Abstract
The identification of new pharmacological approaches to effectively prevent, treat, and cure the metabolic syndrome is of crucial importance. Excessive exposure to dietary lipids causes inflammatory responses, deranges the homeostasis of cellular metabolism, and is believed to constitute a key initiator of the metabolic syndrome. Mammalian Sirt1 is a protein deacetylase that has been involved in resveratrol-mediated protection from high-fat diet-induced metabolic damage, but direct proof for the implication of Sirt1 has remained elusive. Here, we report that mice with moderate overexpression of Sirt1 under the control of its natural promoter exhibit fat mass gain similar to wild-type controls when exposed to a high-fat diet. Higher energy expenditure appears to be compensated by a parallel increase in food intake. Interestingly, transgenic Sirt1 mice under a high-fat diet show lower lipid-induced inflammation along with better glucose tolerance, and are almost entirely protected from hepatic steatosis. We present data indicating that such beneficial effects of Sirt1 are due to at least two mechanisms: induction of antioxidant proteins MnSOD and Nrf1, possibly via stimulation of PGC1α, and lower activation of proinflammatory cytokines, such as TNFα and IL-6, via down-modulation of NFκB activity. Together, these results provide direct proof of the protective potential of Sirt1 against the metabolic consequences of chronic exposure to a high-fat diet.
Keywords: inflammation, metabolism, NFκB, sirtuins, steatosis
Driven by the need for potent and safe options to treat obesity, diabetes, and the metabolic syndrome, numerous efforts are currently underway to achieve a better understanding of the molecular networks controlling cellular glucose, lipid, and energy metabolism (1–3). It is generally accepted that gene–environment interactions (such as the effect of high-fat diets on the molecular pathways that maintain energy homeostasis) play a key role in the pathogenesis of the metabolic syndrome (4). Intriguingly, several reports recently showed that specific dietary fatty acids can directly activate Toll-like receptors, which are better known as components of the innate immune system recognizing bacteria-derived fatty acids (5–7). The resulting immune response promotes the systemic activation of proinflammatory pathways including NFκB, TNFα, or IL-6 (5, 6). This chain of events is believed to ultimately lead to insulin resistance, setting in motion the vicious cycle of the metabolic syndrome (8).
Recently, a series of studies in several organisms revealed multiple important links of the Sirtuin family of proteins with energy metabolism and inflammation (9–11). Also known as silent information regulator 2 (Sir2)-related enzymes, the Sirtuins have been well conserved throughout evolution and represent a family of nicotinamide adenine dinucleotide-dependent enzymes that deacetylate residues of acetylated lysine. The mammalian sirtuins Sirt1–Sirt7 are implicated in a number of cellular and physiological functions including gene silencing, apoptosis, mitochondrial function, energy homeostasis, and longevity (12). Among its multiple reported targets, Sirt1 deacetylates and thereby activates PGC1α, an essential cofactor in mitochondrial biogenesis driving metabolic rate (10). Thus, activation of Sirt1 with small molecules, such as resveratrol, may represent a promising strategy for preventing and treating metabolic syndrome (13). This hypothesis has gained support recently from newly developed chemical activators of Sirt1 that boost mitochondrial capacity and improve whole-body glucose homeostasis upon high-fat diet (14). However, these small molecules could impinge on multiple members of the Sirtuin family, as well as on other targets yet to be discovered. To directly test the effect of Sirt1 on the metabolic impairment induced by chronic exposure to dietary lipids, we generated mice with moderate overexpression of Sirt1 and analyzed their metabolic phenotype.
Results
Generation of Sirt1 Transgenic Mice.
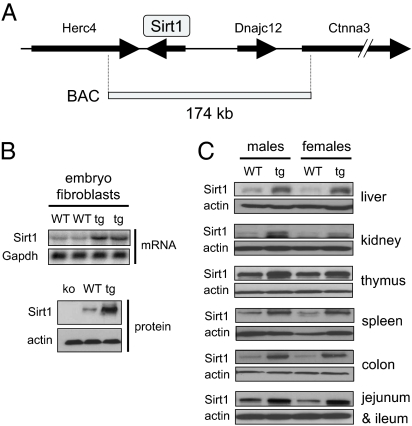
Transgenesis was performed by using a large genomic construct (174 kb) containing the entire Sirt1 gene in its natural genomic context (Fig. 1A). In general, these large genomic transgenes have the advantage of providing a pattern of tissue expression very similar or identical to the endogenous gene (15). The Sirt1 genomic construct used also contains the adjacent gene Dnajc12 (Fig. 1A). This gene belongs to a large family of heat shock proteins, the DnaJ/Hsp40 family, with >40 members in mammalian genomes (16). We reasoned that the likely effect of increasing the expression of Dnajc12 is of little relevance because of (i) the large number of paralogs already present in the genome, (ii) the lack of previous evidence implicating Hsp40 proteins in the processes explored in this report, and (iii) the absence of effect of Dnajc12 inhibition on the molecular pathways affected by Sirt1 (see below). Transgenesis was performed by standard microinjection of fertilized oocytes. One founder mouse was identified that contained a single complete copy of the Sirt1 genomic insert and that transmitted the transgenic allele to the progeny (supporting information (SI) Fig. S1 and Fig. S2). This line of transgenic mice is abbreviated here as Sirt1-tg or simply tg.
Fig. 1.
Generation of a BAC-based transgenic Sirt1 mouse line. (A) Scheme of the Bacterial Artificial Chromosome (BAC) used to generate the Sirt1-tg mouse strain. (B) Levels of Sirt1 mRNA (Upper) and Sirt1 protein (Lower) in mouse embryo fibroblasts. (C) Levels of Sirt1 protein in the indicated tissues.
Expression of Sirt1 was analyzed in mouse embryonic fibroblasts (MEFs) (Fig. 1B), as well as in different tissues from Sirt1-tg mice (Fig. 1C), and it was found that Sirt1 was moderately overexpressed in all of the tissues tested. Quantitative RT-PCR indicated a homogeneous level of overexpression (ranging from 2- to 4-fold in liver, brown adipose tissue, and muscle; for these values, see below). This increase in Sirt1 mRNA was paralleled by a similar increase in protein levels (ranging from 2- to 4-fold in different tissues; see Fig. 1C). In the case of liver, a more extensive quantification of the protein levels indicated an average overexpression of ≈3-fold (2.7 ± 0.35, n = 7). Functionality of the transgene was confirmed genetically by rescue of three overt phenotypes present in Sirt1 knockout mice, namely, partially penetrant perinatal lethality, low weight, and microphthalmia of the surviving mice (17). In brief, Sirt1(−/−;tg) mice were born and viable at the expected Mendelian ratio, with no indication of perinatal lethality, had a normal weight, and did not present with microphthalmia (Fig. S3).
Balanced Energy Homeostasis of Sirt1 Transgenic Mice.
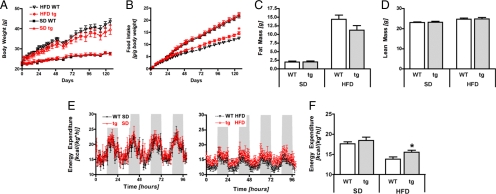
Deficiency for Sirt1 has previously been shown to reduce body weight in mice [(17) and Fig. S3]. Therefore, we began our characterization by examining the effects of moderate Sirt1 overexpression on the regulation of body weight, food intake, and energy balance regulation. Body weights and body lengths of 8- to 10-week-old transgenic Sirt1 mice did not differ from wild-type (WT) control mice on a standard diet (SD) (Fig. 2A, and data not shown). Subsequent exposure for 19 weeks to a high-fat diet (HFD) did not lead to significant differences in body weight (Fig. 2A), fat mass (Fig. 2C), or lean mass (Fig. 2D), although a trend toward lower body weight and fat mass was observed in tg mice on HFD compared with WT controls. Neither plasma leptin levels nor adiponectin levels differed between genotypes on either SD or HFD (Table S1). Noteworthy, tg mice exhibited increased food intake compared with WT mice on HFD (Fig. 2B). This increase in food intake was corroborated by using in-depth analysis with an automated food intake-monitoring system (Fig. S4A). Therefore, we examined next whether the increase in caloric intake of tg mice on HFD was compensated by a concomitant increase in energy expenditure. Consistent with this hypothesis, indirect calorimetry revealed that tg mice showed higher energy expenditure than WT mice on HFD (Fig. 2E Right and F). No differences were observed in mice fed SD (Fig. 2E Left and F). Real-time PCR analysis of UCP1 levels in brown adipose tissue (BAT) suggested that BAT thermogenesis was not the cause for this increase in energy expenditure, despite overexpression of Sirt1 in BAT (Fig. S4B). We next tested activity-induced thermogenesis by monitoring motor activity in a specific home cage beam break system, but failed to find significant changes in total locomotion (Fig. S4C), ambulatory movement, or stationary movement (Fig. S4D). The energetic cost of muscular activity seemed also unchanged, as indicated by a comparable expression of UCP3 and of several mitochondrial respiratory chain enzymes in the quadriceps muscle, despite overexpression of Sirt1 (Fig. S4E). No differences in respiratory quotient were found, suggesting comparable fuel-partitioning patterns in WT and tg mice (Fig. S4F). In summary, although Sirt1 transgenic mice present a modest increase in food intake under HFD, this is compensated by a similarly modest increase in energy expenditure, thus indicating a balanced energy homeostasis.
Fig. 2.
Energy homeostasis of transgenic Sirt1 mice. (A) Body weight of wild type (WT) or transgenic (tg) Sirt1 mice during exposure to a standard chow diet (SD) or a high-fat diet (HFD). (B) Food intake. (C) Fat mass in WT and tg mice after 19 weeks of exposure to SD or HFD. (D) Lean mass. (E and F) Energy expenditure per kg of body weight after 8 weeks of SD or HFD: 5-day measurements in SD mice (E Left) or HFD mice (E Right), and total mean values (F). n = 7–8 per group; means ± SEM; *, P < 0.05.
Sirt1 Transgenic Mice Are Protected from HFD-Induced Hepatic Steatosis.
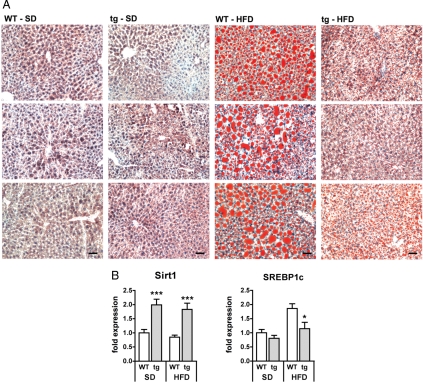
Chronic HFD exposure causes accumulation of lipids in the liver, a process leading to fatty liver disease, also known as Nonalcoholic Fatty Liver Disease (NAFLD), and eventually to Nonalcoholic Steato-Hepatitis (NASH) (19). Sirt1 has been implicated in the control of lipid metabolism (9). We therefore investigated hepatic accumulation of lipids by oil red staining of frozen liver sections (Fig. 3A). As expected, small amounts of lipid droplets were found in liver sections of both WT and tg mice after 19 weeks on SD. In contrast, after 19 weeks of HFD, WT mice presented with severe hepatosteatosis, including massive accumulation of large lipid droplets. Importantly, Sirt1 tg mice were almost entirely protected from NAFLD, showing a low number of lipid droplets with small diameters (Fig. 3A). A common feature of NAFLD is the deregulation of enzymes involved in fat metabolism, a process tightly controlled by a number of specific regulators, being particularly relevant the SREBP transcription factors (20). In agreement with the observed Sirt1-mediated protection from hepatosteatosis, we found that Sirt1 tg mice presented lower levels of SREBP1c mRNA in response to HFD (Fig. 3B). We conclude that Sirt1 protects from NAFLD.
Fig. 3.
Transgenic Sirt1 mice are protected from HFD-induced hepatosteatosis. (A) Oil red staining lipid droplets in frozen liver sections (3 per group) from both wild-type (WT) or transgenic (tg) mice on a standard diet (SD) or after high-fat diet (HFD) exposure for 19 weeks. (Scale bars, 50 μm.) (B) Gene expression analysis (real-time PCR) of hepatic Sirt1 and the transcription factor SREBP1c. n = 7–8 per group; means ± SEM; *, P < 0.05; ***, P < 0.001.
Transgenic Sirt1 Mice Are Protected from HFD-Induced Hepatic Glucose Intolerance.
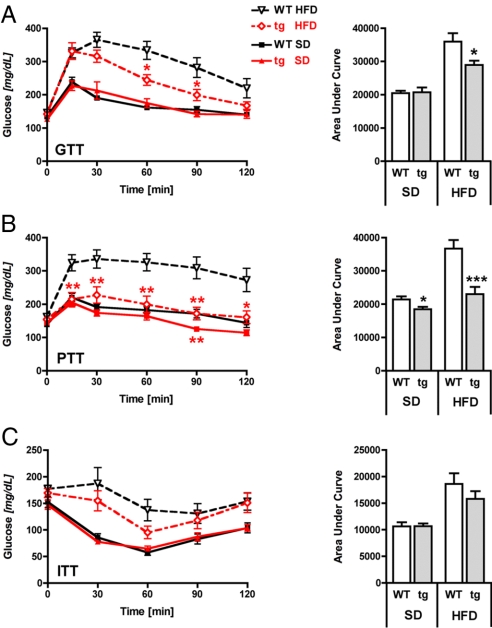
Based on reports showing that NAFLD can also be a direct consequence of peripheral insulin resistance with an elevated transport of free fatty acids from adipose tissue to the liver (21), we next examined whether glucose tolerance and insulin resistance differed between genotypes at different ages and on different diets. After 8 weeks of diet exposure, overnight fasting plasma glucose levels were similar between genotypes on both SD and HFD (Table S1). In addition, insulin, cholesterol, and triglyceride plasma levels did not differ between genotypes and diets (Table S1). Chronic exposure to HFD for 18 weeks increased glucose, insulin, triglyceride, and cholesterol levels, and decreased free fatty acid levels, to the same extent in both WT and tg mice. In addition, blood glucose and free fatty acid levels under ad libitum feeding conditions revealed no differences between genotypes on either SD or HFD (Table S1). To assess the impact of chronic HFD exposure on glucose homeostasis in more detail, the mice were subjected to a glucose tolerance test (GTT) after 7 weeks of SD or HFD exposure (Fig. 4A). Both WT and tg mice on SD were comparable throughout the study, indicating normal glucose tolerance. Intraperitoneal administration of glucose led to a more rapid increase of blood glucose levels in mice fed a HFD, indicating the expected HFD-induced systemic glucose intolerance in both genotypes. However, although peak values were similar for WT and tg mice on the HFD, the transgenic mice on HFD decreased their blood glucose levels more rapidly than WT controls on the same diet. Area-under-the-curve (AUC) values revealed a significantly better preserved glucose tolerance of tg mice on HFD compared with WT mice on HFD (Fig. 4A Right).
Fig. 4.
Transgenic Sirt1 mice are protected from HFD-induced hepatic glucose intolerance. (A) Glucose tolerance test (GTT) after 7 weeks of diet exposure by using an i.p. dose of 2 g of glucose per kg of body weight. (B) Pyruvate tolerance test (PTT) after 16 weeks of diet exposure by using 2 g of pyruvate per kg of body weight. (C) Insulin tolerance test (ITT) after 13 weeks of diet exposure by using 0.75 IU/kg insulin. Curves of glucose levels (Left) and values of the area under the curve (Right). n = 7–8 per group; means ± SEM; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Because we observed substantial protection from hepatic lipid deposition by Sirt1 overexpression, we hypothesized a similar protective effect on hepatic glucose metabolism. We therefore performed a pyruvate tolerance test (PTT; Fig. 4B) in mice after 16 weeks of SD or HFD by intraperitoneally administering pyruvate, a precursor in the synthesis of glucose during gluconeogenesis, a process mainly localized in the liver and, to a smaller extent, the kidney. Subsequent measurement of circulating glucose levels at several time points after pyruvate injection in WT mice on HFD revealed a massive increase of blood glucose levels, which remained high throughout the 2-h study period, whereas blood glucose levels in tg mice on HFD remained significantly lower and in fact were comparable to those measured for WT mice on SD. In addition, overexpression of Sirt1 in mice on SD also decreased 90-min glucose levels (Fig. 4B Left) and AUC values (Fig. 4B Right) when compared with WT mice on SD.
In contrast to the glucose and pyruvate tolerance tests of tg mice on HFD that indicated a substantial protection from HFD-induced glucose intolerance, an insulin tolerance test (ITT) after prolonged exposure to HFD for 13 weeks showed only a tendency toward lower glucose levels in tg mice, which was nonsignificant for AUC values (Fig. 4C). These data indicate that Sirt1 overexpression does not significantly affect HFD-induced insulin resistance in young mice. All together, these data allow us to conclude that increased Sirt1 activity protects from HFD-induced hepatic glucose intolerance.
Transgenic Sirt1 Mice Are Protected from Hepatic Inflammation.
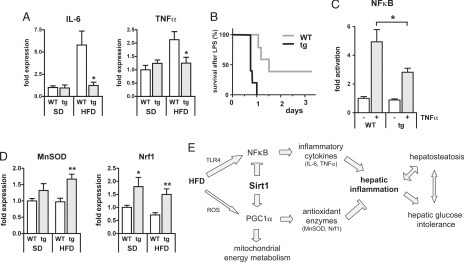
Exposure to a high-fat diet and diet-induced obesity lead to a chronic inflammatory reaction, which is believed to be critical for the development of glucose intolerance and insulin resistance (22). Accordingly, the expression of IL-6 and TNFα, two major proinflammatory cytokines, was significantly enhanced in livers of WT mice on a chronic HFD (Fig. 5A). Transgenic mice on HFD, however, still exhibited low levels of inflammatory markers, comparable to those seen in WT and tg mice on chow diet. IL-6 and TNFα are regulated by NFκB, which in turn is activated by dietary lipids through the Toll-like receptor TLR4 (5). Interestingly, Sirt1 has been described to inhibit NFκB activity (23–25). To evaluate whether NFκB is down-modulated by Sirt1 in our mouse model, we performed the following in vivo and in vitro assays. Lipopolysaccharide (LPS) is an inflammatory agent that activates a wide range of responses partly mediated by TLR4 and NFκB. In the liver, NFκB plays an antiapoptotic role and provides protection against LPS-induced liver failure (26, 27). Consistent with Sirt1 tg mice having lower levels of NFκB activity, Sirt1-tg mice on SD revealed a marked LPS hypersensitivity compared with WT controls (Fig. 5B). In addition, we examined whether NFκB activation differs in mouse embryonic fibroblasts derived from tg and WT mice by using a reporter gene assay with luciferase under the transcriptional control of NFκB responsive elements. Indeed, TNFα stimulation resulted in significantly lower activation of NFκB activation in Sirt1-tg cells (2.8-fold) compared with WT cells (5-fold) (Fig. 5C). As mentioned above, the Sirt1 transgene also carries the adjacent gene Dnajc12 (see Fig. 1A). To rule out the possibility that the lower NFκB activity observed in Sirt1 tg cells could be a consequence of Dnajc12, we performed the same assay in the presence of an shRNA that significantly decreases Dnajc12 mRNA levels (10-fold inhibition) (Fig. S5A). We observed that Sirt1-tg cells exhibited lower NFκB activity both in the absence or presence of shDnajc12 (Fig. S5B). These results implicate Sirt1, and not Dnajc12, as the gene responsible for the lower NFkB activity of Sirt1-tg cells.
Fig. 5.
Transgenic Sirt1 mice are protected from hepatic inflammation. (A) Gene expression analyses (real-time PCR) of the proinflammatory cytokines IL-6 and TNFα in livers of WT and tg mice on SD or HFD (19 weeks) (n = 7–8 per group; means ± SEM; *, P < 0.05). (B) Kaplan–Meier plot of survival curves of WT and tg mice on SD after injection of lipopolysaccharide (LPS) (n = 5 per group). (C) Luciferase reporter gene assay of WT and tg mouse embryonic fibroblasts after transfection with a pNFκB-Luc reporter vector and stimulation with 10 ng/ml of TNFα. Luminescence was measured 6 h after TNFα incubation. (n = 6–8 per group; means ± SEM; *, P < 0.05). (D) Gene expression analyses (real-time PCR) of the antioxidant proteins MnSOD and Nrf1 in livers of WT and tg mice on SD or HFD (19 weeks) (n = 7–8 per group; means ± SEM; *, P < 0.05; **, P < 0.01). (E) Model of Sirt1 effects on known molecular pathways linking dietary lipids, hepatosteatosis, and hepatic glucose intolerance.
In addition to the reduced expression of inflammatory cytokines, the expression of the antioxidant proteins manganese superoxide dismutase (MnSOD) and the nuclear respiratory factor 1 (Nrf1), a master regulator in the protection from reactive oxygen species, were both increased in tg mice on both SD and HFD, compared with WT mice (Fig. 5D). Both genes, MnSOD and Nrf1, have been demonstrated to be induced by PGC1α (28), which in turn is positively regulated by Sirt1 (10, 13). In summary, the overexpression of Sirt1 appears to protect tg mice from HFD-induced hepatic inflammation through decreasing the NFκB-mediated induction of inflammatory cytokines, as well as by the activation of antioxidant proteins, possibly mediated by PGC1α.
Discussion
We show here that modest overexpression of Sirtuin-1 (Sirt1) protects hepatic lipid and glucose metabolism from damage inflicted by high-fat diet. Sirt1 has been implicated in the molecular control of aging and cell proliferation, and more recently has also been involved in protection from metabolic syndrome. However, the proposed involvement of Sirt1 in protection from metabolic syndrome is based mostly on indirect evidence by using chemical activators of Sirt1. In particular, two studies have demonstrated beneficial effects of resveratrol on mice on a high-fat diet, including improved insulin sensitivity, lower steatosis, and increased survival (29, 30). More recently, Sirt1 activators unrelated to resveratrol have been shown to have beneficial effects on mitochondrial and metabolic function in obese rodents and offer potential for treating type 2 diabetes (14). However, although these studies compellingly argue in favor of the role of Sirt1 in protecting against high-fat diet, all of these Sirt1 activators may impinge on other members of the Sirtuin family as well as on a diverse range of pathways, as it is known to be the case of resveratrol (31).
To directly address the role of Sirt1 in protection from the detrimental metabolic consequences of high-fat diet exposure, we have engineered transgenic overexpression of Sirt1 in mice by introducing an additional transgenic copy of the full-length Sirt1 gene in the genome, which thereby remained under the control of its own promoter. Other Sirt1 transgenic mice reported express the transgene in restricted tissues, for example, endocrine pancreas, brain, or heart (32–35). Most recently, Bordone and colleagues (36) described a mouse model with overexpression of Sirt1 in a restricted number of tissues that include white and brown adipose tissue, but not in muscle and liver, which are relevant for the metabolic syndrome. In contrast to these mouse models, ours constitutes a model where Sirt1 is modestly overexpressed under its own promoter, thereby following the physiological pattern of expression, including metabolically relevant tissues, such as liver, muscle, and adipose tissue. These features make our Sirt1-tg mice an ideal model to explore the systemic effects of pharmacological interventions targeted to Sirt1.
Moderate Sirt1 overexpression in our mice led to a small increase in energy expenditure, which, however, did not translate into a significantly lower fat mass. In part, this is likely because of an unexpected increase in food intake, a phenomenon that has not been previously described as a consequence of Sirt1 overexpression. More interestingly, our present findings suggest that Sirt1 activators may hold promise for the treatment of nonalcoholic fatty liver disease (NAFLD). The pathogenesis of NAFLD is complex, but modulation of key transcription factors regulating hepatic lipid metabolism, such as sterol regulatory element binding protein 1 (SREBP1), has been proposed to carry great potential for the treatment of NAFLD (20). Interestingly, Sirt1 overexpression prevents up-regulation of SREBP1 on exposure to a high-fat diet. In addition, oxidative stress has been shown to play a causal role in the development of hepatosteatosis (37) and mice with a defect in hepatic Nrf1 expression develop hepatosteatosis as a result of hepatic oxidative stress (38). We here report an induction of Nrf1 in tg Sirt1 mice, as well as the induction of antioxidant enzymes such as MnSOD. Interestingly, an earlier report demonstrated that PGC1α, a well established target of Sirt1, activates this antioxidant program (28). These effects constitute plausible mechanisms for the protection from HFD-induced hepatosteatosis by Sirt1. Our observations warrant further study to evaluate Sirt1 as a potential target for the treatment of NAFLD.
Concomitant with the development of hepatosteatosis, hepatic inflammation is an established risk factor for the development of hepatic insulin resistance and glucose intolerance (39). Hepatic insulin resistance and glucose intolerance, in turn, can stimulate the development and progression of hepatosteatosis (40), thereby fueling and promoting a detrimental cycle that is believed to represent a key mechanistic component of the metabolic syndrome. Under fasting conditions, Sirt1 has a negative effect on insulin sensitivity and glucose tolerance (41). However, under fed conditions, activation of Sirt1 improves glucose homeostasis and insulin sensitivity (14, 29, 30, 42). Importantly, overexpression of Sirt1 in pancreatic β cells or adipose tissue improves glucose tolerance (32, 36). In our mice, moderate and systemic Sirt1 expression under its own promoter results in protection from HFD-induced glucose intolerance. The improved pyruvate tolerance of our transgenic mice on HFD suggests that the observed glucose tolerance is mainly due to a specific protection from hepatic insulin resistance, rather than to protection from systemic insulin resistance.
NFκB activity has previously been shown to be inhibited by Sirt1 (23–25). We confirmed such inhibition of NFκB in embryonic fibroblasts derived from Sirt1-tg mice. Hepatic NFκB signaling is activated by high-fat diet exposure and triggers insulin resistance (43), thereby linking inflammation with obesity-induced insulin resistance (44). Inhibition of NFκB activity by Sirt1 overexpression may—in conjunction with the activation of antioxidant proteins such as MnSOD and Nrf1—explain the prevention from hepatic inflammation, diet-induced glucose intolerance, and hepatosteatosis observed here (Fig. 5E).
In summary, moderate Sirt1 overexpression under control of its natural promoter in mice prevents HFD-induced glucose intolerance and nonalcoholic fatty liver disease. Our results indicate that this effect may result, in part, from prevention of HFD-induced activation of the proinflammatory NFκB pathway together with up-regulation of PGC1α and its antioxidant targets such as MnSOD or Nrf1. Finally, the anti-inflammatory actions of Sirt1 open up aspects that could link Sirt1 with aging or cancer.
Methods
Transgenesis and Animal Experimentation.
A large genomic DNA segment (174 kb) containing the murine Sirt1 gene and cloned into the BAC (Bacterial Artificial Chromosome) vector pBAC3.6 was obtained from CHORI (identification no. RP23-119G23) (http://www.chori.org). Pronuclei of fertilized oocytes, derived from intercrosses between (C57BL6 × CBA)F1 mice, were injected with ≈2 pl of a DNA solution containing the linearized BAC (see SI Methods). Analysis of integrity and copy number was determined by standard Southern blot methods (see SI Methods). Sirt1-tg mice were backcrossed for four generations with pure C57BL6 mice; in this manner, all of the mice used in this study share a genetic background that is 97% C57BL6. Sirt1-heterozygous mice were kindly obtained from Fred Alt, Harvard Medical School (17). Mice were fed either with a standard chow diet (Harlan Teklad LM-485), or with a high-fat diet (Research Diets 12451, 45% kJ from fat) starting when the mice were ≈2 months old and for a total of 19 additional weeks. To assess LPS susceptibility in each genotype, mice were injected i.p. with 20 mg/kg of Escherichia coli-derived LPS serotype 0111:B4 (Sigma). All studies were approved by and performed according to the ethical guidelines of the University of Cincinnati and the Spanish National Cancer Research Center (CNIO).
DNA, RNA, and Protein Analyses.
Southern and Northern blot analyses were performed using standard procedures (probes and primers are detailed in Table S2). Western blot analyses were performed following standard procedures. For detection of Sirt1, we used rabbit polyclonal antibody ab12193 (AbCam), working dilution 1:2,000; and for β-actin, we used monoclonal antibody AC-15 (Sigma), 1:5,000.
Mouse Embryonic Fibroblasts.
Luciferase assays were done by using Lipofectamine LTX and PLUS reagent (Invitrogen) for the transfections. We used pNFκBLuc (Clontech) reporter vector and E1fα-Renilla vector as transfection control. TNFα (Promega) was added at 10 ng/ml and luminescence was measured 6 h later by using a Glomax luminometer (Promega).
Metabolic Phenotyping.
Tail blood was collected 2 h after the onset of the light phase either after an overnight fast, or in ad libitum fed mice by using EDTA-coated Microvette tubes (Sarstedt), and immediately chilled on ice. Determinations were performed by using standard methods (see SI Methods). Metabolic performance and activity were studied by using an automated combined indirect calorimetry system (TSE Systems GmbH) and a multidimensional infrared light beam system (see SI Methods). Glucose, insulin, and pyruvate tolerance tests were performed according to standard procedures (see SI Methods).
Supplementary Material
Acknowledgments.
We thank Fred Alt for providing us with Sirt1-null mice, Dave D. D'Alessio for critically reviewing the manuscript, and Javier Martin, Maribel Muñoz, Gema Iglesias, and Erin Grant for excellent technical assistance. D.H. is supported by a predoctoral fellowship from the Spanish Ministry of Health and by the “Francisco Cobos” Foundation. S.V. is supported by a “Ramon y Cajal” contract from the Spanish Ministry of Education (MEC). Research at the laboratory of M.S. is funded by the Spanish National Cancer Research Center and by grants from the MEC, the European Union (INTACT and PROTEOMAGE), and the “Marcelino Botin” Foundation. Research at the Obesity Research Center is funded by National Institutes of Health Grants NIDDK59630, NIDDK69987, and NIDDK56863 (to M.H.T.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802917105/DCSupplemental.
References
- 1.Foster-Schubert KE, Cummings DE. Emerging therapeutic strategies for obesity. Endocr Rev. 2006;27:779–793. doi: 10.1210/er.2006-0041. [DOI] [PubMed] [Google Scholar]
- 2.Cooke D, Bloom S. The obesity pipeline: Current strategies in the development of anti-obesity drugs. Nat Rev Drug Discov. 2006;5:919–931. doi: 10.1038/nrd2136. [DOI] [PubMed] [Google Scholar]
- 3.Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 5.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suganami T, et al. Role of the Toll-like receptor 4/NFkappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 7.Tsukumo DM, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 8.Tschop M, Thomas G. Fat fuels insulin resistance through Toll-like receptors. Nat Med. 2006;12:1359–1361. doi: 10.1038/nm1206-1359. [DOI] [PubMed] [Google Scholar]
- 9.Picard F, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 11.Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. 2007;21:1745–1755. doi: 10.1210/me.2007-0079. [DOI] [PubMed] [Google Scholar]
- 13.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1alpha and SIRT1 pathways. FEBS Lett. 2007;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milne JC, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giraldo P, Montoliu L. Size matters: Use of YACs, BACs and PACs in transgenic animals. Transgenic Res. 2001;10:83–103. doi: 10.1023/a:1008918913249. [DOI] [PubMed] [Google Scholar]
- 16.Qiu XB, Shao YM, Miao S, Wang L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci. 2006;63:2560–2570. doi: 10.1007/s00018-006-6192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng HL, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perlemuter G, Bigorgne A, Cassard-Doulcier AM, Naveau S. Nonalcoholic fatty liver disease: From pathogenesis to patient care. Nat Clin Pract Endocrinol Metab. 2007;3:458–469. doi: 10.1038/ncpendmet0505. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed MH, Byrne CD. Modulation of sterol regulatory element binding proteins (SREBPs) as potential treatments for non-alcoholic fatty liver disease (NAFLD) Drug Discov Today. 2007;12:740–747. doi: 10.1016/j.drudis.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Medina J, Fernandez-Salazar LI, Garcia-Buey L, Moreno-Otero R. Approach to the pathogenesis and treatment of nonalcoholic steatohepatitis. Diabetes Care. 2004;27:2057–2066. doi: 10.2337/diacare.27.8.2057. [DOI] [PubMed] [Google Scholar]
- 22.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 23.Yeung F, et al. Modulation of NFkappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao F, Cheng J, Shi T, Yeh ET. Neddylation of a breast cancer-associated protein recruits a class III histone deacetylase that represses NFkappaB-dependent transcription. Nat Cell Biol. 2006;8:1171–1177. doi: 10.1038/ncb1483. [DOI] [PubMed] [Google Scholar]
- 25.Yang SR, et al. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NFkappaB in macrophages in vitro and in rat lungs in vivo: Implications for chronic inflammation and aging. Am J Physiol. 2007;292:L567–L576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- 26.Gadjeva M, et al. A role for NFkappa B subunits p50 and p65 in the inhibition of lipopolysaccharide-induced shock. J Immunol. 2004;173:5786–5793. doi: 10.4049/jimmunol.173.9.5786. [DOI] [PubMed] [Google Scholar]
- 27.Kisseleva T, et al. NFkappaB regulation of endothelial cell function during LPS-induced toxemia and cancer. J Clin Invest. 2006;116:2955–2963. doi: 10.1172/JCI27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St-Pierre J, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 29.Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 32.Moynihan KA, et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Ramsey KM, Mills KF, Satoh A, Imai SI. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in BESTO mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin W, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 35.Alcendor RR, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 36.Bordone L, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 37.Matsuzawa N, et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007;46:1392–1403. doi: 10.1002/hep.21874. [DOI] [PubMed] [Google Scholar]
- 38.Xu Z, et al. Liver-specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proc Natl Acad Sci USA. 2005;102:4120–4125. doi: 10.1073/pnas.0500660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 40.Ota T, et al. Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology. 2007;132:282–293. doi: 10.1053/j.gastro.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci USA. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun C, et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Cai D, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NFkappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arkan MC, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.