Abstract
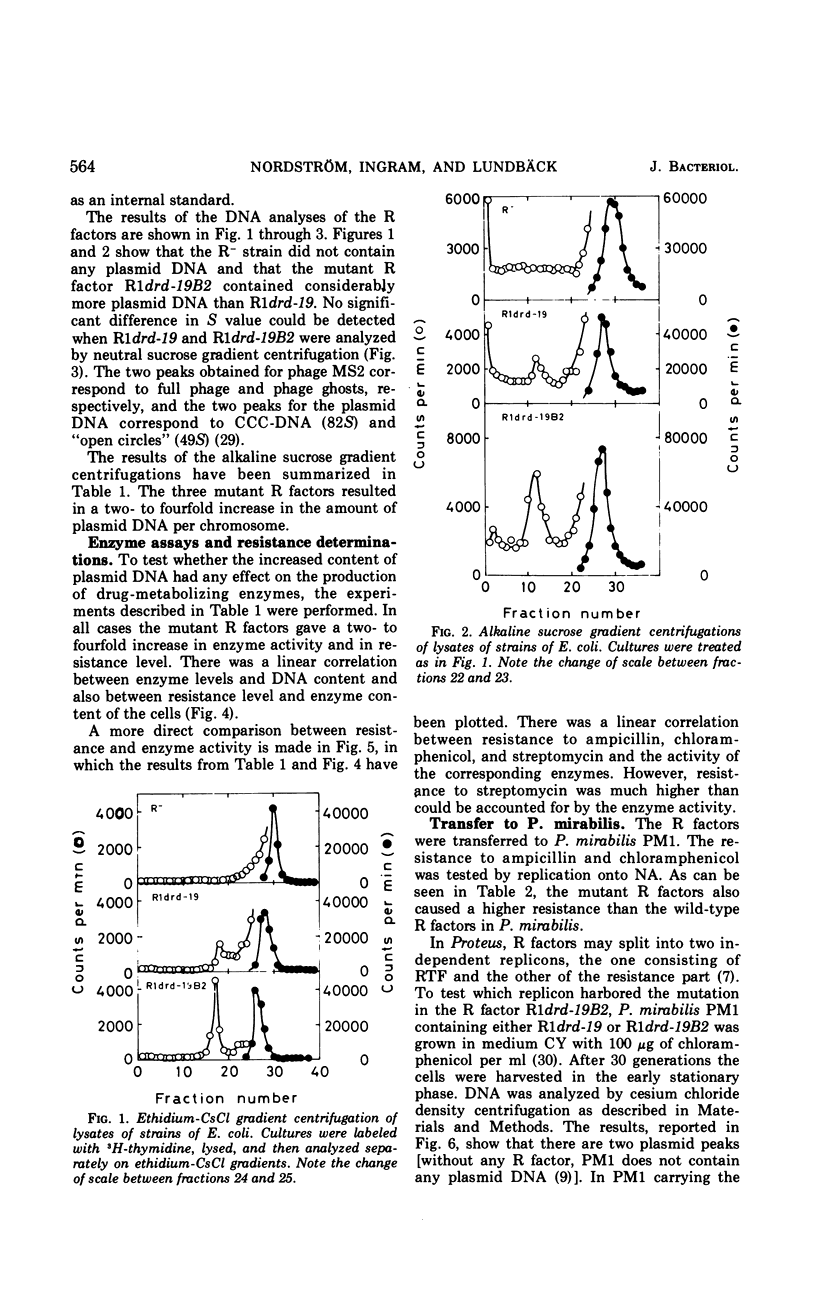
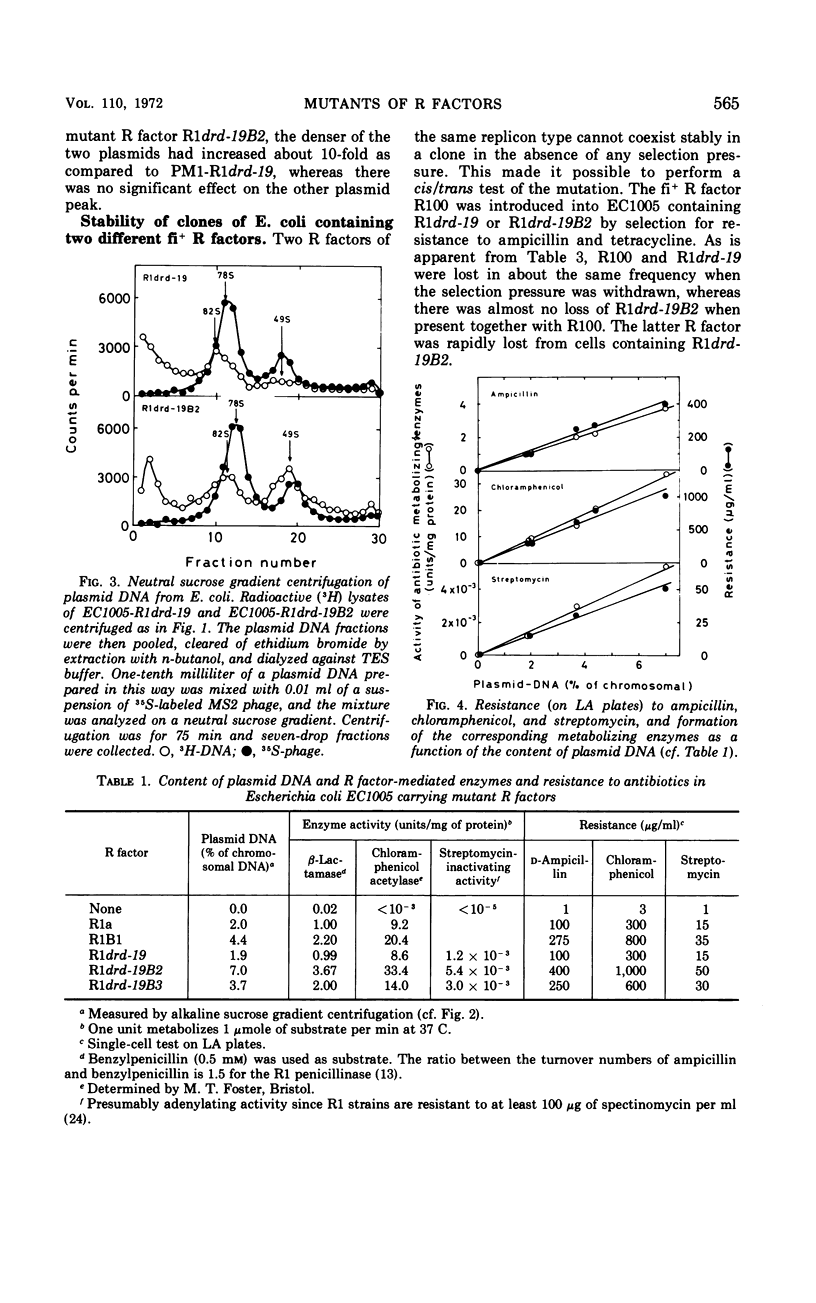
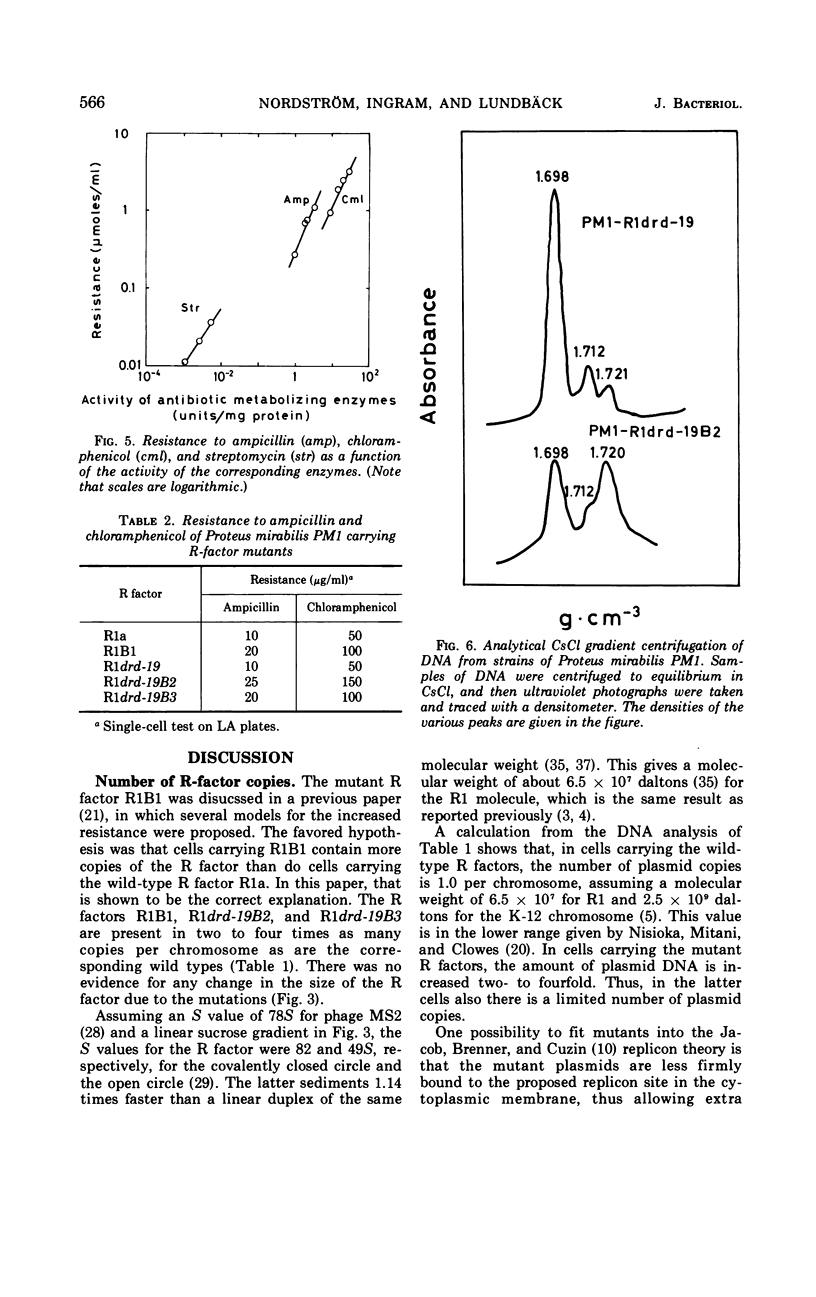
A mutant of the repressed R factor R1a and two mutants of the derepressed R factor R1drd-19 showing a two- to fourfold increase in resistance to all of the antibiotics to which the wild-type R factors mediate resistance were studied. The increased resistance was due to a two- to fourfold increase in the number of R-factor copies per chromosome. The production of drug-metabolizing enzymes was linearly correlated to the gene dosage. There was also a linear correlation between resistance to the drugs and the production of the corresponding enzymes. The mutations were also expressed in Proteus mirabilis PM1. In Proteus, R factors are split into two plasmids, resistance transfer factor and the resistance part. The mutation in one of the mutant R factors seems to be located in the resistance part. A second fi+ R factor (R100) was introduced into strains already carrying R1drd-19 or the mutant R factor R1drd-19B2. In the first case, R100 and R1drd-19 segregated with equal probability when the bacteria were grown on antibiotic-free medium, whereas, in the second case, R100 was rapidly and preferentially excluded.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Nordström K. Colicin tolerance induced by ampicillin or mutation to ampicillin resistance in a strain of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):1–13. doi: 10.1128/jb.106.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Multiple molecular species of circular R-factor DNA isolated from Escherichia coli. Nature. 1969 Dec 27;224(5226):1273–1277. doi: 10.1038/2241273a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Silver R. P., Sharp P. A., McCoubrey A. E. The problems of drug-resistant pathogenic bacteria. Studies on the molecular nature of R factors. Ann N Y Acad Sci. 1971 Jun 11;182:172–187. doi: 10.1111/j.1749-6632.1971.tb30655.x. [DOI] [PubMed] [Google Scholar]
- FALKOW S., WOHLHIETER J. A., CITARELLA R. V., BARON L. S. TRANSFER OF EPISOMIC ELEMENTS TO PROTEUS. I. TRANSFER OF F-LINKED CHROMOSOMAL DETERMINANTS. J Bacteriol. 1964 Jan;87:209–219. doi: 10.1128/jb.87.1.209-219.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontomichalou P., Mitani M., Clowes R. C. Circular R-factor molecules controlling penicillinase synthesis, replicating in Escherichia coli under either relaxed or stringent control. J Bacteriol. 1970 Oct;104(1):34–44. doi: 10.1128/jb.104.1.34-44.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Punch J. D. The problems of drug-resistant pathogenic bacteria. Regulation of R-factor replication in Proteus mirabilis. Ann N Y Acad Sci. 1971 Jun 11;182:201–216. doi: 10.1111/j.1749-6632.1971.tb30657.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindqvist R. C., Nordström K. Resistance of Escherichia coli to penicillins. VII. Purification and characterization of a penicillinase mediated by the R factor R1. J Bacteriol. 1970 Jan;101(1):232–239. doi: 10.1128/jb.101.1.232-239.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström E. B., Nordström K. Automated method for determination of penicillins, cephalosporins, and penicillinases. Antimicrob Agents Chemother. 1972 Feb;1(2):100–106. doi: 10.1128/aac.1.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. C. Molecular recombination between R-factor deoxyribonucleic acid molecules in Escherichia coli host cells. J Bacteriol. 1970 Jul;103(1):166–177. doi: 10.1128/jb.103.1.166-177.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Eriksson-Grennberg K. G., Boman H. G. Resistance of Escherichia coli to penicillins. 3. AmpB, a locus affecting episomally and chromosomally mediated resistance to ampicillin and chlorampheincol. Genet Res. 1968 Oct;12(2):157–168. doi: 10.1017/s0016672300011770. [DOI] [PubMed] [Google Scholar]
- Nordström K. Increased resistance to several antibiotics by one mutation in an R-factor, R 1a. J Gen Microbiol. 1971 May;66(2):205–214. doi: 10.1099/00221287-66-2-205. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Benveniste R., Tipper D., Davies J. Aminoglycoside antibiotics: inactivation by phosphorylation in Escherichia coli carrying R factors. J Bacteriol. 1969 Nov;100(2):1144–1146. doi: 10.1128/jb.100.2.1144-1146.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson P. E. On the possible importance of relaxation processes in enzyme catalysis. J Mol Biol. 1968 Jan 28;31(2):319–321. doi: 10.1016/0022-2836(68)90448-8. [DOI] [PubMed] [Google Scholar]
- Roberts L. M., Reeve E. C. Two mutations giving low-level streptomycin resistance in Escherichia coli K 12. Genet Res. 1970 Dec;16(3):359–365. doi: 10.1017/s0016672300002640. [DOI] [PubMed] [Google Scholar]
- Rohrmann G. F., Krueger R. G. Physical, biochemical, and immunological properties of coliphage MS-2 particles. J Virol. 1970 Sep;6(3):269–279. doi: 10.1128/jvi.6.3.269-279.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T. F., Hayashi M. Allomorphic forms of bacteriophage phiX-174 replicative DNA. Science. 1966 Nov 4;154(3749):658–660. doi: 10.1126/science.154.3749.658. [DOI] [PubMed] [Google Scholar]
- Rownd R., Kasamatsu H., Mickel S. The molecular nature and replication of drug resistance factors of the Enterobacteriaceae. Ann N Y Acad Sci. 1971 Jun 11;182:188–206. doi: 10.1111/j.1749-6632.1971.tb30656.x. [DOI] [PubMed] [Google Scholar]
- Rownd R., Nakaya R., Nakamura A. Molecular nature of the drug-resistance factors of the Enterobacteriaceae. J Mol Biol. 1966 Jun;17(2):376–393. doi: 10.1016/s0022-2836(66)80149-3. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. P., Falkow S. Specific labeling and physical characterization of R-factor deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1970 Oct;104(1):331–339. doi: 10.1128/jb.104.1.331-339.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. P., Falkow S. Studies on resistance transfer factor deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1970 Oct;104(1):340–344. doi: 10.1128/jb.104.1.340-344.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasawa S., Utahara R., Okanishi M., Maeda K., Umezawa H. Studies on adenylylstreptomycin, a product of streptomycin inactivation by E. coli carrying the R-factor. J Antibiot (Tokyo) 1968 Aug;21(8):477–484. doi: 10.7164/antibiotics.21.477. [DOI] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]



