Abstract
Purpose
Retinal prosthetic devices are being developed to bypass degenerated retinal photoreceptors by directly activating retinal neurons with electrical stimulation. However, little is known about retinal activity during such stimulation.
Methods
Whole cell patch-clamp recordings were obtained from ganglion and bipolar cells in the salamander retinal slice preparation. A stimulating electrode was positioned at the vitreal surface of the slice.
Results
Brief pulses of cathodic current evoked transient inward currents in ganglion cells arising from action potentials. Longer pulses (>5 milliseconds) also evoked sustained inward currents in ganglion cells that appeared synaptic in origin because, unlike transient currents, sustained currents were blocked by inhibiting synaptic transmission with Cd2+. These synaptic currents reversed around ECl and were blocked by picrotoxin, strychnine, or both, suggesting they were mediated by GABAa/c and glycine receptors. Synaptic currents were also blocked by the NMDA antagonist MK801 and the KA/AMPA antagonist NBQX, suggesting that epiretinal stimulation evoked glutamate release from bipolar cells, which in turn stimulated the release of GABA and glycine from amacrine cells. Sustained currents were also evoked by epiretinal stimulation in bipolar cells. These currents reversed near ECl and were blocked by picrotoxin, suggesting they arose from GABAa/c receptors.
Conclusions
Pulse duration is an important parameter for effective activation of the inner retina by epiretinal stimulation. Brief pulses evoke action potentials only in ganglion cells. However, longer pulses also evoke sustained synaptic currents by stimulating glutamate release from bipolar cell terminals, which, in turn, evokes the release of GABA and glycine from amacrine cells.
One approach to treat blindness associated with outer retinal degenerative disorders, such as retinitis pigmentosa, Usher syndrome, and age-related macular degeneration, is to develop a multielectrode prosthetic device that will bypass the missing outer retinal elements (e.g., photoreceptors) and will electrically stimulate the remaining inner retinal elements (e.g., bipolar and ganglion cells) in a patterned manner. Indeed, such devices have been developed and implanted in human subjects.1,2 Subjects report that retinal stimulation evokes the perception of phosphenes. However, studies examining the electrophysiological mechanisms engaged by retinal electrical stimulation have been limited in extent. Several in vitro experiments with extracellular electrophysiological recordings in isolated retina preparations have shown that neuronal activity can be evoked by electrical stimulation. Early experiments show that secondary slow-wave retinal potentials can be induced by transretinal electrical stimulation of an amphibian eyecup preparation.3,4 In bullfrog eyecups, the charge-density threshold for evoking responses by epiretinal stimulation was found to be 2.98 μC/cm2.5 Charge-density threshold values were much higher—between 30 and 917 μC/cm2—in isolated rabbit retinas, and similar findings were reported in isolated human retinas.6 Similar threshold values of 500 μC/cm2 using subretinal electrical stimulation have been found in chicken and retinal degenerate Royal College of Surgeons rats (Stett A, et al. IOVS 1999;40:ARVO Abstract 3892).7
However, the main goal of these studies was not to determine underlying mechanisms but to delineate electrode shapes and pulse parameters for safe and effective electrical stimulation in controlled settings. The objective of the present study was to analyze retinal mechanisms during electrical stimulation. To do so, we obtained whole cell recordings from retinal ganglion and bipolar cells in a salamander retina slice preparation. Salamander retina is a widely used model system because its relatively large cells facilitate whole cell recording. In these studies, we tested whether various electrical stimulation parameters induce differential ganglion or bipolar retinal cell responses, and we pharmacologically dissected the retinal circuitry engaged by such stimulation.
Methods
Preparation of Retinal Slices
We used a retinal slice preparation similar to that developed by Werblin8 and described in detail by Wu.9 Aquatic tiger salamanders (Ambystoma tigrinum) 15 to 25 cm in length were handled humanely in accordance with the guidelines of the Association for Research in Vision and Ophthalmology and with the protocols approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center. After rapid decapitation, an eye was enucleated and the anterior segment, including the lens, was removed. The resultant eyecup was cut into thirds, and a section was placed vitreal-side down on a piece of filter paper (2 × 5 mm, Type AAWP, 0.8-μm pores; Millipore, Bedford, MA). After the retina adhered to the filter paper, it was isolated under chilled amphibian superfusate. The retina and the filter paper were cut into 125-μm–thick slices with the use of a razor blade (#121 to 6; Ted Pella Inc., Redding, CA) tissue chopper (Stoelting, Wood Dale, IL). Retinal slices were rotated 90° to permit viewing of the retinal layers when placed under a water-immersion objective (40×, 0.7 NA) and were viewed on an upright fixed-stage microscope (Olympus BHWI, Tokyo, Japan).
Extracellular Solutions
Solutions were delivered to the perfusion chamber at approximately 1 mL/min using a single-pass, gravity-feed perfusion system. The standard amphibian superfusate contained 111 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 0.5 mM MgCl2, 10 mM HEPES, and 5 mM glucose. The pH of the solution was adjusted to 7.8 with NaOH and the osmolarity, if necessary, to 242 ± 5 mOsm. Solutions were continuously bubbled with 100% O2. Drugs were bath applied.
Whole Cell Recording
Patch pipettes were pulled on a vertical puller (PP-830; Narishige USA, East Meadow, NY) from borosilicate glass pipettes (1.2-mm outer diameter [OD], 0.9-mm inner diameter [ID], with internal filament; World Precision Instruments, Sarasota, FL) and had tips of approximately 1-μm OD with resistance values of 8 to 14 MΩ. The pipette electrolyte solution contained 48 mM KCH3SO4, 44 mM KCl, 3.5 mM NaCl, 20 mM HEPES, 3 mM MgCl2, 1 mM CaCl2; 3 mM EGTA; 2 mM glucose; 1 mM MgATP, 0.5 mM GTP, (pH 7.2; 245 ± 5 mOsm). For the low-chloride pipette solution, KCl was omitted and the concentration of KCH3SO4 was increased to 92 mM, resulting in a predicted ECl of −59 mV. Lucifer yellow (2 mg/mL) was also added to the patch pipette solution during some experiments to allow fluorescence visualization of the anatomic structure of the recorded cell.
Ganglion cells were held at −70 mV and bipolar cells at −50 mV near their resting potentials in darkness. Current/voltage relationships were measured using a series of 150-millisecond voltage steps. Passive input resistance averaged approximately 1.5 GΩ in amphibian ganglion and bipolar cells.10,11 Consistent with their high input resistance, charging curves in these cells are typically well fit by single exponentials, indicating that they are isopotential and thus amenable to voltage clamp.
Currents were acquired using interfaces (Digidata 1322; Axon Instruments, Burlingame, CA) with appropriate software (PClamp; Axon Instruments).
Light Stimuli
Light stimuli were generated with the use of a 50-W halogen lamp focused onto a fiberoptic and then reflected through a beam splitter into a condenser path of the microscope. Wratten gel neutral-density filters were used to decrease light intensity.
Stimulating Electrodes
Stimulating electrodes were fabricated from the glass micropipettes used for patch-clamp recording. This pipette was filled with extracellular medium and placed at the ganglion cell side, 10 to 20 μm from the ganglion cell bodies. The stimulating electrode’s ID ranged from 2 to 143 μm. Different diameters were used to test the relationship between amplitude, duration, and charge-density thresholds.
Electrical Stimuli
An electrical stimulator (Isostim 01D; NPI, Tamm, Germany, or S9 stimulator; Grass Instruments, West Warwick, RI) was used to stimulate the retina. For the Grass stimulator, output current was calculated from Ohm’s law after measuring the resistance of the stimulating electrode.
We tested different stimulation parameters to determine the optimal stimuli needed to evoke responses in bipolar and ganglion cells. Test parameters included the amplitude and duration of the stimulating waveform and the electrode’s size.
Stimulus waveforms included a single biphasic or monophasic (cathodic) pulse. Although charge-balanced, biphasic waveforms have been shown to be the safest method of neuronal stimulation, safety was not critical for our short-term experiments; thus, monophasic cathodic stimuli were more commonly used. Stimulus duration varied between 0.1 and 100 milliseconds to test whether certain stimulus durations are more effective at evoking responses in certain cell types.
Cell Identification
Bipolar cells were identified primarily by their response to light and, in some cases, by their dendritic projections into the inner plexiform layer. ON bipolar cells exhibited an outwardly rectifying current/voltage profile and inward light-evoked currents. In contrast, OFF bipolar cells exhibited outward light-evoked currents. OFF bipolar cells could be readily distinguished from horizontal cells by the large outwardly rectifying currents in the former and the inwardly rectifying currents in the latter. Cells were classified as ganglion cells based on their presence in the ganglion cell layer and the presence of prominent fast inward sodium currents.
Results
Whole cell recordings were obtained from ganglion and bipolar cells in a slice preparation of salamander retina, as previously described by Thoreson and Miller.10 In ganglion cells, brief cathodic or bipolar pulses (≥10 μA) evoked transient inward currents (arrows, Fig. 1) shortly after the stimulus was given. Transient inward currents could be evoked by pulses as short as 0.5 milliseconds. The fact that they were not blocked by inhibiting synaptic transmission with Cd2+ (N = 6; Fig. 1) suggested that these transient inward currents reflected action potentials intrinsic to the ganglion cell. Brief cathodic pulses also evoked depolarizing action potentials in ganglion cells recorded extracellularly or under current clamp (not shown). The stimulation threshold for action potentials was closely related to charge per pulse (0.12 μC, SD = 0.09, n = 15). There was no significant correlation between this stimulation charge threshold and the electrode diameter (Fig. 2).
Figure 1.
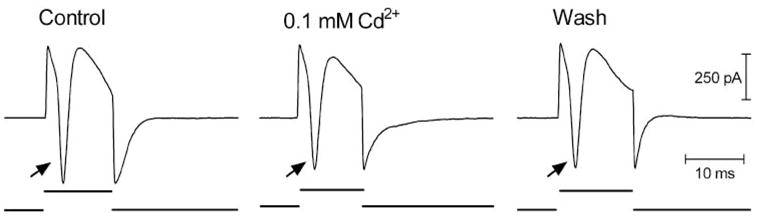
Epiretinal stimulation with cathodic pulses evoke transient inward currents in a retinal ganglion cell (left trace). Transient inward currents (arrow) were not affected by blockade of synaptic transmission with Cd2+ (0.1 mM, middle trace). Wash of Cd2+ did not change these evoked currents (right trace). Stimulus markers are shown at the bottom of each trace. Pulse duration, 12.5 milliseconds. Pulse amplitude, 25 μA.
Figure 2.
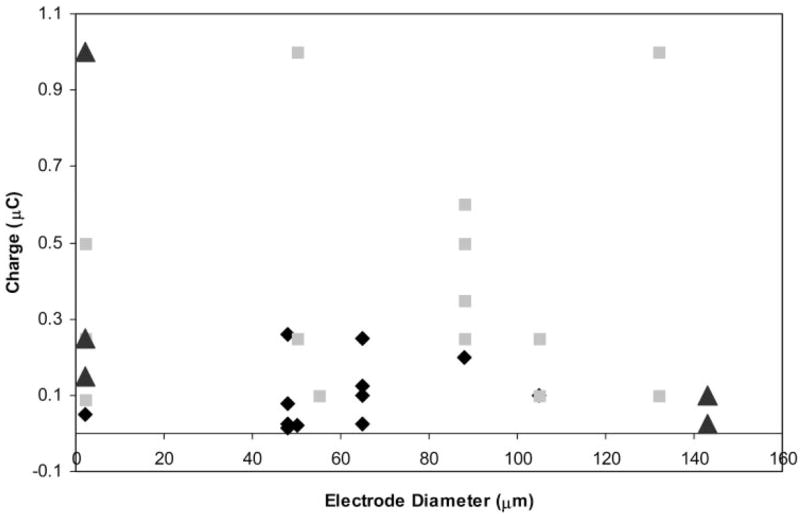
There was no significant relationship between electrode diameter and charge threshold for responses evoked by epiretinal stimulation in retinal ganglion and bipolar cells. Diamonds: Ganglion cell spikes. Squares: Ganglion cell–sustained currents. Triangles: Bipolar cell currents. One data point for a ganglion cell sustained currents, and two data points for bipolar cells sustained currents greater than 2 μC and are not presented on this graph.
In addition to the transient inward currents evoked by brief stimuli, longer pulses (typically >5 milliseconds) evoked more sustained currents that were typically inward under the conditions of our experiments (Fig. 3). This duration of approximately 5 milliseconds appeared to be the minimum necessary to evoke sustained inward currents given that increasing the amplitude of shorter duration stimuli failed to evoke sustained responses. The stimulation threshold for these sustained responses was closely related to charge per pulse (0.46 μC, SD = 0.5, n = 16) and was higher than the threshold for action potentials. Again, there was no significant correlation between electrode diameter and stimulation charge threshold (Fig. 2).
Figure 3.
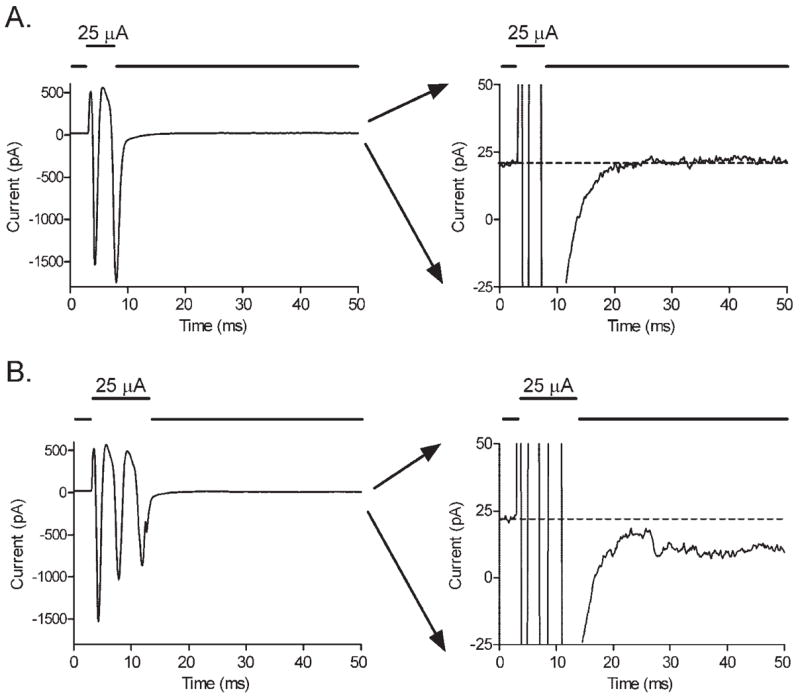
(A) Pulses of extracellular current stimulation (5 milliseconds, 25 μA) evoked transient inward currents arising from action potentials in retinal ganglion cells. No sustained inward currents are seen (the trace in the right panel returned rapidly to baseline). (B) Longer pulses (10 milliseconds, 25 μA) evoked the same transient currents followed by small, sustained inward currents (the trace in the right panel did not return fully to baseline). The ordinate is expanded in panels at the right to better illustrate the small, sustained inward current evoked by 10-millisecond, but not 5-millisecond, steps.
Sustained inward currents reversed polarity at approximately −24.0 ± 2.3 mV (N = 5) with the use of a pipette solution in which ECl was predicted to be −19 mV and reversed at −47.1 ± 1.2 mV (N = 7) when ECl was predicted to be −59 mV (N = 7; Fig. 4). The shift in reversal potential produced by the use of solutions with varying values of ECl suggested that a large fraction of this current was carried by chloride ions. The finding that the reversal potential was less negative than predicted when ECl was −59 mV might have reflected a contribution of cations (e.g., glutamate-gated channels) to sustained inward currents and the possibility of poor space clamp.
Figure 4.
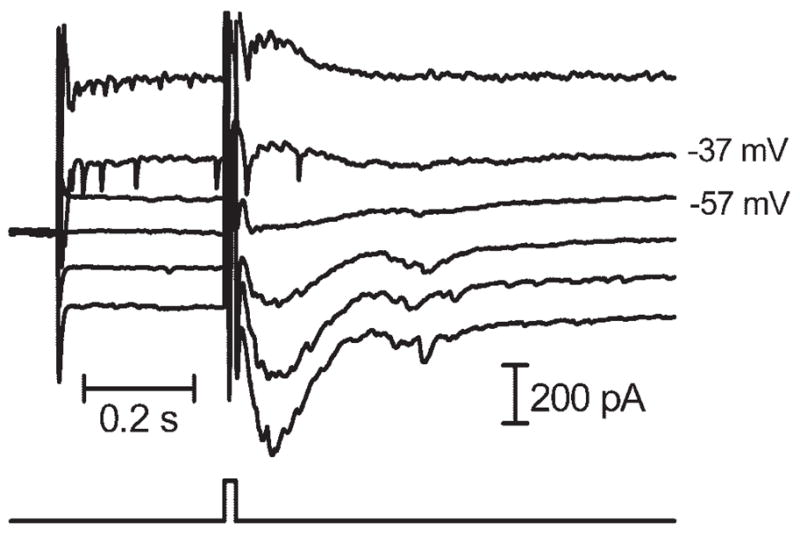
Inward currents evoked by extracellular stimulation (10 milliseconds, 40 μA) in a ganglion cell–reversed polarity between −37 and −57 mV, slightly above the predicted value for ECl of −59 mV.
In most cells, sustained inward currents were inhibited by the GABAa/c antagonist picrotoxin (0.1 mM, N = 10/15; Fig. 5) and the glycine antagonist strychnine (1 μM, N = 6/7; Fig. 5). Picrotoxin had little effect on currents in 3 of 15 cells and increased the amplitude of inward sustained currents evoked by retinal stimulation in 2 of 15 cells. In 1 of 7 cells, strychnine also had little effect. Combined application of picrotoxin and strychnine completely blocked the sustained inward current in both ganglion cells tested, suggesting that retinal stimulation evoked a sustained response in ganglion cells that involved a significant contribution from inhibitory GABAergic and glycinergic inputs but that varied from cell to cell in the balance and composition of those inhibitory inputs.
Figure 5.
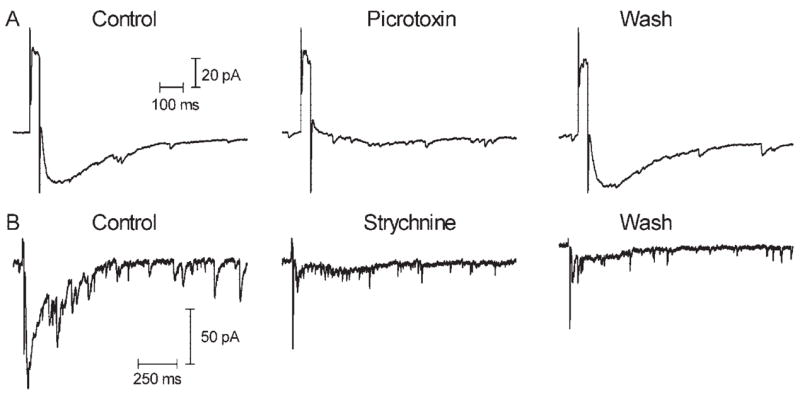
(A) The GABAa/c antagonist picrotoxin (0.1 mM) inhibited inward currents evoked by electrical stimulation (50 milliseconds, 25 μA) in a retinal ganglion cell. After wash-out, the inward current returned. (B) The glycine antagonist strychnine inhibited inward currents evoked by electrical stimulation (10 milliseconds, 10 μA) in a different ganglion cell. As shown here, recovery from the effects of strychnine were typically not observed over the time course of the recording.
These results suggested that retinal electrical stimulation evoked release of the inhibitory transmitters GABA and glycine. However, the reversal potential experiments suggested that there may also be a contribution from excitatory inputs. Does epiretinal stimulation act directly on GABAergic and glycinergic amacrine cells to stimulate release, or does it act primarily to stimulate glutamate release from bipolar cells, which, in turn, stimulates GABA and glycine release? To examine this question, we applied the non–NMDA glutamate antagonist NBQX (10 μM; N = 6) and the NMDA antagonist MK801 (0.1 mM; N = 4). Effects of these drugs are illustrated in Figure 6. In this ganglion cell, a current pulse of 10 milliseconds and 45 μA evoked an inward current that was blocked by NBQX (10 μM). However, this block was overcome by increased duration of the current to 20 milliseconds, suggesting it was not mediated entirely by non–NMDA receptors. The ability of stronger stimulation to overcome the block might have reflected the recruitment of additional bipolar cells with higher thresholds or a spillover of glutamate onto perisynaptic receptors with greater release.12 After washout of NBQX and recovery of the response to a stimulus of 10 milliseconds and 45 μA, MK801 (100 μM) was applied. Like NBQX, MK801 inhibited the response evoked by 10 milliseconds and 45 μA, but the inhibition was overcome by increased current pulse to 20 milliseconds and 90 μA. However, coapplication of NBQX in the continued presence of MK801 blocked the remaining inward current. Coapplication of NBQX and MK801 completely blocked inward currents in all four ganglion cells tested, suggesting that inward currents, though involving a significant inhibitory component, required the release of glutamate from bipolar cell terminals. Blockade of synaptic transmission with Cd2+ (50 μM) also produced a complete blockade of the sustained inward currents in ganglion cells (N = 6).
Figure 6.
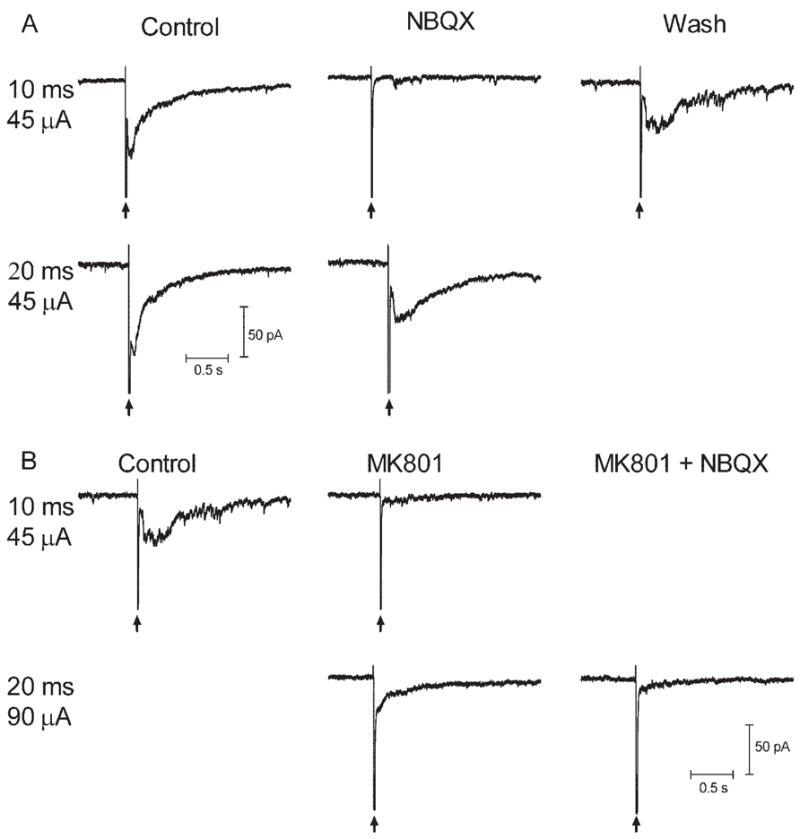
Inward currents evoked by retinal stimulation in a ganglion cell were inhibited by glutamate antagonists. Stimulus pulses produced current artifacts denoted by vertical arrows. (A) Inward currents evoked by a current pulse of 10 milliseconds and 45 μA were blocked by the AMPA/KA antagonist NBQX (10 μM). However, as shown in the second row of A, this block was overcome by increasing the duration of the current to 20 milliseconds. After wash-out, the response to 10 milliseconds and 45 μA recovered (this response is duplicated and shown as the control response in B). (B) Application of the NMDA antagonist MK801 (100 μM) inhibited the response evoked by 10 milliseconds and 45 μA, but, as shown in the second row of B, this inhibition was overcome by increasing the current pulse to 20 milliseconds and 90 μA. Addition of NBQX in the continued presence of MK801 blocked the remaining inward current (lower right trace).
The experiments described here suggest that sustained inward currents in ganglion cells arise from the release of glutamate from bipolar cells. Therefore, we studied the effects of retinal stimulation on bipolar cells. Like ganglion cells, retinal electrical stimulation evoked sustained inward currents in bipolar cell voltage clamped at −50 mV (Fig. 7). Charge threshold for the inward synaptic current in bipolar cells (1.72 μC, SD = 3.06, n = 10) was not correlated with electrode size (Fig. 2). With predicted values for ECl of −19 mV and −59 mV, respectively, inward currents in bipolar cells reversed at −24.1 ± 2.1 mV (N = 4) and −62.6 ± 5.8 mV (n = 4). Thus, currents in bipolar cells appeared to be carried almost exclusively by chloride. Sustained inward currents in bipolar cells were also consistently blocked by the GABAa/c antagonist picrotoxin (Fig. 8A). In contrast, the glycine receptor antagonist strychnine consistently enhanced currents evoked by retinal stimulation in bipolar cells (N = 5), suggesting a serial inhibition of GABAergic amacrine cells by glycinergic amacrine cells (Fig. 8B). Both ON- and OFF-type bipolar cells exhibited a similar response to epiretinal stimulation and were affected similarly by strychnine and picrotoxin.
Figure 7.
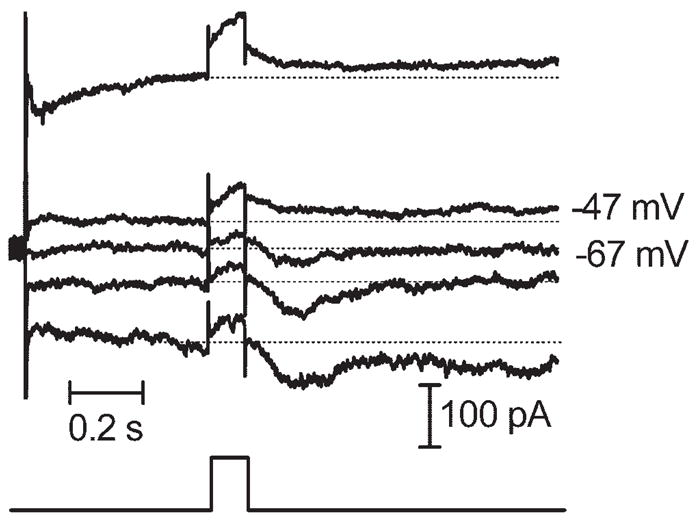
Epiretinal stimulation (100 milliseconds, 4 μA) evoked currents in bipolar cells that reversed between −47 and −67 mV, near the predicted value for ECl of −59 mV.
Figure 8.
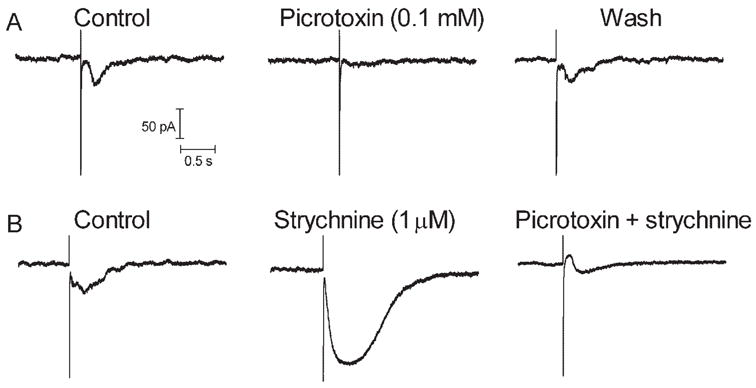
Bipolar cell currents evoked by epiretinal stimulation were inhibited by the GABAa/c antagonist picrotoxin (0.1 mM) and were enhanced by the glycine antagonist strychnine. The same cell is shown in (A) and (B). Stimulation parameters: (A) 5 milliseconds, 10 μA; (B) 5 milliseconds, 20 μA.
Consistent with results in ganglion cells, sustained inward currents in bipolar cells were also inhibited by a combination of the glutamate antagonists NBQX and MK 801 (N = 4; Fig. 9). A small biphasic current often persisted in the presence of picrotoxin and strychnine, MK801 and NBQX, or all four antagonists applied simultaneously. These residual biphasic currents were blocked by Cd2+ (0.1 mM), indicating that they are calcium dependent (Fig. 9). Biphasic currents could not be fully reversed at any potential tested, suggesting the involvement of multiple conductances.
Figure 9.
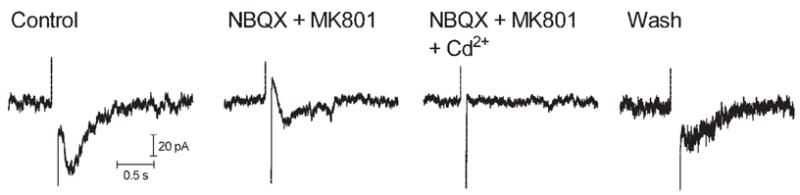
Inward currents evoked by epiretinal stimulation (100 milliseconds, 10 μA) in a bipolar cell were inhibited by the glutamate antagonists NBQX (10 μM) and MK801 (100 μM); residual currents were blocked by the addition of Cd2+ (100 μM).
In the retinal slice preparation, there is little electrical resistance between the stimulating electrode and presynaptic cells, whereas in a flat-mount preparation or in vivo, the inner limiting membrane and other proximal layers of the retina present a resistive barrier to activation. To compare our results with those of more intact preparations, we determined thresholds for evoking responses in ganglion cells using an isolated, flat-mount retina preparation. The average current threshold required to evoke spikes in ganglion cells from the flat-mount preparation (24.4 ± 8.22 μA, N = 6) did not differ significantly (P = 0.4) from that found in the slice preparation (31 ± 3.85 μA, N = 14).
Discussion
Previous studies on the activity of retinal cells under conditions of electrical stimulation have been confined largely to measurements of extracellular electrophysiological activity and designed principally to examine the safe and effective thresholds for evoking responses in ganglion cells under various stimulation conditions.13 One exception is a recent study that found different temporal types of responses to electrical stimulation in ganglion cells. However, this study did not use patch-clamp techniques or pharmacologic means to investigate the mechanisms behind these results.14 Our experiments were designed to analyze physiological mechanisms that initiate responses to retinal electrical stimulation. Results showed that duration is a particularly important stimulus parameter. Previous reports have suggested that the optimum pulse duration is long in the retina because graded potential cells have longer chronaxies than other excitable neurons.15 The chronaxy of a neuron is defined as a pulse width for which the threshold current is twice the rheobase current.16 The rheobase is the minimum threshold current below which an action potential cannot be elicited regardless of pulse duration. This current level can be interpreted as a “leakage” current that can pass through the tissue without inducing depolarization.15 Our results showed that pulses as brief as 0.5 milliseconds evoked transient inward currents and action potentials in ganglion cells. Stimulus durations longer than 5 milliseconds typically evoked sustained currents arising from synaptic inputs in bipolar and ganglion cells. These results are consistent with previous findings that target cells of shorter pulse duration were retinal ganglion cells/axons but that deeper cellular elements, which have longer chronaxies than ganglion cells, were the targets for longer pulse duration.15 Because of the relatively large size of amphibian retinal neurons, the duration required for optimum stimulation in our studies was longer than that found in studies conducted on mammalian retina.9
We found no correlation between electrode size and charge or current amplitude threshold. We found a relatively low charge-density threshold (<2 μC/cm2), similar to results from bullfrog retina.5 As in duration requirements, the relatively large size of amphibian neurons may account for the low charge-density threshold compared with mammalian and chicken retina (Stett A, et al. IOVS 1999;40:ARVO Abstract 3892).6,7 Cell-to-cell variation in the threshold for charge density or current amplitude may be explained by cell-to-cell differences in the balance of inhibitory and excitatory inputs that underlie the evoked response of each cell.
Our results indicated that bipolar cells, both ON- and OFF-type, responded to retinal stimulation with inward currents mediated primarily by GABAa/c receptors. Glycine receptor inhibition with strychnine increased these inward currents, whereas blockade of GABAa/c receptors with picrotoxin blocked these currents. Consistent with the conclusion that they are caused by GABAergic inputs, most likely from amacrine cells, inward currents reversed around ECl. The enhancement by strychnine suggested glycinergic inhibition of the GABAergic amacrine cells. Evidence for serial inhibition of GABAergic amacrine cells by glycinergic amacrine cells has also been obtained in light response experiments.17 However, these light response experiments show a temporal segregation of glycinergic inhibition that was not evident in responses to direct electrical stimulation, perhaps because electrical stimulation acts simultaneously on GABAergic and glycinergic amacrine cells.
In addition to blockade by picrotoxin, a combination of the AMPA antagonist NBQX and the NMDA antagonist MK801 completely abolished inward synaptic currents in bipolar cells, indicating that they required glutamate receptor activation. Small biphasic currents typically remained in bipolar cells after the application of picrotoxin or the combination of NBQX plus MK801. These biphasic currents were blocked by cadmium, suggesting they were calcium dependent, though the current included a prominent outward component, which indicated that calcium ions were not the predominant charge carrier. The outward component could arise from calcium-activated potassium currents in bipolar cells18,19 and in ON bipolar cells by activation of mGluR6 receptors. The inward component may involve calcium and calcium-activated chloride currents in bipolar cells. Amacrine cells contain many neurotransmitters besides GABA and glycine that might also contribute to these biphasic currents. However, we believe that this contribution is small because of the ability of glutamate antagonists to completely block inward currents in ganglion cells, suggesting that retinal stimulation has little direct effect on amacrine cells. Preferential stimulation of bipolar and photoreceptor cells is not unexpected because the lines of force emanating from the stimulation electrode are parallel to bipolar and photoreceptor cells but perpendicular to amacrine cell axons.
In ganglion cells, strychnine and picrotoxin had a greater diversity of effects than they had in bipolar cells. In most cells, responses were inhibited by picrotoxin or strychnine. In some cells, one or the other of these chemicals had no effect, and in two ganglion cells, picrotoxin actually enhanced responses. The presence of displaced amacrine cells in the ganglion cell layer might have accounted for some of the diversity of responses,20 but it is also likely that differences in the presynaptic circuitry of GABAergic and glycinergic synaptic input to a single ganglion cell also contributed. Although the balance of inhibitory inputs differed from cell to cell, inward currents in ganglion cells could be blocked by picrotoxin, strychnine, or a combination of the two, suggesting the sustained responses were dominated by inhibitory inputs from amacrine cells. Consistent with this, the reversal potential for responses in ganglion cells showed chloride dependence. However, responses reversed at potentials slightly more positive than ECl, suggesting that cationic excitatory currents may also contribute to these inward currents. One possible reason for the difficulty in detecting purely excitatory currents when ganglion cells were held at the chloride reversal potential was that the space clamp might not have been adequate to fully separate excitation and inhibition.
Blocking glutamate receptors with NBQX and MK801 abolished responses in ganglion cells, indicating that glutamate release from bipolar cells is required to evoke synaptic currents, even those mediated by inhibitory GABA and glycine receptors. This is consistent with the suppression of responses evoked by subretinal stimulation in the chicken retina using the nonselective glutamate receptor antagonist kynurenic acid.7 Taken together, experiments on the reversal potential and pharmacology of ganglion cell responses suggest that epiretinal stimulation promotes the release of glutamate from bipolar cell terminals. Some of this glutamate acts directly on ganglion cells, and some acts on amacrine cells to stimulate the release of GABA and glycine. The relatively large inhibitory component to the response of ganglion cells suggested by its chloride dependence and sensitivity to picrotoxin and strychnine may result from the fact that largely inhibitory amacrine cell synapses greatly outnumber bipolar cell synapses on ganglion cells.21
In addition to synaptic effects, retinal stimulation evoked action potentials in voltage-clamped retinal ganglion cells, indicating a fast, direct effect on these cells. Thus, the ganglion cell output produced by retinal stimulation appears to consist of an initial spike (or spikes) followed by sustained inhibition. In ON ganglion cells, spikes would presumably produce a perception of light and the subsequent inhibition a perception of reduced intensity; the converse would be true in OFF ganglion cells. We found no clear evidence for differences in the responses of ON, OFF, and ON/OFF ganglion cells to retinal electrical stimulation, though given the greater diversity of ganglion cell responses to epiretinal stimulation, a larger sample is needed to verify this conclusion.
In summary, the results show that brief pulses of cathodic epiretinal stimulation selectively evoke action potentials in ganglion cells, whereas longer pulses also evoke sustained synaptic currents. Synaptic currents in ganglion cells appeared to result from the stimulation-evoked release of glutamate from bipolar cell terminals, which, in turn, evoked the release of GABA and glycine from amacrine cells. Because the fundamental structure of the retina is conserved across vertebrate species, it seems likely that epiretinal stimulation will engage fundamentally similar mechanisms in mammalian retina. However, differences in cell size are likely to alter the optimal stimulation parameters. The diversity of responses among amphibian ganglion cells suggests that detailed differences in the circuitry among different types of ganglion cells in the mammalian retina are also likely to produce diverse responses varying in their balance of inhibitory and excitatory inputs. Although healthy amphibian and mammalian retina may be expected to respond similarly to stimulation, the retinal circuitry undergoes significant remodeling during retinal degeneration, suggesting that responses to epiretinal stimulation are likely to differ in retinas that have begun to degenerate (e.g., during retinitis pigmentosa).22 Further experiments in normal and degenerating mammalian retina are needed to extend our conclusions about the mechanisms that underlie responses to retinal electrical stimulation.
Acknowledgments
The authors thank Eric Bryson for technical assistance.
Supported in part by an unrestricted grant from Research to Prevent Blindness and by National Eye Institute Grant EY10546.
Footnotes
Disclosure: E. Margalit, None; W.B. Thoreson, None
References
- 1.Humayun MS, Weiland JD, Fujii GY, et al. Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Res. 2003;43:2573–2581. doi: 10.1016/s0042-6989(03)00457-7. [DOI] [PubMed] [Google Scholar]
- 2.Chow AY, Chow VY, Packo KH, Pollack JS, Peyman GA, Schuchard R. The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa. Arch Ophthalmol. 2004;122:460–469. doi: 10.1001/archopht.122.4.460. [DOI] [PubMed] [Google Scholar]
- 3.Knighton RW. An electrically evoked slow potential of the frog’s retina, I: properties of response. J Neurophysiol. 1975;38:185–197. doi: 10.1152/jn.1975.38.1.185. [DOI] [PubMed] [Google Scholar]
- 4.Knighton RW. An electrically evoked slow potential of the frog’s retina, II: identification with PII component of electroretinogram. J Neurophysiol. 1975;38:198–209. doi: 10.1152/jn.1975.38.1.198. [DOI] [PubMed] [Google Scholar]
- 5.Humayun M, Propst R, de Juan E, Jr, McCormick K, Hickingbotham D. Bipolar surface electrical stimulation of the vertebrate retina. Arch Ophthalmol. 1994;112:110–116. doi: 10.1001/archopht.1994.01090130120028. [DOI] [PubMed] [Google Scholar]
- 6.Grumet AE, Wyatt JL, Rizzo JF. Multi-electrode stimulation and recording in the isolated retina. J Neurosci Methods. 2000;101:31–42. doi: 10.1016/s0165-0270(00)00246-6. [DOI] [PubMed] [Google Scholar]
- 7.Stett A, Barth W, Weiss S, Haemmerle H, Zrenner E. Electrical multisite stimulation of the isolated chicken retina. Vision Res. 2000;40:1785–1795. doi: 10.1016/s0042-6989(00)00005-5. [DOI] [PubMed] [Google Scholar]
- 8.Werblin FS. Transmission along and between rods in the tiger salamander retina. J Physiol. 1978;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu SM. Synaptic connections between neurons in living slices of the larval tiger salamander retina. J Neurosci Methods. 1987;20:139–149. doi: 10.1016/0165-0270(87)90046-x. [DOI] [PubMed] [Google Scholar]
- 10.Thoreson WB, Miller RF. Membrane currents evoked by excitatory amino acid agonists in ON bipolar cells of the mudpuppy retina. J Neurophysiol. 1993;70:1326–1338. doi: 10.1152/jn.1993.70.4.1326. [DOI] [PubMed] [Google Scholar]
- 11.Coleman PA, Miller RF. Measurement of passive membrane parameters with whole-cell recording from neurons in the intact amphibian retina. J Neurophysiol. 1989;61:218–230. doi: 10.1152/jn.1989.61.1.218. [DOI] [PubMed] [Google Scholar]
- 12.Matsui K, Hosoi N, Tachibana M. Excitatory synaptic transmission in the inner retina: paired recordings of bipolar cells and neurons of the ganglion cell layer. J Neurosci. 1998;18:4500–4510. doi: 10.1523/JNEUROSCI.18-12-04500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen RJ, Ziv OR, Rizzo JF., III Thresholds for activation of rabbit retinal ganglion cells with relatively large, extracellular microelectrodes. Invest Ophthalmol Vis Sci. 2005;46:1486–1496. doi: 10.1167/iovs.04-1018. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Hayashida Y, Yagi T. Temporal properties of retinal ganglion cell responses to local transretinal current stimuli in the frog retina. Vision Res. 2005;45:263–273. doi: 10.1016/j.visres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg RJ. PhD thesis. Baltimore, MD: The Johns Hopkins University; 1998. Analysis of Electrical Stimulation of the Vertebrate Retina—Work Towards a Retinal Prosthesis. [Google Scholar]
- 16.Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975;98:417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- 17.Roska B, Nemeth E, Werblin FS. Response to change is facilitated by a three-neuron disinhibitory pathway in the tiger salamander retina. J Neurosci. 1998;18:3451–3459. doi: 10.1523/JNEUROSCI.18-09-03451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lasater EM. Membrane currents of retinal bipolar cells in culture. J Neurophysiol. 1988;60:1460–1480. doi: 10.1152/jn.1988.60.4.1460. [DOI] [PubMed] [Google Scholar]
- 19.Connaughton VP, Maguire G. Differential expression of voltage-gated K+ and Ca2+ currents in bipolar cells in the zebrafish retinal slice. Eur J Neurosci. 1998;10:1350–1362. doi: 10.1046/j.1460-9568.1998.00152.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Yang Z, Wu SM. Immuocytochemical analysis of spatial organization of photoreceptors and amacrine and ganglion cells in the tiger salamander retina. Vis Neurosci. 2004;21:157–166. doi: 10.1017/s0952523804042075. [DOI] [PubMed] [Google Scholar]
- 21.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 22.Jones BW, Marc RE. Retinal remodeling during retinal degeneration. Exp Eye Res. 2005;81:123–137. doi: 10.1016/j.exer.2005.03.006. [DOI] [PubMed] [Google Scholar]


