Abstract
Understanding how the cell uses a limited set of proteins to transduce very different signals into specific cellular responses is a central goal of cell biology and signal transduction disciplines. Although multifunctionality in signal transduction is widespread, the mechanisms that allow differential modes of signaling in multifunctional signaling pathways are not well defined. In legume plants, a common symbiosis signaling pathway composed of at least seven proteins mediates infection by both mycorrhizal fungi and rhizobial bacteria. Here we show that the symbiosis signaling pathway in legumes differentially transduces both bacterial and fungal signals (inputs) to generate alternative calcium responses (outputs). We show that these differential calcium responses are dependent on the same proteins, DMI1 and DMI2, for their activation, indicating an inherent flexibility in this signaling pathway. By using Lyapunov and other mathematical analyses, we discovered that both bacterial-induced and fungal-induced calcium responses are chaotic in nature. Chaotic systems require minimal energy to produce a wide spectrum of outputs in response to marginally different inputs. The flexibility provided by chaotic systems is consistent with the need to transduce two different signals, one from rhizobial bacteria and one from mycorrhizal fungi, by using common components of a single signaling pathway.
Keywords: chaos, mycorrhization, nodulation
During the interaction of legumes with nitrogen-fixing rhizobial bacteria, the plant develops unique structures, namely nodules, that provide a suitable environment for bacterial nitrogen fixation. This symbiotic process is established through plant perception of bacterially derived Nod factor. The signal transduction pathway responsible for perception of Nod factor has been well characterized, and at the core of this signaling pathway are oscillations in cytosolic calcium associated with the nuclear region (1). The receptor-like kinases NFP/NFR5 and LYK3/NFR1 are necessary for Nod factor perception (2–6). Additionally, a receptor-like kinase (DMI2/SYMRK), core components of the nuclear pore (NUP133 and NUP85) and cation channels (DMI1/Pollux and Castor) located on the nuclear membrane are all involved in Nod factor induction of calcium spiking (7–14). A calcium and calmodulin-dependent protein kinase (CCaMK) is necessary for the perception of the calcium signal, and a suite of transcription factors (NSP1, NSP2 and ERNs) transduce this signal to activate gene expression (15–20).
In addition to the nitrogen-fixing rhizobial symbiosis, legumes are also able to establish an interaction with beneficial arbuscular mycorrhizal fungi that aid the plant with nutrient uptake, in particular phosphate. The developmental ramifications of mycorrhizal fungal infection in the root are profoundly different from the formation of nodules and the mechanisms by which rhizobial bacteria gain entry into the plant. In addition, the profile of genes activated by mycorrhizal fungi is very different from the set of genes induced upon rhizobial inoculation (21). Despite these developmental differences, these two symbioses utilize the same symbiosis signaling (Sym) pathway, with components of the Nod factor signaling pathway playing a key role in the mycorrhizal association (1, 22); DMI2/SYMRK, DMI1/Pollux, Castor, NUP133, NUP85 and CCaMK are all components of the Sym pathway. The receptor-like kinases NFP/NFR5 and LYK3/NFR1 have nodulation specific functions as do the transcription factors NSP1, NSP2 and ERN1. This suggests that Nod factor signaling has inputs and outputs unique from the common Sym pathway, and it seems likely that mycorrhizal signaling will also have analogous mycorrhizal specific components.
Utilization of a common signaling pathway, despite differences in the developmental outcomes of these two symbioses presents a specificity problem. This is highlighted by recent work showing that the activation of CCaMK is sufficient to induce nodule formation (23, 24). CCaMK must also be activated during the mycorrhizal association and lead to early mycorrhizal responses, not nodule initiation. Hence, despite conservation of the genetic components that underpin signal transduction, there must be mechanisms that allow specific activation of CCaMK during rhizobial or mycorrhizal infection. Here we show that mycorrhizal fungi induce repetitive calcium oscillations with a signature that differs from Nod factor-induced calcium spiking. Both Nod factor and mycorrhizal fungi require DMI1 and DMI2 for activation of calcium oscillations. This indicates that these proteins or other components of the common Sym pathway must be differentially activated to produce the different calcium responses. Mathematical analysis of Nod factor-induced and mycorrhizal-induced calcium oscillations indicates that both of these responses are chaotic in nature. Chaos provides a possible mechanism for flexibility that must be an inherent component of this common Sym pathway.
Results
The Mycorrhizal-Induced Calcium Response.
To better ascertain the mechanism governing specificity in symbiosis signaling, we assessed mycorrhizal-induced calcium changes in Medicago truncatula plants transformed with the calcium reporter cameleon (25). We examined many cells on many root systems, but no calcium changes were detected in noninoculated roots (Fig. 1a), in inoculated roots where fungal structures were not immediately adjacent to the root tissues under observation (Fig. 1b), or in root cells in close association with nonsymbiotic fungal hyphae (Fig. 1c). Perception by the fungus of the plant diffusible signal strigolactone (26) leads to formation of highly branched hyphae. We found that root cells in close association with these ‘symbiotic’ fungal hyphae showed oscillatory calcium responses (Fig. 1d). Calcium oscillations were observed in a number of cells in close proximity to these plant/fungal contact points. In most cases, we could clearly see responsive cells were not in direct contact with the fungus. Measurements taken at the same sites on the following days showed that once fungal attachment to the root surface had occurred, calcium oscillations could no longer be detected (Fig. 1e). This initial work was performed on hairy root cultures, but we saw an equivalent calcium response in whole plants at an equivalent stage of mycorrhizal infection (Fig. 2a). This work indicates that mycorrhizal fungi activate calcium oscillations in M. truncatula root hair cells before direct fungal contact, and this is likely due to diffusible mycorrhizal factor(s).
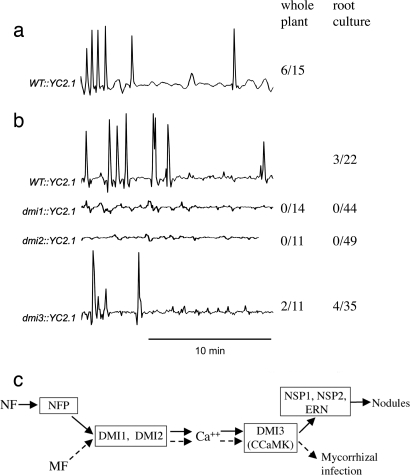
Fig. 1.
Calcium oscillations were observed in M. truncatula hairy root cultures at a specific stage during the interaction with G. intraradices. (a–c) No calcium changes were observed in root hair cells in the absence of G. intraradices (a), in a region of a G. intraradices infected root distant from the site of plant/fungal contact (b), or in regions of the root associated with G. intraradices runner hyphae (c). (d) Calcium oscillations were observed in root hair cells associated with highly branched hyphae of G. intraradices. Continued observations at sites such as d indicate that oscillations are no longer observed when the fungus attaches to the root (e) and forms an appresorium. The traces indicate calcium levels in the cell marked with an asterisk. The numbers indicate number of cells with calcium oscillations/total number of cells analyzed at each stage. The y axis is the ratio of CFP to YFP in arbitrary units.
Fig. 2.
Mycorrhizal-induced calcium oscillations require components of the Sym pathway. (a and b) Root hair cells on whole plants (a) and on root cultures (b) showed calcium oscillations when in close proximity to highly branched G. intraradices hyphae. (b) Plants mutated in dmi1 or dmi2 did not show any calcium changes in response to G. intraradices, whereas dmi3 mutants did show mycorrhizal-induced calcium oscillations. (c) The symbiosis signaling pathway of M. truncatula. DMI1, DMI2 and DMI3 are necessary for both Nod factor and mycorrhizal factor signaling, whereas NFP, NSP1, NSP2 and ERN are only required for Nod factor signaling. The y axis in a and b represents the ratio of YFP to CFP in arbitrary units. NF, Nod factor; MF, mycorrhizal factor; WT, wild type; YC2.1, cameleon. The numbers indicate numbers of cells with calcium ocillations/total numbers of cells analyzed.
Mycorrhizal-Induced Calcium Oscillations Require DMI1 and DMI2.
To ascertain whether these mycorrhizal-induced calcium oscillations were a component of the Sym pathway, we analyzed whether mycorrhizal fungi could activate calcium oscillations in dmi1, dmi2 and dmi3 mutants, which define the Sym pathway in M. truncatula. Glomus intraradices did not induce calcium oscillations in dmi1 or dmi2, but calcium oscillations similar to those observed in wild-type roots were seen in dmi3 (Fig. 2b). The responses in the mutant lines were observed in both root cultures and whole plants (Fig. 2b). Hence, mycorrhizal-induced calcium oscillations and Nod factor-induced calcium spiking function at equivalent positions in the Sym pathway (14) (Fig. 2c). The dmi1, dmi2 and dmi3 mutants are defective in mycorrhizal infection, but the fungus does form appresoria on the root surface (27), indicating that the mycorrhizal-induced calcium oscillations are likely to function in fungal infection after appresoria formation. Considering the dual nature of the Sym pathway, we assessed how a cell may respond to cotreatment with mycorrhizal fungi and Nod factor. Cells on whole plants undergoing mycorrhizal-induced calcium oscillations were treated with 1 nM Nod factor. Nod factor-induced calcium spiking was never observed in cells already responding to the mycorrhizal fungus (n = 3), whereas cells away from the site of fungal contact did show Nod factor-induced calcium spiking (n = 3).
Rhizobial Bacteria and Mycorrhizal Fungi Induce Differential Calcium Responses.
Differences in the frequency and amplitude of calcium oscillations have been linked to differences in gene expression and enzymatic activities in several systems (28). A comparison of Nod factor-induced and mycorrhizal-induced calcium responses revealed many differences (Fig. 3). The duration of a single mycorrhizal-induced calcium transient is considerably shorter than a Nod factor-induced calcium spike, and the shapes of the individual transients are also different (Fig. 3 a–c). In a manner analogous to Nod factor-induced calcium spiking (29, 30), the mycorrhizal-induced calcium oscillations are predominantly restricted to the nuclear region [supporting information (SI) Fig. S1]. Fourier analysis is a valuable method for assessing repetition within a signal by deconvoluting a signal into its frequency components and detecting periodicity (31). This and further analyses were performed on traces from which the basal noise levels (the noise present in untreated cells) had been removed. Fourier analysis revealed a highly significant periodicity in Nod factor-induced calcium spiking, with the major significant peak at 90 second intervals (Fig. 3d). In mycorrhizal-treated cells, the Fourier amplitudes were less significant and showed a major significant peak at 30 second intervals (Fig. 3d). This variation in the period may be partially attributed to harmonics recognized by the Fourier analysis. It is difficult to ascertain an exact value for the calcium transient amplitude by using cameleon. However, we used the basal fluctuations in fluorescence to compare the relative value of Nod factor-induced and mycorrhizal-induced calcium transients. This analysis suggested that mycorrhizal-induced transients were much lower in amplitude than Nod factor-induced calcium spiking; mycorrhizal calcium transients were only ≈17% of Nod factor-induced calcium spikes. It has been calculated that Nod factor-induced calcium spiking represents a calcium change of 443–669 nM (30). This suggests that the mycorrhizal-induced calcium transients produce a 75–113 nM calcium change. These analyses confirm that Nod factor, and to a lesser extent mycorrhizal fungi, activate repetitive oscillations in calcium, but the signatures of these responses are very different. These differential calcium oscillations might provide a mechanism for the observed changes in gene expression and physiological differences in response to rhizobial bacteria and mycorrhizal fungi.
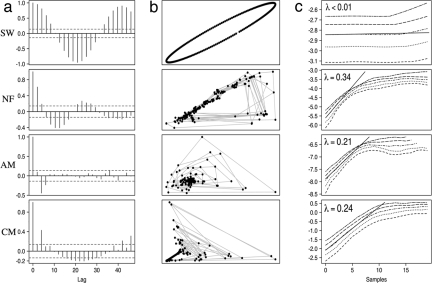
Fig. 3.
Nod factor and mycorrhizal fungi produce different calcium signatures. (a) A root hair cell showing mycorrhizal-induced calcium oscillations. (b) A root hair cell showing Nod factor-induced calcium spiking. (c) Analysis of individual transients in arbuscular mycorrhizal-induced and Nod factor-induced calcium changes. The upward phase of the spike (white) is compared with the downward phase (gray). (d) A histogram showing significant peaks from Fourier analysis of 10 Nod factor-treated cells and 10 mycorrhizal-treated cells. Components within each trace that showed significance were separated and plotted by the oscillation period. Nod factor-induced calcium changes show significance at longer periods between oscillations than mycorrhizal-induced oscillations. The y axis in a and b represents the ratio of YFP to CFP in arbitrary units. The y axis in d gives the ratio of the power of the components over the 99% significance level, which was calculated by using multitaper spectral analysis. AM, arbuscular mycorrhizal; NF, Nod factor.
To better understand the nature of Nod factor-induced and mycorrhizal-induced calcium oscillations, we performed a number of mathematical tests to measure the repetitiveness and periodicity of these two calcium responses. To screen for repeating patterns, we used autocorrelation (32) with the following controls: a sine wave to represent a linear stable system, and a mathematical model of calcium spiking to represent chaos (33). Both the Nod factor traces and the sinewave showed periodicity (Fig. 4a), with the Nod factor traces showing a decay in autocorrelation because of their unstable cycle time. Neither the chaotic model nor the mycorrhizal calcium response appeared to be periodic, although some repetition could be seen in both systems. To further ascertain the nature of the repetition, we constructed phase portraits (34) that graph each event (i.e. a calcium oscillation) on top of each other and thus provide insight into the dynamic structure of the system (Fig. 4b). During Nod factor-induced calcium spiking, the spikes return to a shifting baseline that creates a diagonal line across the trace. In contrast, mycorrhizal-induced calcium changes return to a stable baseline, creating a dark center with many data points (Fig. 4b). Nod factor-induced oscillations that deviate from the baseline are similar to each other, whereas mycorrhizal calcium oscillations are quite different from each other and do not graph on top of each other like the sine wave and to a lesser extent the Nod factor response. These tests confirm that Nod factor-induced calcium spiking is repetitive and periodic, and all of the spikes are similar. In contrast, the mycorrhizal-induced calcium oscillations differ with each repetition.
Fig. 4.
Measures of determinism and chaos in Nod factor-induced and mycorrhizal-induced calcium responses. Analysis of a sine wave, a Nod factor-induced calcium trace, a mycorrhizal-induced calcium trace, and a chaotic model of calcium spiking by using autocorrelation (a), phase portraits (b) and the maximal Lyapunov exponent (c). Autocorrelation (a) uncovers linear repeating patterns, with the y axis representing the correlation of the signal with time shifted versions of itself. Data points above and below the dotted line are significant at the 95% confidence interval. (b) For phase portraits, the value of traces at sample time points (t) on the x axis are graphed against the value at the next sample time point (t + 1) on the y axis. This provides a dynamic image of the structure of each response. The fit of a straight line to a plot of stretching factor against number of samples in the future is shown in (c). The gradient of this line gives the maximal Lyapunov exponent (λ). The line is fitted to sections of curve that show >0.999 correlation, except for the sine wave where it was reduced to 0.995. SW, sine wave; NF, Nod factor; AM, arbuscular mycorrhizal; CM, chaotic model.
Both Nod Factor-Induced and Mycorrhizal-Induced Calcium Oscillations Are Chaotic in Nature.
In mathematical terms, the random-looking, poorly periodic oscillations observed in the mycorrhizal-induced calcium response originate from either a stochastic (nondeterministic and random) or a chaotic (deterministic and predictable) system (35). Stochastic behavior is nondeterministic in that precise knowledge of the state of the system is not sufficient to determine the next state; no underlying law exists, and the outcome is random and unpredictable. In contrast, chaotic behavior originates from deterministic systems, which implies that a well-defined law governs the evolution of the system and each state is sufficient to determine the next. However, sensitivity to the initial condition in chaotic systems means that tiny differences can have drastic effects, thus limiting predictability to a short time-scale. Importantly, chaotic systems are able to produce an infinite number of dynamic behaviors, both periodic and nonperiodic, in nature (36). A common feature of determinism is that one state of the system is similar to a previous state, and this can be measured mathematically by using a number of tests, including recurrence plots (37). Analysis revealed recurrent behavior, suggestive of determinism, in Nod factor-induced calcium spiking and to a lesser extent in mycorrhizal-induced calcium changes (Fig. S2). Additional tests for determinism have been defined by Aparicio (37). Applying these, we found significant evidence for determinism in all Nod factor-treated and mycorrhizal-treated cells analyzed (Table 1). These tests indicate that both Nod factor-induced and mycorrhizal-induced calcium signals are deterministic, suggesting that the mycorrhizal response results from chaotic, rather than stochastic, behavior.
Table 1.
Mathematical tests performed on fluorescence traces from individual cells treated with Nod factor or arbuscular mycorrhizal fungi
| Cell treatment | Length of trace, min | Determinism test | Barahona test | Lyapunov exponent |
|---|---|---|---|---|
| None | 27.17 | + | − | − |
| None | 27.17 | − | − | 0.13 |
| None | 16.67 | + | − | 0.11 |
| None | 66.42 | − | − | − |
| None | 66.42 | + | − | − |
| None | 38.33 | + | − | − |
| AM | 17.92 | + | + | 0.26 |
| AM | 25.17 | + | + | 0.17 |
| AM | 26.00 | + | + | 0.33 |
| AM | 20.33 | + | + | 0.25 |
| AM | 19.83 | + | + | 0.23 |
| AM | 16.83 | + | + | 0.17 |
| AM | 16.83 | + | + | 0.21 |
| AM | 22.58 | + | + | 0.14 |
| AM | 51.17 | + | + | − |
| AM | 20.58 | + | + | 0.14 |
| NF | 45.08 | + | + | 0.32 |
| NF | 125.08 | + | + | 0.39 |
| NF | 66.75 | + | + | 0.34 |
| NF | 66.75 | + | − | 0.31 |
| NF | 36.58 | + | + | 0.30 |
| NF | 50.00 | + | + | 0.33 |
| NF | 20.67 | + | + | − |
| NF | 22.92 | + | + | 0.34 |
NF, Nod factor; AM, arbuscular mycorrhizal.
The maximal Lyapunov exponent is the accepted measure for chaotic systems (34). Stable or stochastic oscillations have a zero exponent, whereas chaotic systems show positive Lyapunov exponents. The use of this measure is widespread and has been used to reveal chaotic behavior in bacterial population dynamics (38), as well as economical and climatological systems (39, 40). We analyzed 10 cells undergoing a mycorrhizal calcium response and found that 9 of the 10 cells showed positive Lyapunov exponents, indicating chaotic behavior (Fig. 4c and Table 1). Surprisingly, we also found evidence for chaotic behavior in 7 of 8 cells undergoing Nod factor-induced calcium spiking (Fig. 4c and Table 1). Hence, the somewhat noisy periodic Nod factor signals represent near-periodic chaotic behavior. The detection of chaos implies that both Nod factor-induced and mycorrhizal-induced calcium responses originate from nonlinear systems. By using the Barahona test (41), we found clear evidence for nonlinearity in all mycorrhizal-treated cells and 7 of 8 Nod factor-treated cells (Table 1). Thus, the tests for determinism, the Lyapunov exponents and indicators of nonlinearity strongly suggest that both Nod factor-induced and mycorrhizal-induced calcium responses are chaotic in nature.
Discussion
Legumes use a common Sym pathway for the establishment of both rhizobial and mycorrhizal symbioses. Despite conservation in signal transduction, specificity must be maintained to allow for the appropriate responses to the symbiotic microorganism. We have shown that mycorrhizal fungi activate calcium oscillations in M. truncatula root hair cells and that this requires DMI1 and DMI2, components of the Sym pathway that are also necessary for Nod factor-induced calcium spiking. A comparison between Nod factor-induced calcium spiking and mycorrhizal-induced calcium oscillations reveals significant differences between these two calcium signatures. Hence, despite conservation in the genetic components underpinning symbiosis signaling, mycorrhizal fungi and rhizobial bacteria both activate this signaling pathway. Therefore, there must be an inherent flexibility in the action of the Sym pathway that allows differential responses. This may function at DMI1, DMI2 or other as yet unidentified components. Some of the differences we have observed may be caused by differences in experimental approaches: Nod factor treatment versus fungal infection of the plant. However, treatment of cells with rhizobial bacteria gives a response comparable to treatment with Nod factor (42), indicating that there are still differences between rhizobial-induced and mycorrhizal-induced calcium oscillations. However, it will be interesting to compare Nod factor-induced calcium spiking to the calcium response induced by isolated mycorrhizal factor, when this becomes available.
We have shown that mycorrhizal fungi activate an oscillatory calcium response before direct contact with the plant. Mutations in DMI1 and DMI2 abolish both mycorrhizal infection (27) and mycorrhizal-induced calcium oscillations. This correlation indicates that the calcium oscillations are necessary for the invasion of the plant by the mycorrhizal fungus. The dmi1, dmi2 and dmi3 mutants show fungal attachment to the surface of the root and the formation of appresoria, but no fungal infection of epidermal cells. In rare cases, fungal infection is successful in dmi1 and dmi2, and when this happens, extensive fungal colonization of the root cortex occurs with no apparent defects in arbuscule formation (27). This suggests that the Sym pathway is only necessary for fungal infection of the root epidermis, and once this has occurred there is no further need for the Sym pathway during fungal colonization of the root cortex. Thus, we can infer the function of the mycorrhizal-induced calcium oscillations. A diffusible mycorrhizal factor is responsible for the induction of calcium oscillations before direct fungal contact with the root. These oscillations are likely to prime epidermal root cells for fungal colonization. In the absence of this function, the fungus is unable to gain entry to epidermal cells.
Nod factor-induced and mycorrhizal-induced calcium oscillations are near-periodic and chaotic in nature. Processes that appear to be periodic can be inherently chaotic, for instance the beat-to-beat intervals of the human heart (43). Interestingly, patients suffering from congestive heart failure show a reduction in the chaotic nature of their heart beat, indicating that a too stable heart rate is associated with disease, because rigid periodicity is less able to adapt to differing physiological conditions (41). In a similar fashion, we have found chaotic behavior in rhythmic responses, Nod factor-induced and mycorrhizal-induced calcium oscillations. This indicates that although there is measurable and significant periodicity in these responses, the spike duration is sufficiently varied to indicate chaos. This suggests that activity of the calcium channel(s) that underpin these calcium responses is inherently chaotic, which has been predicted by mathematical models of calcium oscillations induced by calcium-induced calcium release (44). In this model, Haberichter et al. (44) found that varying a single parameter, the endoplasmic reticulum calcium channel, could switch the mode of calcium oscillations from stable and rhythmic to chaotic. In support of this model, neuronal cells have been shown to oscillate calcium chaotically under certain conditions (45).
Biological systems are generally considered nonchaotic, with a single major mode of action. However, such linear systems are trapped in a single activity, with a large amount of energy required to shift them into an alternative state, the equivalent of kicking a ball out of a bowl. In contrast, chaotic systems can produce extremely variable responses, because of their sensitivity to perturbations. Chaotic systems can be made to respond differently with very little energetic input, the equivalent of kicking a ball around a plate. Hence, chaotic systems provide a means for flexibility. However, this is not the only mechanism by which flexibility can be generated. Our work reveals that a single signal transduction pathway has the capacity to produce two different outputs, both of which are chaotic in nature. This indicates that mycorrhizal fungi and Nod factor can differentially activate the same proteins in the Sym pathway but produce significantly different effects on the calcium response. The inherent nonlinear nature of the calcium machinery within the Sym pathway ensures that small changes in the mode of activation can lead to major differences in the response.
We have shown that Nod factor and mycorrhizal fungi can induce differential calcium responses by using the same signal transduction pathway. These differential calcium responses provide a possible mechanism for linking specific activation of CCaMK to discreet downstream responses. We cannot currently conclude that the differential calcium responses encode specificity. However, they provide an attractive model, and defining how CCaMK can respond to these chaotic calcium responses will give insight into the nature of specificity in this signaling pathway. We can conclude that differential activation of DMI1, DMI2 or other components in the Sym pathway leads to alternative outcomes with regard to the nature of the calcium response, and we propose that the inherent chaotic behavior provides the necessary flexibility for this differential activation. We believe that chaotic behavior is likely to be a common feature of signal transduction, and chaos permits greater flexibility in the regulation of protein activity than either stochastic or stable systems, allowing differential responses in multifunctional signaling pathways. In 1907, well before mathematical adoption of the term ‘chaos,’ the American historian Henry Adams said “chaos often breeds life, when order breeds habit” (46). Biological systems require flexibility, and as such, chaos is preferable to habitual behavior.
Materials and Methods
Measuring Calcium.
Hairy roots of cameleon YC2.1-expressing M. truncatula R108-1 line Mtyc21-2-14 (25) were produced by using Agrobacterium rhizogenes Arqua1 (47) and subcultured weekly on M medium (48) with 0.5% Phytagel (Sigma, St. Louis). The apical region (3–4 cm) of fast-growing hairy roots was axenically subcultured to the fungal mat portion of G. intraradices-carrot hairy root co-cultures on M medium, propped vertically and incubated in the dark at 24°C for 2–6 days. Roots were examined daily using a Leica stereo-microscope to identify sites where fungal-root contact might occur. For whole plant analysis, overnight germinated seedlings were transferred to G. intraradices-carrot hairy root co-cultures and treated as described for the hairy roots. For observation, a 10-cm × 10-cm block of solid medium containing roots and fungus was removed from the Petri dish so that the fine hyphal network surrounding the roots was not disturbed. The block was wrapped in cling film, inverted and placed on the microscope stage to settle for 20 min before observation. Cameleon was imaged on a Nikon TE2000U inverted microscope as described in ref. 25.
Mathematical Analyses.
Most traces had a low frequency component caused by movement of the root hair, movement of the nucleus and photobleaching. The low frequency component was removed and the traces de-trended by keeping the residuals after applying either a 19- or 25-point moving average (32). Traces were made stationary by shortening the time series until they appeared stationary in a recurrence plot and passed a statistical test for nonstationarity (49). Traces smaller than 200 data points were then rejected as it was possible to get a positive Lyapunov exponent from a time series generated by a linear autoregressive model fitted to traces smaller than 200 data points. Simple nonlinear noise reduction was applied to the traces (50). The level of the noise reduction, controlled by the neighborhood size, was particular to each trace and was chosen based on autocorrelation and cross-correlation of the obtained signal and noise components. If the noise component showed a slowly decaying autocorrelation, or significant correlation with the signal, then a smaller neighborhood size was used.
Autocorrelation plots and phase portraits were generated within the R statistical environment. Fourier analysis was performed using the multitaper technique (31, 51) as implemented by the SSA-MTM toolkit (52). This form of analysis estimates the background power spectrum and provides a 99% significance level for frequency components based on a red noise model. The value of peaks in the spectrum whose value exceeded that of the significance level was divided by the significance level. The peaks were separated into frequency ranges, which were chosen to highlight interesting areas of the spectrum. The mean ratio of the peak over significance level was then plotted for each frequency range (Fig. 3e).
Three tests for determinism (37) were run on 1 dimensional recurrence plots of the time series. A recurrence was identified at 0.5 times the standard deviation, or in cases where this produced more recurrences than non-recurrence, the tolerance was set to 0.1 times the standard deviation. The null hypothesis of the test is that a random process generates the recurrence plot. A trace was considered deterministic if P values <0.01 were obtained from each of the three tests. The “Barahona” test for nonlinearity (41) is based on a Volterra-Weiner series, which can be fitted either linearly or nonlinearly. If a nonlinear fit was more accurate than a linear fit, and the ratio of normalized residuals was significant in an F test with a P value of <0.05, the test was considered as positive.
In preparation for estimating the maximal Lyapunov exponent, space time separation plots were used to get a Theiler window for each time series. The window sizes were used as parameter values to generate plots of stretching factor against time span. The plot was done in a set of embedding dimensions starting at 4 and ending in 8. A straight line was fitted to the curve, generated in dimension 6. This line was only fitted, and a maximal Lyapunov exponent reported from the gradient, if the section of curve being fitted had a correlation >0.999. Checks were made to confirm the linear trend was also evident in the neighboring dimensions. The analysis was performed in the R statistical environment by using the tseriesChaos library and the RTisean library that wraps functionality of the Tisean nonlinear time series analysis package (version 3.01).
Supplementary Material
Acknowledgments.
This work was supported by a Biotechnology and Biological Sciences Research Council David Phillips Fellowship and a Royal Society Wolfson Research Merit award to G.O., and a grant in aid from the Biotechnology and Biological Sciences Research Council to G.O., R.M., and J.A.D.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803499105/DCSupplemental.
References
- 1.Oldroyd GE, Downie JA. Nuclear calcium changes at the core of symbiosis signalling. Curr Opin Plant Biol. 2006;9:351–357. doi: 10.1016/j.pbi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Arrighi JF, et al. The Medicago truncatula lysine motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiol. 2006;142:265–279. doi: 10.1104/pp.106.084657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Limpens E, et al. LysM domain receptor kinases regulating rhizobial Nod factor-induced infection. Science. 2003;302:630–633. doi: 10.1126/science.1090074. [DOI] [PubMed] [Google Scholar]
- 4.Madsen EB, et al. A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature. 2003;425:637–640. doi: 10.1038/nature02045. [DOI] [PubMed] [Google Scholar]
- 5.Radutoiu S, et al. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature. 2003;425:585–592. doi: 10.1038/nature02039. [DOI] [PubMed] [Google Scholar]
- 6.Radutoiu S, et al. LysM domains mediate lipochitin-oligosaccharide recognition and Nfr genes extend the symbiotic host range. EMBO J. 2007;26:3923–3935. doi: 10.1038/sj.emboj.7601826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ane JM, et al. Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science. 2004;303:1364–1367. doi: 10.1126/science.1092986. [DOI] [PubMed] [Google Scholar]
- 8.Endre G, et al. A receptor kinase gene regulating symbiotic nodule development. Nature. 2002;417:962–966. doi: 10.1038/nature00842. [DOI] [PubMed] [Google Scholar]
- 9.Imaizumi-Anraku H, et al. Plastid proteins crucial for symbiotic fungal and bacterial entry into plant roots. Nature. 2005;433:527–531. doi: 10.1038/nature03237. [DOI] [PubMed] [Google Scholar]
- 10.Kanamori N, et al. A nucleoporin is required for induction of Ca2+ spiking in legume nodule development and essential for rhizobial and fungal symbiosis. Proc Natl Acad Sci USA. 2006;103:359–364. doi: 10.1073/pnas.0508883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miwa H, Sun J, Oldroyd GE, Downie JA. Analysis of Nod-factor-induced calcium signaling in root hairs of symbiotically defective mutants of Lotus japonicus. Mol Plant Microbe Interact. 2006;19:914–923. doi: 10.1094/MPMI-19-0914. [DOI] [PubMed] [Google Scholar]
- 12.Saito K, et al. NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses, and seed production in Lotus japonicus. Plant Cell. 2007;19:610–624. doi: 10.1105/tpc.106.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schauser L, Roussis A, Stiller J, Stougaard J. A plant regulator controlling development of symbiotic root nodules. Nature. 1999;402:191–195. doi: 10.1038/46058. [DOI] [PubMed] [Google Scholar]
- 14.Wais RJ, et al. Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula. Proc Natl Acad Sci USA. 2000;97:13407–13412. doi: 10.1073/pnas.230439797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andriankaja A, et al. AP2-ERF transcription factors mediate Nod factor-dependent MtENOD11 activation in root hairs via a novel cis-regulatory motif. Plant Cell. 2007;19:2866–2885. doi: 10.1105/tpc.107.052944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalo P, et al. Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science. 2005;308:1786–1789. doi: 10.1126/science.1110951. [DOI] [PubMed] [Google Scholar]
- 17.Levy J, et al. A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science. 2004;303:1361–1364. doi: 10.1126/science.1093038. [DOI] [PubMed] [Google Scholar]
- 18.Middleton PH, et al. An ERF transcription factor in Medicago truncatula that is essential for Nod factor signal transduction. Plant Cell. 2007;19:1221–1234. doi: 10.1105/tpc.106.048264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitra RM, et al. A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development: Gene identification by transcript-based cloning. Proc Natl Acad Sci USA. 2004;101:4701–4705. doi: 10.1073/pnas.0400595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smit P, et al. NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science. 2005;308:1789–1791. doi: 10.1126/science.1111025. [DOI] [PubMed] [Google Scholar]
- 21.Kistner C, et al. Seven Lotus japonicus genes required for transcriptional reprogramming of the root during fungal and bacterial symbiosis. Plant Cell. 2005;17:2217–2229. doi: 10.1105/tpc.105.032714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oldroyd GE, Downie JA. Calcium, kinases and nodulation signalling in legumes. Nat Rev Mol Cell Biol. 2004;5:566–576. doi: 10.1038/nrm1424. [DOI] [PubMed] [Google Scholar]
- 23.Gleason C, et al. Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature. 2006;441:1149–1152. doi: 10.1038/nature04812. [DOI] [PubMed] [Google Scholar]
- 24.Tirichine L, James EK, Sandal N, Stougaard J. Spontaneous root-nodule formation in the model legume Lotus japonicus: A novel class of mutants nodulates in the absence of rhizobia. Mol Plant Microbe Interact. 2006;19:373–382. doi: 10.1094/MPMI-19-0373. [DOI] [PubMed] [Google Scholar]
- 25.Miwa H, Sun J, Oldroyd G, Downie A. Analysis of calcium spiking using a cameleon calcium sensor reveals that nodulation gene expression is regulated by calcium spike number and the developmental status of the cell. The Plant J. 2006;48:883–894. doi: 10.1111/j.1365-313X.2006.02926.x. [DOI] [PubMed] [Google Scholar]
- 26.Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 27.Catoira R, et al. Four genes of Medicago truncatula controlling components of a Nod factor transduction pathway. Plant Cell. 2000;12:1647–1665. doi: 10.1105/tpc.12.9.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kummer U, et al. Transition from stochastic to deterministic behavior in calcium oscillations. Biophys J. 2005;89:1603–1611. doi: 10.1529/biophysj.104.057216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw SL, Long SR. Nod factor elicits two separable calcium responses in Medicago truncatula root hair cells. Plant Physiol. 2003;131:976–984. doi: 10.1104/pp.005546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehrhardt DW, Wais R, Long SR. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 1996;85:673–681. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- 31.Thomson DJ. Spectrum estimation and harmonic analysis. Proc. IEEE. 1982;70:1055–1096. [Google Scholar]
- 32.Brockwell PJ, Davis RA. Chapter 1. Introduction to Time Series and Forecasting. New York: Springer; 2002. [Google Scholar]
- 33.Kummer U, et al. Switching from simple to complex oscillations in calcium signaling. Biophys J. 2000;79:1188–1195. doi: 10.1016/S0006-3495(00)76373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kantz H, Schreiber T. Chapter 1. Nonlinear Time Series Analysis. New York: Cambridge Univ Press; 1997. [Google Scholar]
- 35.Casdagli M. Chaos and deterministic versus stochastic non-linear modelling. J R Stat Soc B. 1992;54:303–328. [Google Scholar]
- 36.Grebogi C, Ott E, Yorke JA. Unstable periodic orbits and the dimensions of multifractal chaotic attractors. Phys Rev A. 1988;37:1711–1724. doi: 10.1103/physreva.37.1711. [DOI] [PubMed] [Google Scholar]
- 37.Aparicio T, Pozo EF, Saura D. Detecting determinism using recurrence quantification analysis: Three test procedures. J Econ Behav Organ. 2008;65:768–787. [Google Scholar]
- 38.Becks L, Hilker FM, Malchow H, Jurgens K, Arndt H. Experimental demonstration of chaos in a microbial food web. Nature. 2005;435:1226–1229. doi: 10.1038/nature03627. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Chen G, Lu J. Complex dynamical behaviors of daily data series in stock exchange. Phys Lett A. 2004;333:246–255. [Google Scholar]
- 40.Tiwari RK, Rao KNN. Phase space structure, attractor dimension, Lyapunov exponent and nonlinear prediction from Earth's atmospheric angular momentum time series. Pure Appl Geophys. 1999;156:719–736. [Google Scholar]
- 41.Barahona M, Poon C-S. Detection of nonlinear dynamics in short, noisy time series. Nature. 1996;381:215–217. [Google Scholar]
- 42.Wais R, Wells D, Long S. Analysis of differences between Sinorhizobium meliloti 1021 and 2011 strains using the host calcium spiking response. Mol Plant–Microbe Interact. 2002;15:1245–1252. doi: 10.1094/MPMI.2002.15.12.1245. [DOI] [PubMed] [Google Scholar]
- 43.Poon C-S, Merrill CK. Decrease of cardiac chaos in congestive heart failure. Nature. 1997;389:492–495. doi: 10.1038/39043. [DOI] [PubMed] [Google Scholar]
- 44.Haberichter T, Marhl M, Heinrich R. Birhythmicity, trirhythmicity and chaos in bursting calcium oscillations. Biophys Chem. 2001;90:17–30. doi: 10.1016/s0301-4622(01)00127-2. [DOI] [PubMed] [Google Scholar]
- 45.Korn H, Faure P. Is there chaos in the brain? II. Experimental evidence and related models. C R Biol. 2003;326:787–840. doi: 10.1016/j.crvi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Adams H. Chapter 16. Education of Henry Adams: An Autobiography. New York: Houghton and Mifflin Company; 1918. [Google Scholar]
- 47.Boisson-Dernier A, et al. Agrobacterium rhizogenes-transformed roots of Medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Mol Plant–Microbe Interact. 2001;14:695–700. doi: 10.1094/MPMI.2001.14.6.695. [DOI] [PubMed] [Google Scholar]
- 48.Becard G, Fortin JA. Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol. 1988;108:211–218. doi: 10.1111/j.1469-8137.1988.tb03698.x. [DOI] [PubMed] [Google Scholar]
- 49.Kennel MB. Statistical test for dynamical nonstationarity in observed time-series data. Phys Rev E. 1997;56:316–321. [Google Scholar]
- 50.Schreiber T. Extremely simple nonlinear noise-reduction method. Phys Rev E. 1993;47:2401–2404. doi: 10.1103/physreve.47.2401. [DOI] [PubMed] [Google Scholar]
- 51.Mann ME, Lees JM. Robust estimation of background noise and signal detection in climatic time series. Climatic Change. 1996;33:409–445. [Google Scholar]
- 52.Ghil M, et al. Advanced Spectral Methods for Climatic Time Series. Reviews of Geophysics. 2002;40:1–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.