Abstract
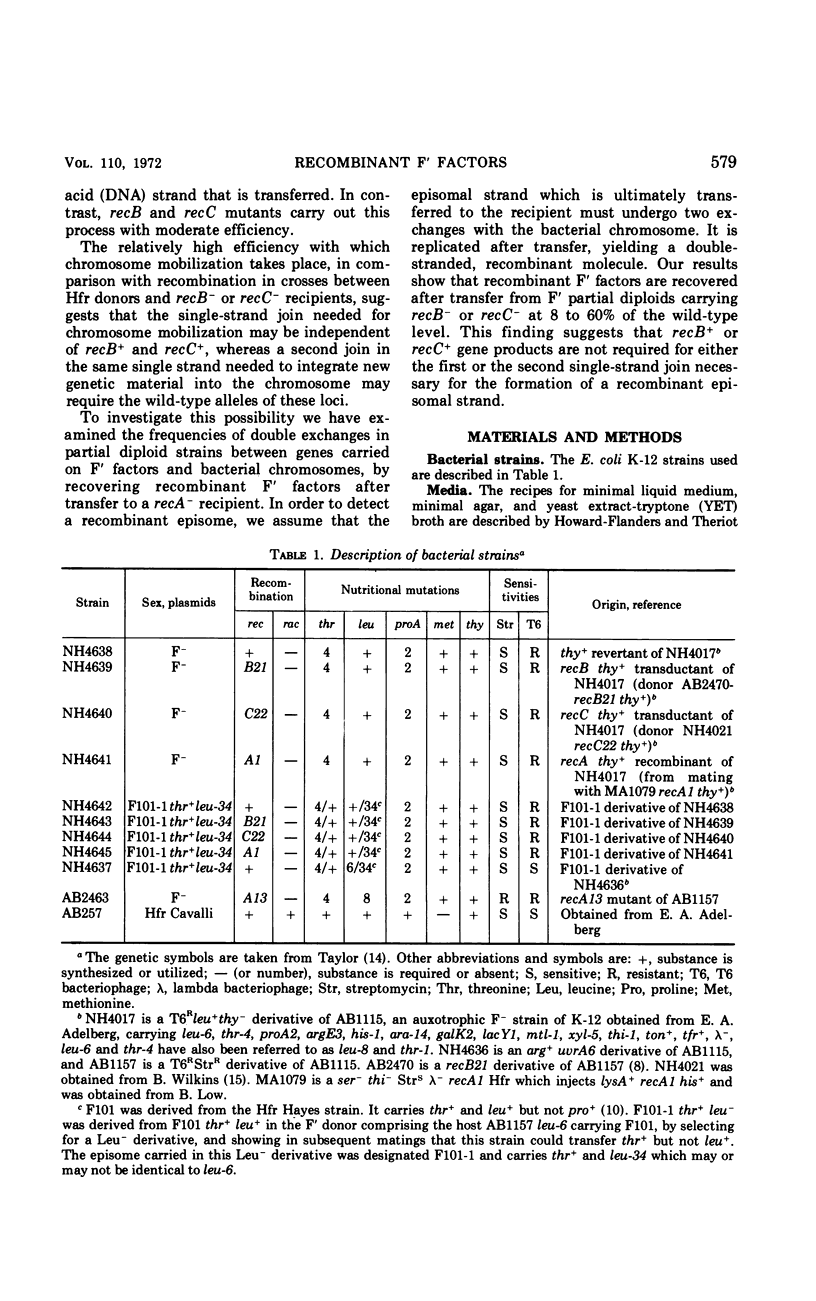
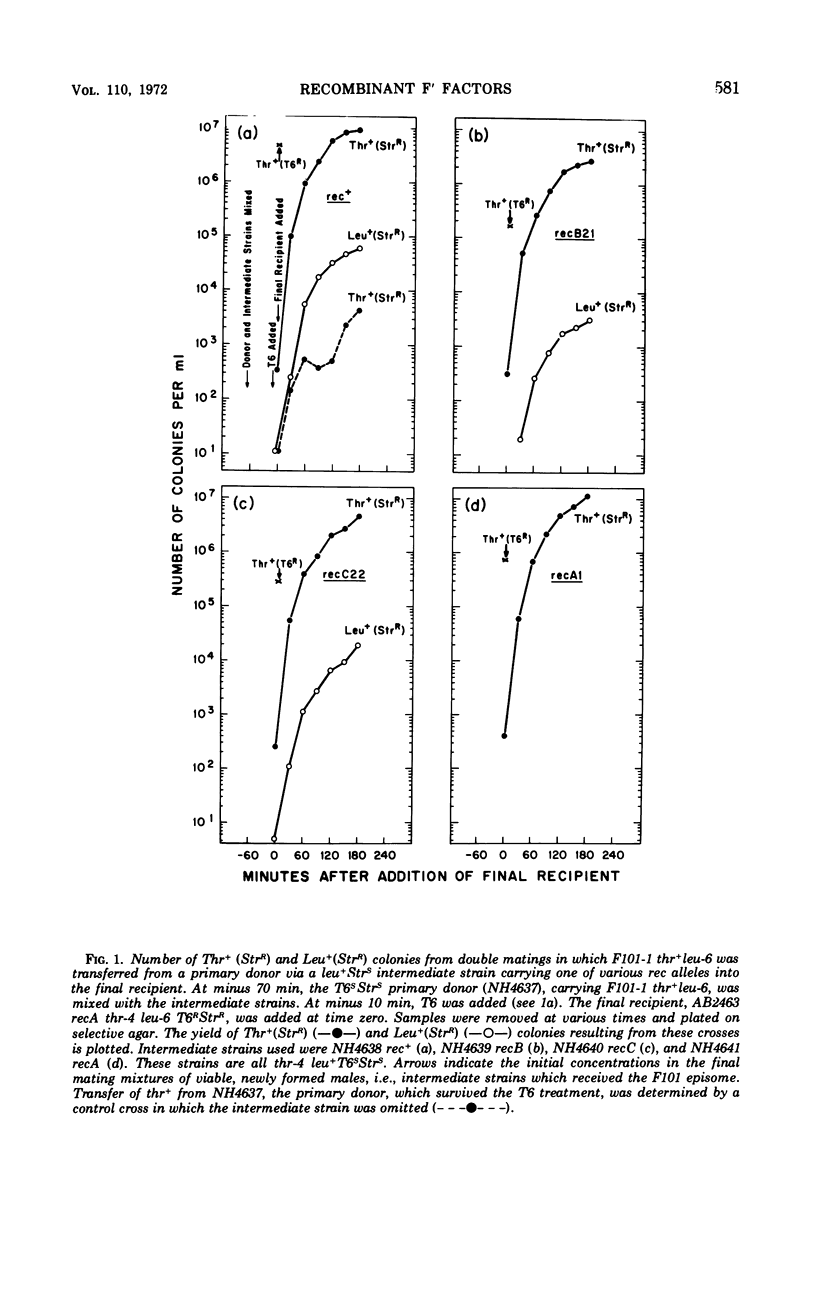
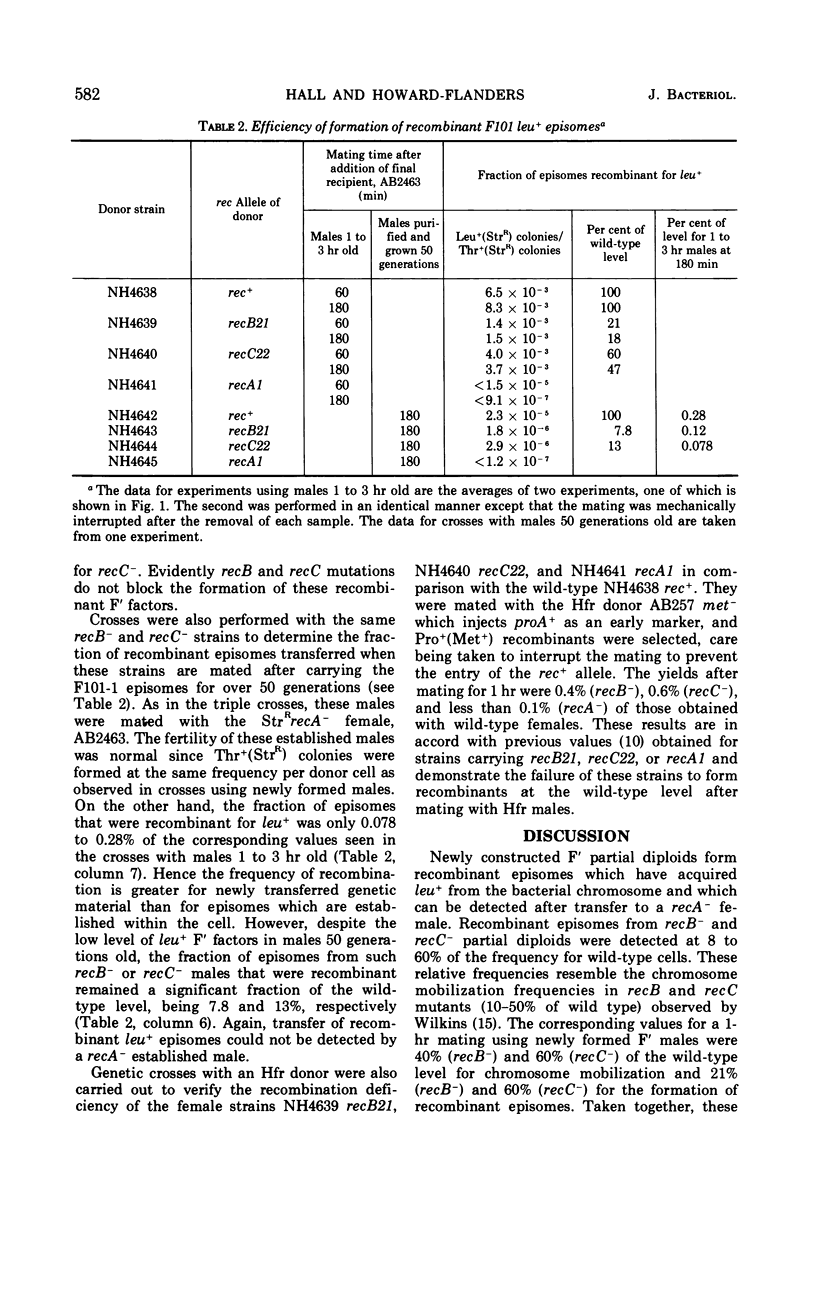
The frequency of genetic exchanges between F′ factors and the bacterial chromosome was studied in recombination-deficient Escherichia coli mutants under conditions in which the recombinant F′ factors were immediately transferred to new hosts. In a series of double matings, F101-1 thr+leu− episomes were first transferred into each of four intermediate F−thr−leu+ strains carrying various rec alleles. After the original F′ donors were killed with phage T6, the F101-1 episomes were then transferred from the intermediate cells to F−thr−leu−StrRrecA− females. Recipients of nonrecombinant episomes formed Thr+ (StrR) colonies, and recipients of recombinant episomes formed Leu+(StrR) colonies. A comparison of the numbers of Leu+(StrR) and Thr+(StrR) colonies shows that recB− males formed 18 to 21% and recC− formed 47 to 60% of the wild-type level of recombinant episomes that could be detected after transfer. No recombinant episomes were detected using a recA− intermediate strain. If the intermediate strains harboring the F101 episomes were purified, allowed to grow for 50 generations, and then mated with the recA− recipient, recombinant episomes were transferred at 8% of the wild-type level for recB− and 13% for recC−. In contrast, only 0.4 and 0.6% of the normal number of recombinants were obtained from crosses between Hfr Cavalli donors and the same recB− and recC− strains. Recombinant episomes were detected with greater frequency among newly formed rec+, recB−, and recC− partial diploids than in those which were 50 generations old.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Nagaishi H., Templin A., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. II. Rec+ revertants caused by indirect suppression of rec- mutations. Proc Natl Acad Sci U S A. 1970 Sep;67(1):128–135. doi: 10.1073/pnas.67.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. An endonuclease activity from Escherichia coli absent from certain rec- strains. Proc Natl Acad Sci U S A. 1970 Sep;67(1):434–441. doi: 10.1073/pnas.67.1.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. K. Identification of recombinant chromosomes and F-merogenotes in merodiploids of Escherichia coli. J Bacteriol. 1968 Jul;96(1):173–179. doi: 10.1128/jb.96.1.173-179.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Rupp W. D., Wilkins B. M., Cole R. S. DNA replication and recombination after UV irradiation. Cold Spring Harb Symp Quant Biol. 1968;33:195–207. doi: 10.1101/sqb.1968.033.01.023. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTARD J., ADELBERG E. A. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI K-12. II. INTERACTION BETWEEN F-MEROGENOTE AND CHROMOSOME DURING TRANSFER. J Bacteriol. 1963 Jun;85:1402–1408. doi: 10.1128/jb.85.6.1402-1408.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTARD J., ADELBERG E. A. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI K-12. III. AN ANALYSIS OF THE RECOMBINATION EVENTS OCCURRING IN THE F' MALE AND IN THE ZYGOTES. Genetics. 1964 Jun;49:995–1007. doi: 10.1093/genetics/49.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins B. M. Chromosome transfer from F-lac+ strains of Escherichia coli K-12 mutant at recA, recB, or recC. J Bacteriol. 1969 May;98(2):599–604. doi: 10.1128/jb.98.2.599-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



