–302. doi: 10.1007/BF00894773. [DOI] [PubMed] [Google Scholar]
- Ben-Hamida F., Schlessinger D. Synthesis and breakdown of ribonucleic acid in Escherichia coli starving for nitrogen. Biochim Biophys Acta. 1966 Apr 18;119(1):183–191. doi: 10.1016/0005-2787(66)90049-9. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Control of RNA synthesis in Escherichia coli. I. Amino acid dependence of the synthesis of the substrates of RNA polymerase. J Mol Biol. 1968 Jul 14;34(2):317–330. doi: 10.1016/0022-2836(68)90256-8. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Cashel M., Kalbacher B. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem. 1970 May 10;245(9):2309–2318. [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Fiil N., Friesen J. D. Isolation of "relaxed" mutants of Escherichia coli. J Bacteriol. 1968 Feb;95(2):729–731. doi: 10.1128/jb.95.2.729-731.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J., Harada B. The control of ribonucleic acid synthesis in Escherichia coli. 3. The functional relationship between purine ribonucleoside triphosphate pool sizes and the rate of ribonucleic acid accumulation. J Biol Chem. 1969 Jun 25;244(12):3125–3132. [PubMed] [Google Scholar]
- Gallant J., Irr J., Cashel M. The mechanism of amino acid control of guanylate and adenylate biosynthesis. J Biol Chem. 1971 Sep 25;246(18):5812–5816. [PubMed] [Google Scholar]
- Goldberg A. L. A role of aminoacyl-tRNA in the regulation of protein breakdown in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Feb;68(2):362–366. doi: 10.1073/pnas.68.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irr J., Gallant J. The control of ribonucleic acid synthesis in Escherichia coli. II. Stringent control of energy metabolism. J Biol Chem. 1969 Apr 25;244(8):2233–2239. [PubMed] [Google Scholar]
- KLEIN H. P. Synthesis of enzymes in resting cells. Ann N Y Acad Sci. 1963 Jan 21;102:637–654. doi: 10.1111/j.1749-6632.1963.tb13665.x. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Lazzarini R. A., Nakata K., Winslow R. M. Coordinate control of ribonucleic acid synthesis during uracil deprivation. J Biol Chem. 1969 Jun 10;244(11):3092–3100. [PubMed] [Google Scholar]
- MANDELSTAM J. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol Rev. 1960 Sep;24(3):289–308. doi: 10.1128/br.24.3.289-308.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDERATH E., RANDERATH K. RESOLUTION OF COMPLEX NUCLEOTIDE MIXTURES BY TWO-DIMENSIONAL ANION-EXCHANGE THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1964 Oct;16:126–129. doi: 10.1016/s0021-9673(01)82446-8. [DOI] [PubMed] [Google Scholar]
- Salivar W. O., Sinsheimer R. L. Intracellular location and number of replicating parental DNA molecules of bacteriophages lambda and phi-X174. J Mol Biol. 1969 Apr 14;41(1):39–65. doi: 10.1016/0022-2836(69)90124-7. [DOI] [PubMed] [Google Scholar]



