Abstract
A noteworthy aspect of melanoma differentiation-associated gene-7/interleukin-24 (mda-7/IL-24) as a cancer therapeutic is its ability to selectively kill cancer cells without harming normal cells. Intracellular MDA-7/IL-24 protein, generated from an adenovirus expressing mda-7/IL-24 (Ad.mda-7), induces cancer-specific apoptosis by inducing an endoplasmic reticulum (ER) stress response. Secreted MDA-7/IL-24 protein, generated from cells infected with Ad.mda-7, induces growth inhibition and apoptosis in surrounding noninfected cancer cells but not in normal cells, thus exerting an anti-tumor “bystander” effect. The present studies reveal a provocative finding that recombinant MDA-7/IL-24 protein can robustly induce expression of endogenous mda-7/IL-24, which generates the signaling events necessary for bystander killing. To evaluate the mechanism underlying this positive autocrine feedback loop, we show that MDA-7/IL-24 protein induces stabilization of its own mRNA without activating its promoter. Furthermore, this posttranscriptional effect depends on de novo protein synthesis. As a consequence of this autocrine feedback loop MDA-7/IL-24 protein induces sustained ER stress as evidenced by expression of ER stress markers (BiP/GRP78, GRP94, GADD153, and phospho-eIF2α) and reactive oxygen species production, indicating that both intracellular and secreted proteins activate similar signaling pathways to induce apoptosis. Thus, our results clarify the molecular mechanism by which secreted MDA-7/IL-24 protein (generated from Ad.mda-7-infected cells) exerts cancer-specific killing.
Keywords: bystander antitumor activity, endoplasmic reticulum stress, reactive oxygen species, mRNA stabalization, cancer-specific killing
Melanoma differentiation-associated gene-7 (mda-7) was first identified as a gene associated with terminal differentiation of metastatic human melanoma cells (1, 2). Based on its structure, chromosomal location, and biochemical properties, mda-7 has been classified as a member of the IL-10 family of cytokines that includes IL-10, IL-19, IL-20, IL-22, and IL-26 and has been redesignated IL-24 (3, 4). When expressed at supraphysiological levels, by means of an adenoviral (Ad) expression system (Ad.mda-7), MDA-7/IL-24 induces growth suppression and apoptosis in a broad spectrum of human cancer cells, including those from melanoma, malignant glioma, fibrosarcoma, and carcinomas of the breast, cervix, colon, rectum, liver, lung, ovary, and prostate, without exerting any deleterious effects on their normal counterparts (5–13). A phase I trial evaluating Ad.mda-7 (INGN 241) activity by intratumoral injection in patients with advanced solid tumors was performed, indicating that mda-7/IL-24 was safe and could induce as much as 70% apoptosis in tumors after a single injection of recombinant virus and that multiple injections elicited clinical responses (14–16). The successful transition of Ad.mda-7 into the clinic in a phase I clinical trial reinforces the hypothesis that mda-7/IL-24 is safe and affords remarkable potential as a cancer gene therapeutic. Moreover, secreted MDA-7/IL-24 protein, generated from Ad.mda-7-infected cells, promotes antiangiogenic, immunostimulatory, radiosensitizing and “bystander” antitumor activities (6, 11, 17, 18).
mda-7/IL-24 expression is detected in human tissues and cells associated with the immune system such as spleen, thymus, peripheral blood leukocytes, and normal melanocytes (19). Secreted MDA-7/IL-24 stimulates monocytes and specific populations of T lymphocytes and promotes proinflammatory cytokine production. When expressed at low, presumably physiological levels, MDA-7/IL-24 binds to currently recognized MDA-7/IL-24 receptor complexes consisting of two sets of heterodimeric chains, IL-20R1/IL-20R2 or IL-22R1/IL-20R2 (20–22). Most human tissues express the IL-20R1/IL-20R2 complex. IL-22R is found in a few tissues lacking IL-20R2, such as adult and fetal liver, colon, small intestine, and pancreas. A functional set of cell surface receptors can also be found in the majority of human tumor cells (23). Upon ligand binding, both receptors induce activation of STAT3 (20–22). However, our previous studies demonstrate that activation of the JAK/STAT pathway is dispensable for Ad.mda-7-induced apoptosis, and cell death triggered by intracellular MDA-7/IL-24 protein occurs through a receptor-independent mechanism (23). Intracellular MDA-7/IL-24 localizes to the endoplasmic reticulum (ER) and induces an ER stress response, also known as unfolded protein response (UPR), thus eliciting tumor-specific apoptosis (24).
A highly conserved UPR signal transduction pathway is activated by ER stress caused by misfolded protein accumulation (25). The UPR can be triggered by unfolding proteins in the lumen of the ER, resulting in de novo synthesis of ER proteins (such as the “glucose-regulated proteins” BiP/GRP78 and GRP94) that assist in protein folding. Cell death is an inevitable consequence of persistent ER stress, and ER stress can lead to apoptosis through both mitochondria-dependent and -independent pathways. Production and integration of apoptotic signals may occur in the ER, generating the death response. Mechanisms involved in this process include PERK/α-subunit of eukaryotic translation initiation factor-2 (eIF2α)-dependent induction of the proapoptotic transcription factor GADD153, BAK/BAX-regulated Ca2+ release from the ER, and cleavage and activation of procaspase 12 (25).
Ad.mda-7 infection of cancer cells induces growth arrest and DNA damage-inducible (GADD) gene family, classically associated with the stress response, including the ER stress pathways (9, 26). Induction of GADD genes and further upstream events such as activation of p38MAPK and PKR are promoted by mda-7/IL-24 in a transformed cell-specific manner, and these events occur independently of the glycosylation of MDA-7/IL-24. Ad.mda-7 also specifically activates the p44/42MAPK pathway and up-regulates the inositol 1,4,5-trisphosphate receptor (IP3R) in H1299 cells (27). IP3R is an intracellular calcium-release channel implicated in apoptosis and localized in the ER. Hsp70-like chaperone (BiP/GRP78)-binding sites are present in helices C and F of MDA-7/IL-24, and mutations in these sites prevent this cytokine from inducing cancer cell-specific apoptosis (17). Additionally, a microarray study indicated that mda-7/IL-24 is able to induce the expression of ER stress response genes, including BiP/GRP78. Overall, these findings suggest a series of events mediated by MDA-7/IL-24 that promote apoptosis by up-regulation of specific signal transduction pathways and gene products involved in the ER stress response. MDA-7/IL-24 also generates reactive oxygen species (ROS) in the mitochondria (8, 12, 28).
Secreted MDA-7/IL-24 protein, generated from Ad.mda-7-infected cells, induces growth inhibition and apoptosis in surrounding noninfected cancer cells, but not in normal cells, thus exerting a cancer-specific bystander effect (12, 18, 28). We showed that conditioned medium generated from Ad.mda-7-infected cells but not from Ad.SP−mda-7 (lacking the signal peptide) display the bystander effect (24). These results indicate that the bystander effect is likely caused by secreted MDA-7/IL-24 protein and not by other secreted factor(s). It has been demonstrated that partially purified MDA-7/IL-24 protein, obtained from supernatants of HEK293 cells, induces apoptosis only in cancer cells in a receptor-dependent and STAT3-independent manner (29). A recent study demonstrated that purified MDA-7/IL-24 protein induces cell death in melanoma cells (29). However, a detailed analysis of the molecular mechanism underlying this effect was not performed. Understanding these significant properties of MDA-7/IL-24 will facilitate the development of rational combinatorial approaches with potential to enhance therapeutic activity.
We have now used a recombinant bioactive MDA-7/IL-24 protein to delineate the mechanism mediating the bystander effect. We show that purified MDA-7/IL-24 protein induces a robust expression of endogenous mda-7/IL-24 and subsequent induction of tumor-specific killing through an ER stress-mediated pathway as well as by ROS production. Furthermore, we demonstrate that the molecular mechanism of the cancer-specific killing effect observed in Ad.mda-7-infected cells involves the same pathways as those triggered by secreted MDA-7/IL-24 protein. These findings provide a unified mechanism of mda-7/IL-24 action evoking apoptosis selectively in cancer cells.
Results
Recombinant MDA-7/IL-24 Protein Induces Endogenous mda-7/IL-24 Expression.
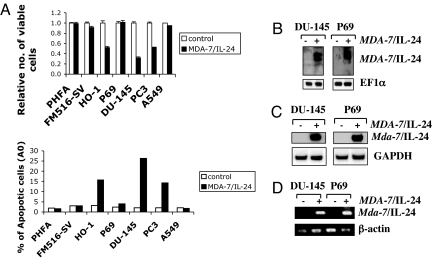
To understand the molecular mechanism by which MDA-7/IL-24 protein induces apoptosis, we purified recombinant MDA-7/IL-24 protein and treated diverse normal and cancer cells and evaluated cell viability and induction of apoptosis. Normal cells included early passage (<5) primary human fetal astrocytes (PHFA), immortal human melanocytes (FM516-SV), and immortal human prostate epithelial cells (P69). Human melanoma cells HO-1, prostate cancer cells DU-145 and PC-3, and lung carcinoma cells A549 were used as representative cancer cells. Recombinant MDA-7/IL-24 protein (20 ng/ml) decreased viability and induced apoptosis in susceptible tumor cell lines (HO-1, DU-145, and PC-3) without affecting the viability of comparable normal cells (Fig. 1A). MDA-7/IL-24 protein effectively inhibited growth and induced apoptosis in PC-3 cells lacking STAT3, indicating that the effect of MDA-7/IL-24 is STAT3-independent. Additionally, MDA-7/IL-24 protein did not affect A549 cells that lack cognate MDA-7/IL-24 receptors, IL-20R2 and IL-22R1 (11, 29), indicating that the response is mediated by binding of MDA-7/IL-24 to its cognate receptors.
Fig. 1.
MDA-7/IL-24 protein induces expression of endogenous MDA-7/IL-24 and growth-inhibitory effects. (A) Growth-inhibitory effects of recombinant MDA-7/IL-24 protein in different tumor cell lines. (Upper) Cells were treated with MDA-7/IL-24 protein, and cell viability was determined by the MTT proliferation assay 4 days after treatment. Numbers represent a ratio of specific treatments versus values in untreated cells. An average of three independent experiments is shown ± SD. (Lower) Cells were treated as described in Upper, and the percentage of cells displaying hypodiploidy (Ao), a measure of apoptosis, was determined 24 h later by FACS analysis using the CellQuest software (Becton Dickinson). (B) Endogenous induction of MDA-7/IL-24 protein after treatment with MDA-7/IL-24. DU-145 and P69 cells were treated with MDA-7/IL-24 protein for 48 h, and cell lysates were collected. Samples were run on 12% SDS/PAGE, transferred onto nitrocellulose membranes, and stained with anti-MDA-7/IL-24 or anti-EF1α antibodies. (C) DU-145 and P69 cells were treated with MDA-7/IL-24 for 48 h, and expression of the indicated mRNAs was analyzed by Northern blotting. (D) DU-145 and P69 cells were treated with MDA-7/IL-24 protein for 48 h. Total RNA was isolated from the cells. RT-PCR was performed with mda-7/IL-24 and β-actin-specific primers.
We next analyzed whether MDA-7/IL-24 affects the expression of endogenous mda-7/IL-24 (18, 24). DU-145 and P69 cells were treated with MDA-7/IL-24 protein (15 ng/ml) for 48 h, and expression of MDA-7/IL-24 protein was analyzed by Western blotting using anti-MDA-7/IL-24 antibody (Fig. 1B). MDA-7/IL-24 induced production of endogenous MDA-7/IL-24 protein (Fig. 1B). These findings were confirmed at an mRNA level by Northern blot analysis and by RT-PCR (Fig. 1 C and D, respectively). MDA-7/IL-24 protein treatment for 48 h augmented mda-7/IL-24 mRNA expression in both DU-145 and P69 cells without affecting the expression of housekeeping genes GAPDH and β-actin. These findings suggest that exogenous MDA-7/IL-24 protein induces endogenous mda-7/IL-24 expression in normal and cancer cells.
Autocrine Feedback Loop Is Required for Growth-Inhibitory effect of MDA-7/IL-24.
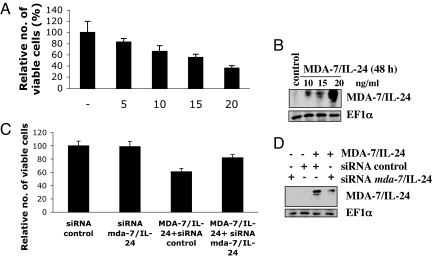
A direct correlation was evident with level of induction of endogenous MDA-7/IL-24 protein and reduction in tumor cell viability upon MDA-7/IL-24 treatment (Fig. 2 A and B). Exposing DU-145 cells to increasing concentrations of MDA-7/IL-24 resulted in a nonlinear dose-dependent induction of endogenous MDA-7/IL-24 protein. Similarly, MDA-7/IL-24 exposure also reduced viability of DU-145 cells in a concentration-dependent manner. To assess the importance of autoregulation of mda-7/IL-24 in mediating growth-inhibitory activity, an siRNA approach was used to knock down expression of MDA-7/IL-24. HeLa cells were chosen for these studies because of high transient transfection efficiency. HeLa cells were transiently transfected with mda-7/IL-24 siRNA or control siRNA and then challenged with recombinant MDA-7/IL-24 protein (10 ng/ml). Western blotting confirmed the ability of mda-7/IL-24 siRNA to knock down protein expression (Fig. 2D). A significant reduction in the MDA-7/IL-24 protein level was observed with mda-7/IL-24 siRNA but not with control siRNA. As a corollary, mda-7/IL-24 siRNA, but not control siRNA, significantly protected cancer cells from MDA-7/IL-24-induced reduction in viability, indicating a direct correlation between endogenous expression of MDA-7/IL-24 and recombinant MDA-7/IL-24-mediated killing (Fig. 2C).
Fig. 2.
Induction of endogenous mda-7/IL-24 is required for growth-inhibitory activity of recombinant MDA-7/IL-24. (A) DU-145 cells were treated with MDA-7/IL-24 (5, 10, 15, 20 ng/ml) for 72 h, and cell viability was evaluated by standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. (B) DU-145 cells were treated with MDA-7/IL-24 (5, 10, 15, 20 ng/ml) protein for 48 h, and expression of MDA-7/IL-24 and EF-1α protein was analyzed by Western blotting. (C) HeLa cell viability assay showing a significant decrease in the number of dead cells in cultures treated with mda-7/IL-24 siRNA plus MDA-7/IL-24 compared with MDA-7/IL-24 plus control siRNA. (D) HeLa cells were transfected with control or mda-7/IL-24 siRNA (100 nmol/liter) and untreated or treated with recombinant MDA-7/IL-24 protein, and expression of MDA-7/IL-24 protein was analyzed by Western blotting 48 h later.
Recombinant MDA-7/IL-24 Increases mda-7/IL-24 mRNA Stability.
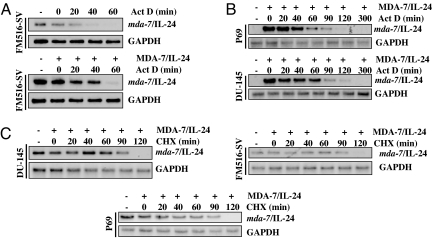
To explore the molecular mechanism by which MDA-7/IL-24 protein induces endogenous mda-7/IL-24 expression, we analyzed mda-7/IL-24 promoter function and mda-7/IL-24 mRNA stability because other known inducers of mda-7/IL-24 work predominantly by stabilizing mda-7/IL-24 mRNA. Indeed, the 3′-UTR region of mda-7/IL-24 mRNA contains several AU-rich elements (ARE), conferring rapid mRNA turnover, and the robust induction of mda-7/IL-24 mRNA during terminal differentiation of melanoma cells involves stabilization of its mRNA rather than direct transcriptional control. FM516-SV cells, which endogenously express mda-7/IL-24 mRNA, were used to determine whether treatment with MDA-7/IL-24 altered the half-life of this mRNA. FM516-SV cells were treated with vehicle or MDA-7/IL-24 protein (20 ng/ml) for 48 h followed by actinomycin D (Act D) (0.5 μg/ml). Cells were then incubated further and harvested at different times points. Total RNA was isolated, and mda-7/IL-24 mRNA expression was examined by Northern blotting to measure mRNA decay rates. Without any treatment, the half-life of mda-7/IL-24 mRNA was <20 min (Fig. 3A Upper). MDA-7/IL-24 treatment extended the half-life of the mRNA to ≈40 min (Fig. 3A Lower). In DU-145 and P69 cells, no basal expression of mda-7/IL-24 mRNA was detected. Combination of MDA-7/IL-24 and Act D treatment revealed that, similar to FM516-SV cells, the half-life of mda-7/IL-24 mRNA was also ≈40 min in these cells (Fig. 3B).
Fig. 3.
Recombinant MDA-7/IL-24 protein increases stability of endogenous mda-7/IL-24 mRNA that requires de novo protein synthesis. (A) FM516-SV cells were untreated or treated with MDA-7/IL-24 (20 ng/ml) for 48 h and then with Act D (0.5 μg/ml). At the indicated times after treatment, total RNA was extracted and subjected to Northern blotting for the indicated mRNAs. (B) P69 and DU-145 cells were treated with MDA-7/IL-24 for 48 h and then with Act D (0.5 μg/ml). At the indicated times after treatment, total RNA was extracted and subjected to Northern blot analysis for the indicated mRNAs. (C) P69, FM516-SV, and DU-145 cells were treated with or without MDA-7/IL-24 protein for 48 h and then with CHX (10 μM). At the indicated times after treatment, total RNA was extracted and subjected to Northern blot analysis for the indicated mRNAs.
We next analyzed the role of new protein synthesis in regulating MDA-7/IL-24-induced mda-7/IL-24 mRNA expression. FM516-SV and DU-145 cells were treated with cycloheximide (CHX, 10 μM) for different periods of time after treatment with MDA-7/IL-24 protein for 48 h. The cells were harvested, total RNA was extracted, and Northern blotting was performed by using a radiolabeled mda-7/IL-24 cDNA probe (Fig. 3C). CHX treatment increased the mda-7/IL-24 mRNA half-life to ≈120 min (Fig. 3C). These findings indicate that MDA-7/IL-24 protein increases the stability of mda-7/IL-24 mRNA. Inhibition of protein synthesis further increased the mRNA half-life, indicating that new proteins binding to the mRNA and reducing stability may play a critical role in maintaining low (or undetectable) basal expression of mda-7/IL-24 mRNA. Our data demonstrate that protein synthesis is necessary for promoting MDA-7/IL-24-mediated mda-7/IL-24 message stability.
Induction of mda-7/IL-24 mRNA by MDA-7/IL-24 Protein Occurs Independently of mda-7/IL-24 Promoter Activation.
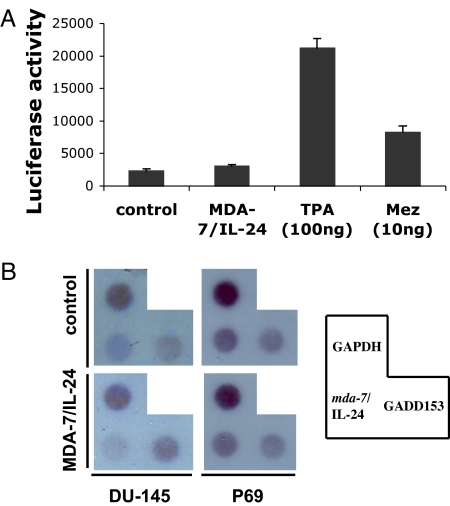
To analyze potential transcriptional induction of mda-7/IL-24 mRNA by MDA-7/IL-24 protein, we performed mda-7/IL-24 promoter analysis by using a luciferase reporter-based approach. A plasmid was generated containing ≈2.2 kb of the mda-7/IL-24 promoter sequence upstream of a luciferase gene (mda-7-Prom-luc). HeLa cells were transfected with mda-7-Prom-luc along with a β-galactosidase expression plasmid and treated with MDA-7/IL-24 protein (20 ng/ml) for 48 h. Luciferase and β-galactosidase activity was measured, and luciferase activity was normalized by β-galactosidase activity (Fig. 4A). Phorbol 12-tetradecanoate 13-acetate (TPA) and mezerein (MEZ), known inducers of mda-7/IL-24 expression, significantly increased mda-7/IL-24 promoter activity. MDA-7/IL-24 protein treatment did not augment promoter activity beyond the basal level. Nuclear run-on assays substantiated the results obtained by promoter analysis (Fig. 4B). MDA-7/IL-24 did not elevate the transcription of mda-7/IL-24 mRNA in isolated nuclei from DU-145 or P69 cells, whereas the transcription of GADD153, a known downstream target of MDA-7/IL-24, was augmented only in DU-145. These results confirm that mda-7/IL-24 is induced by MDA-7/IL-24 protein at a posttranscriptional level.
Fig. 4.
Induction of mda-7/IL-24 by MDA-7/IL-24 protein occurs posttranscriptionally. (A) HeLa cells were transfected with mda-7-Prom-luc and treated with MDA-7/IL-24 protein (20 ng/ml), TPA, or MEZ. Luciferase activity was measured after 48 h. (B) Nuclei were prepared from P69 and DU-145 cells. The isolated nuclei were used to label preinitiated RNA transcription with [α-32P]UTP in vitro, and the purified RNA was hybridized to a dot blot carrying an equivalent amount of cDNA probes. The transcription rate of GAPDH served as control.
MDA-7/IL-24 Protein Stimulates the UPR Pathway and Generates ROS.
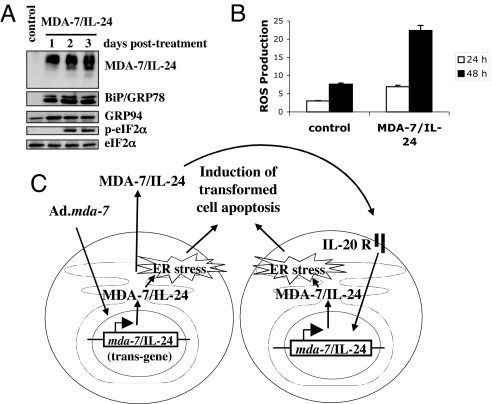
We reported that Ad.mda-7 induces an UPR pathway (17, 18, 24). The robust expression of MDA-7/IL-24 protein and the subsequent killing effect suggest that similar to Ad.mda-7, MDA-7/IL-24 may induce an UPR pathway. To determine whether chaperone proteins were activated after MDA-7/IL-24 treatment, we measured steady-state levels of specific proteins (BiP/GRP78, and GRP94) up-regulation of which frequently correlates with UPR. We also determined the phosphorylation of eIF2α, a key downstream event of UPR that mediates inhibition of protein translation (Fig. 5A). In addition, we measured de novo synthesis of GADD153, a proapoptotic transcription factor (Fig. 4B). Enhanced BiP/GRP78 and GRP94 protein levels were apparent at 1 or 2 days after MDA-7/IL-24 treatment, indicating selective modulation of specific chaperone proteins (Fig. 5A). In addition, MDA-7/IL-24 treatment induced phosphorylation of eIF2α (Fig. 5A).
Fig. 5.
Recombinant MDA-7/IL-24 protein triggers ER stress and ROS production. (A) Cells were treated with recombinant MDA-7/IL-24 protein (20 ng/ml), and changes in BiP/GRP78, GRP94, and phospho-eIF2α proteins were evaluated by using Western blot analyses. (B) Cells were untreated or treated with MDA-7/IL-24 protein (20 ng/ml) for 24 and 48 h, and ROS production was determined as described. Results are average from at least three independent experiments. (C) Model illustrating the possible molecular mechanism of cancer cell-selective apoptosis induction by recombinant MDA-7/IL-24 protein.
We determined the time course of mitochondrial changes (ROS generation) after treatment of DU-145 cells with MDA-7/IL-24 protein. Cells were treated with MDA-7/IL-24 protein, collected at 24 and 48 h, and stained for ROS production with dichlorofluorescin diacetate (DCFH-DA). Fig. 5B shows that MDA-7/IL-24 protein increased ROS production similar to Ad.mda-7 infection.
Discussion
Defining the molecular/biochemical basis of bystander cancer-selective activity of MDA-7/IL-24 provides an important entry point for rationally devising combinatorial approaches to enhance the therapeutic impact of this intriguing multifunctional antitumor molecule. MDA-7/IL-24 stimulates the immune system to generate secondary cytokines, such as TNF-α, IFN-γ, and IL-1 that might evoke an antitumor immune response (30, 31). Secreted MDA-7/IL-24 protein, generated from Ad.mda-7-infected cells, exerts antiangiogenic activity by inhibiting endothelial cell differentiation and by blocking the activities of VEGF and TGF-β via inhibition of src activity within tumor cells (7, 32). This effect of MDA-7/IL-24 may be mediated by the IL-20 receptor and has proven to be 20- to 50-fold more potent than endostatin or angiostatin (33). MDA-7/IL-24 protein exerts the cancer-specific bystander effect in a receptor-dependent and STAT3-independent manner (11, 29). Here, we define a unique mechanism by which bioactive MDA-7/IL-24 stimulates its own expression in a positive-feedback loop (Figs. 1 and 2). Furthermore, we also demonstrate that exogenous MDA-7/IL-24-mediated mda-7/IL-24 up-regulation is essential for the MDA-7/IL-24-induced apoptotic effect. Blocking mda-7/IL-24 expression by RNA interference inhibits extracellular MDA-7/IL-24-mediated apoptosis (Fig. 2B).
Our data provide strong evidence that the self-stimulatory effect of MDA-7/IL-24 is to a great extent, based on stabilization of mda-7/IL-24 mRNA (Figs. 3 and 4). mda-7/IL-24 mRNA shares with the mRNAs of several interleukins and cytokines the feature of being very unstable. Delay of mRNA degradation is a common posttranscriptional mechanism to up-regulate the expression of these proteins. The half-life of mda-7/IL-24 mRNA in unstimulated cells is <20min, well in line with data reported earlier in other cells (34, 35). The instability is conferred by ARE located in the 3′-UTR (35). The ARE is a well studied instability signal present in many relevant messages that is specifically bound by trans-acting RNA-binding proteins, which ultimately determine whether mRNA decay is delayed or facilitated. We observed that inhibition of translation by CHX blocked mda-7/IL-24 mRNA decay (Fig. 3C). This effect could be caused by either the involvement of highly unstable factors (trans-acting RNA binding proteins) in the degradation of the transcript or by the fact that translation per se is necessary for the decay of mda-7/IL-24 mRNA. Additionally, we show that MDA-7/IL-24 protein also causes ER stress, as shown by ER stress-specific apoptotic markers (Figs. 4 and 5). These findings support the hypothesis that exogenous MDA-7/IL-24 protein induces endogenous mda-7/IL-24 expression in normal as well as cancer cells, and similar to Ad.mda-7 infection, MDA-7/IL-24 protein causes tumor-specific killing, which is most likely ER stress-mediated. These findings provide a direct link between extracellular and intracellular pathways in ER stress induction and apoptosis induction by MDA-7/IL-24.
It is unclear why MDA-7/lL-24 induces its apoptotic effect through ER stress mechanisms exclusively in cancer cells. One possible reason for this differential killing effect involves inherent biochemical differences between normal and cancer cells. ER stress activates both prosurvival and proapoptotic pathways. Thus, by activating elements of the adaptive stress response and by attenuating apoptotic pathways, ER stress can be protective during tumor development. However, a particularly strong or prolonged ER stress can overwhelm the prosurvival mechanisms, tipping the balance toward apoptotic pathways, preventing tumor development, growth, and invasion. A cascade effect can lead to activation of multiple apoptotic signals, thus amplifying this effect up to a point of irreversible commitment and cell death. This could explain why MDA-7/IL-24 induces apoptosis in cancer cells that have higher ER stress levels compared with normal cells, rendering cancer cells more susceptible to ER stress by mda-7/IL-24 than normal cells. Such an explanation would also be compatible with the fact that by blocking one single pathway, induced by MDA-7/IL-24, it is possible to block the killing effect. ROS, key mediators of MDA-7/IL-24 effects, have been shown to have differential effects on cancer cells versus normal cells (12, 13, 28). Multiple studies have demonstrated that the basal ROS level in cancer cells is higher than that in normal cells (36). As such, exogenous agents that promote ROS production, such as MDA-7/IL24, could overcome natural antioxidants much more efficiently in cancer cells, as opposed to normal cells, thus inducing apoptosis and cell death.
We demonstrated that a nonreplicating adenovirus expressing a nonsecreted version of the MDA-7/IL-24 protein (Ad.SP−mda-7) is as effective as wild-type (Ad.mda-7) in promoting apoptosis through exclusively, intracellular mechanisms (24). Therefore, mda-7/IL-24-mediated apoptosis can be triggered efficiently in the absence of protein secretion. Nevertheless, we cannot exclude the possibility that certain cancer cells that are more resistant would only be vulnerable to higher levels of intracellular MDA-7/IL-24, in which case the positive autocrine loop we describe would be essential to generate enough MDA-7/IL-24 protein to reach a threshold needed to induce apoptosis. Moreover, we could infer that the autocrine loop is important in in vivo systems where the therapeutic effect of Ad.mda-7 treatment could be amplified. Indeed, clinical trials in which Ad.mda-7 was administered to 28 patients with resectable solid tumors had transgenic MDA-7/IL-24 beyond the injection site, and this protein persisted longer than the transgene and showed enhanced distribution (14, 15). Moreover, apoptosis correlated spatially with MDA-7/IL-24 protein expression, and both MDA-7/IL-24 protein expression and apoptosis had similar intratumoral dynamics. This in vivo observation is directly compatible with induction of an MDA-7/IL-24 autocrine loop.
Nonsteroidal antiinflammatory drugs (NSAIDs), such as sulindac, induce apoptosis in cancer cells (37). NSAIDs, on one hand, up-regulate MDA-7/IL-24 and, on the other hand, down-regulate cyclooxygenase-2 (COX-2). It is well established that increased levels of prostaglandin E2 (PGE2) production and matrix metalloproteinase-2 (MMP-2) activation are associated with enhanced expression of COX-2 in many cancers. Administration of COX-2 inhibitors has been reported to suppress PGE2 production and MMP-2 activation in various cancers, and suppression of COX-2 expression results in significant inhibition of cell motility and tumor growth and angiogenesis. These observations coupled with our present findings suggest that MDA-7/IL-24-mediated mda-7/IL-24 induction could involve COX-2 down-regulation as an event downstream of IL-20R activation by extracellular MDA-7/IL-24. Our results also raise the intriguing notion that MDA-7/IL-24-mediated ER stress through a robust activation of its own protein expression might also induce antiangiogenic responses through the same signal transduction pathways.
Taken together, our results indicate that MDA-7/IL-24 protein induces the bystander antitumor effect through an ER stress mechanism mediated by a robust activation of its own protein expression. Our results, outlined in the model in Fig. 5C, clearly reveal that exogenous MDA-7/IL-24 protein induces growth inhibition and apoptosis only in cancer cells through a mechanism identical to Ad.mda-7 infection. Having purified functional MDA-7/IL-24 protein provides insights into the mechanism by which this molecule exerts the bystander antitumor effects and supports alternative therapeutic approaches in addition to adenovirus delivery. These could include protein delivery systems employing antibody-targeted liposomes, nanoparticles, microbubbles, stem cells, and/or dendritic cells with conventional therapies such as radiation or chemotherapy. All of these possibilities merit future research efforts as new therapeutic approaches using this protein-based reagent for developing an effective and enduring treatment for cancer.
Materials and Methods
Cell lines, Viability and Apoptosis Assays, and ROS Production.
Cancer cells, DU-145, A549, PC-3, HO-1, and HeLa, and normal cells, FM516-SV immortalized normal human melanocytes, P69 immortalized normal human prostate epithelial cells, and PHFA, were cultured as described (11–13). MTT assays and apoptosis assays were performed by flow cytometry (11–13, 18). For analysis of ROS production, cells were stained with DCFH-DA and analyzed by flow cytometry (12).
Protein Purification.
Ad.MDA-7/IL-24 with a His6 tag on the C terminus (Ad.His-mda-7) was generated by standard protocols. Immortal primary human fetal astrocytes (IM-PHFA) (9) were infected with Ad.His-mda-7 (100 pfu per cell) for 2 h, washed, and then cultured in complete growth medium. IM-PHFA were used because they are well infected and generate high levels of MDA-7/IL-24 protein that is secreted without being killed (9). After 96 h, medium was collected and centrifuged to remove any cell debris. Supernatant was mixed with a nickel-nitrilotriacetic (Ni-NTA) acid slurry and incubated overnight to allow binding of MDA-7/IL-24 to the Ni-NTA beads. After 24 h, the Ni-NTA slurry-containing medium was allowed to pass through a column to collect the beads. The beads were washed with sodium phosphate buffer (pH 6.0), and MDA-7/IL-24 protein was eluted in sodium phosphate buffer (pH 9.0) containing 250 mM imidazole. The protein was dialyzed against DMEM to remove imidazole. The size and purity of the recombinant MDA-7/IL-24 protein were determined by Coomassie blue staining gel and Western blot analysis (6).
Northern Blot Analysis and RT-PCR.
Total RNA was isolated by using the RNAeasy kit (Qiagen). Reverse transcription (RT) was performed on 5 μg of total RNA with an oligo(dT) primer (18, 23). cDNA corresponding to 5 ng of total RNA was amplified for 35 cycles by PCR with specific primers for mda-7/IL-24 (23). For Northern blotting, 15 μg of total RNA was denatured, electrophoresed in a 1.2% agarose gel with 3% formaldehyde, and transferred onto a nylon membrane (11). The blots were probed with an [α-32P]dCTP full-length human mda-7/IL-24 cDNA probe and then stripped and reprobed with an [α-32P]dCTP human gapdh probe (11).
Western Blot Analysis.
Protein extracts were prepared with RIPA buffer containing a mixture of protease inhibitors as described (6, 11). Fifty micrograms of protein was applied to a 12% SDS/PAGE and transferred to nitrocellulose membranes. The membranes were probed with polyclonal antibodies to MDA-7/IL-24, eIF2α, BiP/GRP78, p-eIF2α, and GRP94.
siRNA Assay.
HeLa cells were transfected with 100 nM mda-7/IL-24 siRNA (Dharmacon, Inc.) or control siRNA (38). After 24 h of siRNA transfection, cells were washed, and each plate was split in two and untreated or treated with MDA-7/IL-24 protein. Cells were harvested 48 h later, counted, and cell lysates were prepared and analyzed for MDA-7/IL-24 protein expression by Western blotting (6).
Nuclear Run-On Assays.
Nuclear run-on assays for mda-7/IL-24, gapdh, and gadd153 were performed as described in ref. 39.
Promoter Analysis.
A plasmid containing ≈2.2 kb of mda-7/IL-24 promoter sequence upstream of luciferase gene (mda-7-Prom-luc) was generated (35). HeLa cells were transfected with mda-7-Prom-luc along with a β-galactosidase expression plasmid by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. The cells were treated with MDA-7/IL-24 protein for 48 h, and luciferase and β-galactosidase activity was measured (35). Luciferase activity was normalized by β-galactosidase activity.
Acknowledgments.
This work was supported in part by National Institutes of Health Grants R01 CA097318 and P01 CA104177 and the Samuel Waxman Cancer Research Foundation. P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research and is a Samuel Waxman Cancer Research Foundation Investigator.
Footnotes
The authors declare no conflict of interest.
References
- 1.Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation-associated gene, mda-7, modulated during human melanoma differentiation, growth, and progression. Oncogene. 1995;11:2477–2486. [PubMed] [Google Scholar]
- 2.Jiang H, et al. The melanoma differentiation-associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pestka S, et al. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 4.Chaiken IM, Williams WV. Identifying structure–function relationships in four-helix bundle cytokines: Toward de novo mimetics design. Trends Biotechnol. 1996;14:369–375. doi: 10.1016/0167-7799(96)10050-0. [DOI] [PubMed] [Google Scholar]
- 5.Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res. 2005;65:10128–10138. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- 6.Sarkar D, et al. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci USA. 2005;102:14034–14039. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta P, et al. mda-7/IL-24: Multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol Ther. 2006;111:596–628. doi: 10.1016/j.pharmthera.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebedeva IV, et al. mda-7/IL-24: Exploiting cancer's Achilles' heel. Mol Ther. 2005;11:4–18. doi: 10.1016/j.ymthe.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Su ZZ, et al. Melanoma differentiation-associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene. 2003;22:1164–1180. doi: 10.1038/sj.onc.1206062. [DOI] [PubMed] [Google Scholar]
- 10.Yacoub A, et al. mda-7 (IL-24) inhibits growth and enhances radiosensitivity of glioma cells in vitro via JNK signaling. Cancer Biol Ther. 2003;2:347–353. doi: 10.4161/cbt.2.4.422. [DOI] [PubMed] [Google Scholar]
- 11.Su Z, et al. Unique aspects of mda-7/IL-24 antitumor bystander activity: Establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene. 2005;24:7552–7566. doi: 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- 12.Lebedeva IV, et al. Melanoma differentiation-associated gene-7, mda-7/interleukin-24, induces apoptosis in prostate cancer cells by promoting mitochondrial dysfunction and inducing reactive oxygen species. Cancer Res. 2003;63:8138–8144. [PubMed] [Google Scholar]
- 13.Lebedeva IV, et al. Targeting inhibition of K-ras enhances Ad. mda-7-induced growth suppression and apoptosis in mutant K-ras colorectal cancer cells. Oncogene. 2007;26:733–744. doi: 10.1038/sj.onc.1209813. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham CC, et al. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: A phase I study. Mol Ther. 2005;11:149–159. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Tong AW, et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation-associated gene-7 (mda-7/IL24): Biologic outcome in advanced cancer patients. Mol Ther. 2005;11:160–172. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Fisher PB, et al. Melanoma differentiation-associated gene-7/interleukin-24 (mda-7/IL-24): Novel gene therapeutic for metastatic melanoma. Toxicol Appl Pharmacol. 2007;224:300–307. doi: 10.1016/j.taap.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta P, et al. BiP/GRP78 is an intracellular target for MDA-7/IL-24 induction of cancer-specific apoptosis. Cancer Res. 2006;66:8182–8191. doi: 10.1158/0008-5472.CAN-06-0577. [DOI] [PubMed] [Google Scholar]
- 18.Sauane M, et al. N-glycosylation of MDA-7/IL-24 is dispensable for tumor cell-specific apoptosis and “bystander” antitumor activity. Cancer Res. 2006;66:11869–11877. doi: 10.1158/0008-5472.CAN-06-1887. [DOI] [PubMed] [Google Scholar]
- 19.Huang EY, et al. Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation-associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene. 2001;20:7051–7063. doi: 10.1038/sj.onc.1204897. [DOI] [PubMed] [Google Scholar]
- 20.Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol. 2001;167:3545–3549. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- 21.Wang M, Tan Z, Zhang R, Kotenko SV, Liang P. Interleukin 24 (MDA-7/MOB-5) signals through two heterodimeric receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2. J Biol Chem. 2002;277:7341–7347. doi: 10.1074/jbc.M106043200. [DOI] [PubMed] [Google Scholar]
- 22.Parrish-Novak J, et al. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor–ligand interactions mediate unique biological functions. J Biol Chem. 2002;277:47517–47523. doi: 10.1074/jbc.M205114200. [DOI] [PubMed] [Google Scholar]
- 23.Sauane M, et al. mda-7/IL-24 induces apoptosis of diverse cancer cell lines through JAK/STAT-independent pathways. J Cell Physiol. 2003;196:334–345. doi: 10.1002/jcp.10309. [DOI] [PubMed] [Google Scholar]
- 24.Sauane M, et al. Melanoma differentiation-associated gene-7/interleukin-24 promotes tumor cell-specific apoptosis through both secretory and nonsecretory pathways. Cancer Res. 2004;64:2988–2993. doi: 10.1158/0008-5472.can-04-0200. [DOI] [PubMed] [Google Scholar]
- 25.Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- 26.Sarkar D, et al. mda-7 (IL-24) mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci USA. 2002;99:10054–10059. doi: 10.1073/pnas.152327199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mhashilkar AM, et al. MDA-7 negatively regulates the β-catenin and PI3K signaling pathways in breast and lung tumor cells. Mol Ther. 2003;8:207–219. doi: 10.1016/s1525-0016(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 28.Lebedeva IV, et al. Bcl-2 and Bcl-xL differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation-associated gene-7, mda-7/IL-24. Oncogene. 2003;22:8758–8773. doi: 10.1038/sj.onc.1206891. [DOI] [PubMed] [Google Scholar]
- 29.Chada S, et al. Bystander activity of Ad-mda7: Human MDA-7 protein kills melanoma cells via an IL-20 receptor-dependent but STAT3-independent mechanism. Mol Ther. 2004;10:1085–1095. doi: 10.1016/j.ymthe.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Poindexter NJ, Walch ET, Chada S, Grimm EA. Cytokine induction of interleukin-24 in human peripheral blood mononuclear cells. J Leukocyte Biol. 2005;78:745–752. doi: 10.1189/jlb.0205116. [DOI] [PubMed] [Google Scholar]
- 31.Caudell EG, et al. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol. 2002;168:6041–6046. doi: 10.4049/jimmunol.168.12.6041. [DOI] [PubMed] [Google Scholar]
- 32.Inoue S, Branch CD, Gallick GE, Chada S, Ramesh R. Inhibition of Src kinase activity by Ad-mda7 suppresses vascular endothelial growth factor expression in prostate carcinoma cells. Mol Ther. 2005;12:707–715. doi: 10.1016/j.ymthe.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Ramesh R, et al. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res. 2003;63:5105–5113. [PubMed] [Google Scholar]
- 34.Madireddi MT, Dent P, Fisher PB. AP-1 and C/EBP transcription factors contribute to mda-7 gene promoter activity during human melanoma differentiation. J Cell Physiol. 2000;185:36–46. doi: 10.1002/1097-4652(200010)185:1<36::AID-JCP3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 35.Madireddi MT, Dent P, Fisher PB. Regulation of mda-7 gene expression during human melanoma differentiation. Oncogene. 2000;19:1362–1368. doi: 10.1038/sj.onc.1203424. [DOI] [PubMed] [Google Scholar]
- 36.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Zerbini LF, et al. A novel pathway involving melanoma differentiation-associated gene-7/interleukin-24 mediates nonsteroidal anti-inflammatory drug-induced apoptosis and growth arrest of cancer cells. Cancer Res. 2006;66:11922–11931. doi: 10.1158/0008-5472.CAN-06-2068. [DOI] [PubMed] [Google Scholar]
- 38.Lebedeva IV, et al. Molecular target-based therapy of pancreatic cancer. Cancer Res. 2006;66:2403–2413. doi: 10.1158/0008-5472.CAN-05-3510. [DOI] [PubMed] [Google Scholar]
- 39.Lee SG, et al. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc Natl Acad Sci USA. 2006;103:17390–17395. doi: 10.1073/pnas.0608386103. [DOI] [PMC free article] [PubMed] [Google Scholar]