Abstract
We report a case of T-cell lymphoma metastatic to the eye, with an accompanying review of the literature. A 78-year-old Caucasian male with bilateral vitritis was diagnosed with primary cutaneous peripheral T-cell lymphoma (PCPTCL) unspecified, via vitreous biopsy. The tumor was found to be clonally related to the prior cutaneous malignancy using cytology, immunophenotyping and molecular analysis. The vast majority of primary intraocular lymphomas are malignant B-cells, while intraocular T-cell lymphomas are uncommon. This case demonstrates the utility of immunophenotyping and molecular analysis with microdissection and polymerase chain reaction, as critical adjunctive studies, in patients presenting with a masquerade syndrome, and later diagnosed with T-cell intraocular lymphomas. Vitreo-retinal without uveal involvement in this case, similar to many ocular metastatic T-cell lymphomas reported in the literature, is particularly intriguing since the uvea, not retina, is the typical ocular tissue involvement in the majority of metastatic B-cell lymphomas.
Keywords: metastatic, primary cutaneous peripheral T-cell lymphoma, primary intraocular lymphoma, T-cell lymphoma
Introduction
There are two main distinct forms of intraocular lymphoma. One originates from outside the central nervous system (CNS) and metastasizes to the eye, usually to the uvea.16,32,41 The second type arises within the CNS and eye, usually involving the retina and vitreous, that is usually referred to as primary CNS lymphoma (PCNSL). When PCNSL initially involves the eye it is called primary intraocular lymphoma (PIOL).8 Most PIOLs are malignant B-cells. Intraocular T-cell lymphomas are uncommon; some of them are secondary to metastatic systemic T-cell lymphomas, including primary cutaneous peripheral T-cell lymphoma (PCPTCL) and rarely adult T-cell leukemia/lymphoma (ATL).3,27,30,36,40
The intraocular manifestations of PCPTCL are rare and diverse. Previously reported findings included retinal infiltrates and hemorrhages, optic nerve infiltrates and non-specific uveitis.26,49,34,17,18,45,29,13 Herein we report a patient with bilateral vitritis, diagnosed with metastatic T-cell lymphoma via vitreous biopsy. The tumor was found to be clonally related to a prior PCPTCL, using immunophenotyping and molecular analysis. There are only a few published cases of metastatic intraocular PCPTCL with confirmatory histopathology and immunologic studies, from an ocular specimen. Additionally, we present a review of the available literature with reports of metastatic intraocular PCPTCL.
Case Report
A 78-year-old Caucasian male with a 4-month history of bilateral vitritis was seen at the National Eye Institute (NEI) to be evaluated for a possible intraocular lymphoma. His past medical history was significant for a PCPTCL on his left shin 3 years prior to initial presentation that was resected and treated with external beam radiation, at a local hospital. On examination his visual acuity was 20/25 OD and 20/500 OS. Slit-lamp biomicroscopy revealed no keratic precipitates, quiet anterior chamber, normal iridies and pseudophakia OU. Dilated fundus examination revealed trace cells without haze in the vitreous OD, and 2+ cells and 3+ haze in the vitreous OS (Fig.1). There were no clinically evident retinal/choroidal lesions OU. A systemic evaluation including a complete blood count with differential, serum chemistries, ESR and MRI of the brain were unremarkable. CSF analysis revealed pleocytosis with 94 % lymphocytes (40–80%). Cytology demonstrated lymphocytosis with no malignant lymphoid cells. The patient was seen by the oncology service at the National Cancer Institute, and further evaluated with a CT of the chest, abdomen and pelvis, bone marrow biopsy, analysis of peripheral blood by flow cytometry, with no evidence of systemic involvement. The patient then underwent a diagnostic vitrectomy.
Figure 1.
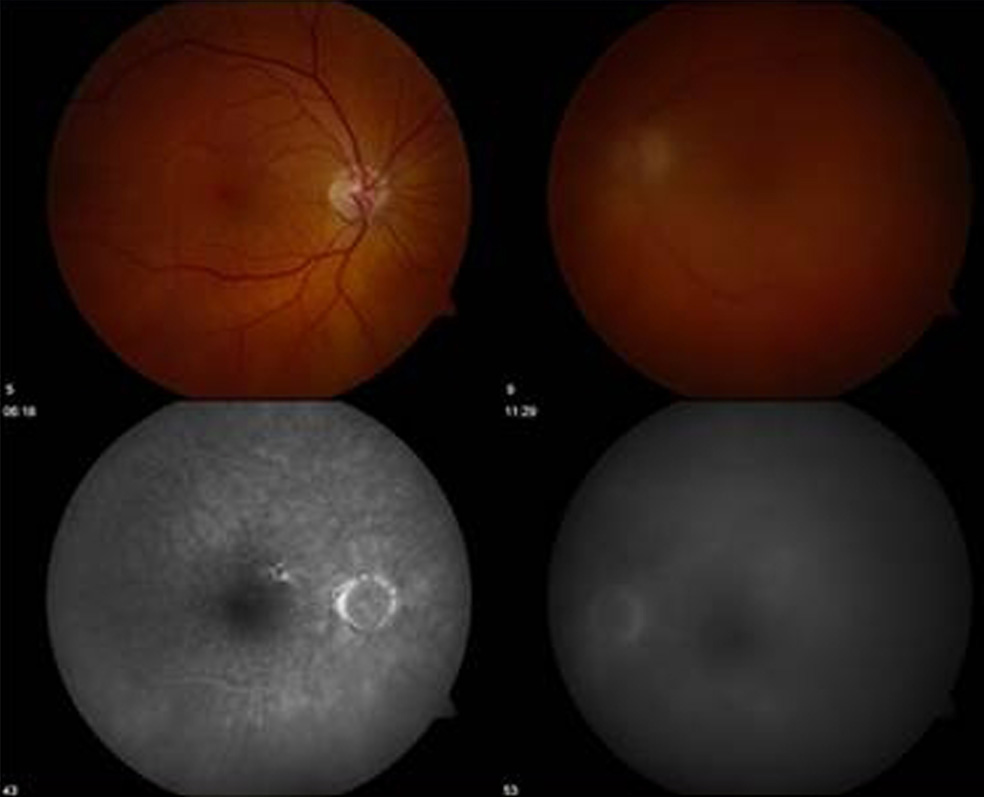
Fundus photograph showing vitreous haze OS and early angiograph showing no retinal or choroidal lesions OU
The immuno-pathological analysis of the vitreous specimen was consistent with a diagnosis of intraocular T-cell lymphoma. Additional studies included a bone marrow biopsy which was normal and immunophenotyping of a peripheral blood sample, which showed no evidence of an aberrant T-cell population. A repeat spinal tap was performed, and the CSF analysis again revealed only reactive lymphocytosis, by cytology and flow cytometry. The paraffin block from the previous excisional skin biopsy in 2002 was obtained, for comparative pathological analysis.
The patient received oral prednisone, initiated at 40 mg, and tapered slowly, sub tenon kenalog and intravitreal methotrexate as previously described.15,44 Nine weeks following the initial course of therapy, he developed mental status changes, with cytological studies of the CSF indicative of leptomeningeal involvement of lymphoma. An MRI of the brain, showed no parenchymal lesions. Clinical examination revealed persistent ocular disease. He went into hospice and expired one week later, 4 months after his initial presentation. No autopsy was performed.
PATHOLOGICAL FINDINGS
Skin Biopsy
The skin biopsy showed a marked infiltration of atypical polymorphic lymphoid cells, involving the dermis and the epidermal and dermal junction (Fig. 2C). Immunophenotyping of the infiltrating lymphoid cells demonstrated CD3+, CD4+, CD8−, CD30− and CD20− cells in the skin. The atypical lymphoid cells obtained by microdissection were subjected for polymerase chain reaction amplification (PCR) and detected clonal TCR-γ gene rearrangement (Fig. 2D). The pathological findings were consistent with a PCPTCL.
Figure 2.

Figure 2A: Cytology of the vitreous specimen revealed many atypical, large lymphoid cells, with large, round, irregular nuclei, visible nucleoli and basophilic cytoplasm (Giemsa, original magnification, x640).
Figure 2B: Immunohistochemistry showed that most atypical cells were CD3 positive (avidin-biotin-complex immunoperoxidase, original magnification, x200).
Figure 2C: The skin biopsy showed a marked infiltration of atypical polymorphic lymphoid cells, involving the dermis and the epidermal and dermal junction (hematoxylin & eosin, original magnification, x100).
Figure 2D: The atypical lymphoid cells (vitreous and skin) obtained by microdissection were subjected to polymerase chain reaction amplification and detected clonal TCR-γ gene rearrangement.
Vitreous Specimen
Cytology of the vitreous revealed many atypical, polymorphic, and small to large lymphoid cells, with large, round, irregular nuclei, visible nucleoli and basophilic cytoplasm (Fig. 2A). Immunohistochemistry showed that most atypical cells were CD3 and CD4 positive (Fig. 2B). There were few scattered CD8 positive cells, negative CD20, with both κ and λ positive cells (Fig. 2D). These results were compatibly found using flow cytometry, which also demonstrated an aberrant T-cell population of 65%, CD2− and CD5−; CD3+ and CD7+. The data are considered highly abnormal T-cell immunophenotypes. Molecular analysis of the microdissected lymphoid cells revealed no IgH rearrangement, but detected clonal TCR-γ rearrangement with a similar size compared to the previous skin biopsy (Fig.2D). In addition, cytokine analysis of the vitreous fluid demonstrated an IL-10 (137 pg/ml): IL-6 (143 pg/ml) <1.
The pathological findings confirmed the diagnosis of metastatic intraocular T-cell lymphoma.
Discussion
Most PIOLs are monoclonal populations of malignant B-cells and demonstrate monoclonality with either kappa or lambda light chain restrictions.14,32 Intraocular T-cell lymphomas are uncommon; some of them are secondary to metastatic systemic T-cell lymphomas including PCPTCL and rarely ATL.7,20,23,30,38,49
EPIDEMIOLOGIC, DEMOGRAPHIC AND CLINICAL FEATURES
Primary intraocular lymphoma, typically affects an older population, the median age of onset is usually the late 50s and 60s. A total of 29 cases (including the current) of intraocular metastatic T-cell lymphomas, confirmed with ocular biopsy, was reviewed in the literature (Table 1). The age of the patients described, ranged from 24 to 83 years, with a mean of 57.86 and a median of 57 years. There were 14 males and 15 females, without any definitive gender predominance. Previously reported reviews, indicate a slight male predominance.22,35 This series is unique, in describing only cases with pathological analysis of ocular tissue, and thus may defer from previously reported cases, in some of its demographic features.
Table 1.
29 Cases of Intraocular Metastatic T-cell Lymphomas Confirmed with Ocular Biopsy
| Author | Age/Sex | Previous Systemic Site (years prior to ocular involvement) | Clinical characteristics | Ocular Tissue | Diagnostic Studies performed | Systemic Diagnosis | Symptom duration | Concurrent CNS/Systemic Site | Treatment and survival. |
|---|---|---|---|---|---|---|---|---|---|
| Foerster18 1960 (1) | 59 M |
Skin (14) | Non-granulomatous anterior uveitis Vitritis Unilateral |
Enucleated globe | Histopathology | Mycosis fungoides | 12m | None | Died 12months after enucleation of diffuse systemic metastases |
| Keltner26 1977 (2) | 58 M |
Skin (5) | Papillitis Macular Edema Vitritis Retinal lesions Bilateral |
Globe autopsy | Histopathology | Mycosis fungoides | 3m | CNS | WBRT GRT SCT ICT Died 7 months after ocular involvement |
| Saga38 1984 (3) | 45 M |
Nasal cavity (1.5) | Non-granulomatous anterior uveitis Hypopyon Hyphema Iris lesion Lesion in the angle Unilateral |
Enucleated globe | Histopathology Immunohistochemistry |
Peripheral T cell lymphoma | 3m | Skin Inguinal node, Testis |
GRT SCT Died at 10 months |
| Char9 1988 (4,5) | 82 F 53 F |
CNS Not reported |
Anterior uveitis Vitritis Bilateral Anterior uveitis Vitritis Retinal and choroidal infiltrates Unilateral |
Vitreous Vitreous |
Cytology Immunophenotyping |
Unspecified T-cell lymphoma | Not reported | Not reported Not reported |
GRT Alive at 6 months GRT Alive at 95 months |
| Goldey20 1989 (6) | 54 F |
None | Non-granulomatous anterior uveitis Unilateral |
Aqueous | Cytology Flow cytometry Molecular analysis |
Non Hodgkin’s T-cell lymphoma | 4m | Abdomen, Bone marrow | GRT SCT ICT Survival time Not reported |
| Erny17 1991 (7) | 48 M |
Skin (30) | Papillitis Sub-retinal lesions Bilateral |
Globe autopsy | Histopathology Immunohistochemistry |
Mycosis fungoides | 1m | Skin, Lymph nodes, Retroperitoneal tissue | Died 2 months after ocular complaints, prior to initiation of therapy |
| Leitch29 1993 (8) | 61 F |
Skin (5) | Granulomatous anterior uveitis Stromal corneal scars Vitritis Creamy-white retinal infiltrates Retinal hemorrhages Bilateral |
Vitreous | Histopathology, Immunophenotyping | Mycosis fungoides | 4m | Skin | GRT Lost to f/u at 4 months |
| Jensen25 1994 (9) | 51 F |
None | Granulomatous anterior uveitis Iris lesion with bombe Unilateral |
Enucleated globe | Histopathology Immunohistochemistry Flow cytometry |
Non-mycosis fungoides, T-cell | 3m | Bone marrow | SCT Died in 12 months |
| Kumar27 1994 (10) | 38 M |
BM, Diffuse skeletal metastasis (0.33) | Sub-retinal creamy lesion Cotton wool spots Blurred disc margin Unilateral |
Chorioretinal biopsy | Histopathology, Immunohistochemistry | ATL | 1m | Not reported | GRT Died at 7 months |
| Davis14 1997 (11,12) | 52 M 74 F |
Skin (8) None |
Vitritis Vitritis |
Vitreous Vitreous |
Flow cytometry Flow cytometry Cytology |
Mycosis fungoides None |
Not reported Not reported |
Not reported None |
No data on survival No data on survival |
| Shibata40 1997 (13) | 51 M |
None | Vitritis Retinal exudates Papillitis Bilateral |
Globe autopsy | Histopathology | ATL | 1m | CNS | Died 5 months of ATL progression, after ocular diagnosis |
| Wylen50 1998 (14) | 76 F |
Skin (1) | Vitritis Corneal opacification Dilated pupil Bilateral |
Vitreous | Presumed histopathology immunophenotyping | Mycosis fungoides | 1m | Skin CNS |
WBRT Died at 4 months |
| Goeminne19 1998 (15) | 57 F |
Skin CNS |
Granulomatous anterior and intermediate uveitis Bilateral |
Vitreous | Cytology | None | 7m | Skin, Breast, CNS | SCT Died at 4 months |
| White47 1999 (16,17) | 83 F 73 F |
None None |
Vitritis Unilateral Vitritis Bilateral |
Vitreous Vitreous |
Cytology Molecular analysis Cytology Molecular analysis |
None None |
4 Months 4 months |
CNS None |
GRT Alive 30 months WBRT, GRT SCT |
| Yeh52 1999 (18) | 57 M |
Nasal cavity (3) | Vitritis Bilateral |
Vitreous | Cytology Immunophenotyping |
T/NK-cell lymphoma | Approximately 4 months | CNS | WBRT, ICT, SCT Died at 6 months |
| Coupland12 1999 (19,20) | 82 M 66 F |
Testicle (1) None |
Indurated conjunctiva Retinal hemorrhages CW spots, RD Unilateral Preretinal infiltrates, perivascular streaks, proptosis, diplopia Bilateral |
Globe autopsy Enucleated globe |
Histopathology Immunohistochemistry Molecular analysis Histopathology Immunohistochemistry Molecular analysis |
Peripheral T-cell lymphoma, unspecified T/NK-cell Lymphoma |
3 months 3 weeks |
Tonsil, BM, Skin, lymph node Skin |
SCT, ICT Dued 3m after starting chemo GRT Died at 4 months |
| Lois34 2000 (21) | 82 M |
Skin (7 yrs prior to ocular involvement) | Vitritis Retinal hemorrhage |
Vitreous | Histopathology Immunohistochemistry |
Mycosis fungoides | Not reported | None | GRT Early demise (assumed 1 month) |
| Hunyor23 2000 (22) | 24 F |
None | Corneal edema with subepithelial opacity Anterior uveitis Thickened, hemorrhagic iris Exudative RD Unilateral |
Aqueous | Cytology Immunocytochemistry |
NK-T | 4.5m | CNS, Liver, Bone marrow | Died in 2 weeks |
| Williams49 2000 (23) | 59 M |
Skin (4) | Vitritis Epiretinal plaques Papillitis Sub-retinal/Choroidal infiltrates Bilateral |
Vitreous | Cytology, Immunohistochemistry Flow cytometry |
Mycosis fungoides | 2m | CNS | ICT SCT Died 3 months |
| Yahalom51 2002 (24) | 31 M |
None | Ciliary body mass, Granulomatous anterior uveitis, iris mass Unilateral |
Iris | Cytology Immunohistochemistry Immunophe notyping |
Large T-cell lymphoma | 4 weeks | Skin | SCT, ICT |
| Levy-Clarke30 2002 (25) | 40 F |
None | Anterior uveitis Vitritis Deep white retinal lesions Perivascular infiltrates Bilateral |
Retina | Immunohistochemistry Molecular analysis |
Adult Tcell Leukemia / HTLV-1 | 2m | CNS | SCT Died 3 months |
| Hoffman22 2003 (26) | 64 M |
Presumed Skin |
Anterior uveitis Vitritis Multifocal choroiditis Vascular/Perivascular infiltrates Papillitis Bilateral |
Vitreous | Cytology Immunohistochemistry |
Peripheral T-cell | Not reported | None | GRT SCT Alive, no eye disease 101 months of f/u |
| Lobo33 2003 (27) | 30 F |
None | Anterior uveitis Corneal edema Elevated IOP Pseudo-hypopyon Iris nodules Unilateral |
Iris-surgical peripheral iridectomy | Histopathology Immunophenotyping |
Peripheral T-cell lymphoma, unspecified | Few days (0.5 months) | None | GRTM Died at 60 months with systemic involvement of tonsil and palate |
| Coupland11 2005 (28) | 50 F |
None | Vitritis Retinal lesions with exudation Unilateral |
Vitreous, Chorioretinal biopsy | Cytology Immuno-cytology, Histopathology Immunohistochemistry Molecular analysis |
None | 15m | Not reported | GRT 10 month f/u Alive |
| Levy-Clarke (29, current) | 78 M |
Skin (3) | Vitritis Bilateral |
Vitreous | Cytology Immunophenotyping Molecular analysis |
PCPTCL, unspecified | 4m | None | IVCT Died 4 months |
Key: WBRT- whole brain radiotherapy, GRT-globe radiotherapy, SCT-systemic chemotherapy, ICT-intra-thecal chemotherapy, IVCT-intravitreal chemotherapy
The duration of presenting symptoms, ranged from a few days to 15 months, with a mean duration of 3.68 months. A past history of a peripheral T-cell lymphoma was available in 13 cases (44.8 %). The mean time between onset of peripheral T-cell lymphoma and the ocular disease was 76 months (median 48 months, range 4–360 months).
Intraocular T-cell lymphoma is typically secondary to metastatic primary cutaneous T-cell lymphoma, of the mycosis fungoides sub-type (MF). Cutaneous T-cell lymphoma is a common adult lymphoma in the United States. This terminology designates a wide spectrum of diseases, typically characterized by clonal proliferation of T lymphocytes, arising or predominantly involving the skin. This disease is more common in men than women, and occurs most frequently in patients over age 45. The two most common variants, of this disease are MF and Sézary syndrome.22 In the current case series, 8/29 (27.6%) patients had a diagnosis of MF. The World Health Organization, and the European Organization for Research and Treatment of Cancer, published a new classification for cutaneous lymphomas in 2005, which delineates 8 types of cutaneous T-cell lymphomas, which now includes, more specific designation, compared to previous reports.43,48 Using this recently published classification, the case that we are reporting, would be classified as PCPTCL, unspecified. This designation is a heterogeneous group, which requires in all cases, that the diagnosis of MF be ruled out by clinical and physical examination.43
Ocular manifestations of cutaneous T-cell lymphoma are rare, and generally occur in the more advanced stages of the disease.4,10 The most frequent ophthalmic finding reported is blepharo-conjunctivitis, with intraocular involvement occurring only in rare cases.28 Previously reported intraocular findings included retinal infiltrates and hemorrhages, optic nerve infiltrates and non-specific uveitis.10 In the current series, the most common presenting clinical features were vitritis (19/29, 65.5%) and non granulomatous ant uveitis (13/29, 44.8%). The vitreous was the most common site of biopsy, 15/29 (51.7%). In the cases where documentation was given, 12 were unilateral and 14 bilateral. Previous systemic primary site reported indicated that the skin was the most common site, 8/29 (27.6%). Concurrent CNS involvement were reported in 9/29 (31.0%) cases. All of the reported demographic and clinical features are delineated in Table I.
DIAGNOSIS
In general the diagnostic approach in a patient with a suspected intraocular lymphoma when evaluated at the NEI usually follows the published algorithm.32 The gold standard for diagnosing intraocular lymphoma remains cytopathologic examination of the ocular specimen.6,9,46 This technique has many well documented limitations that include, but not limited to the skill and experience of the cytopathologists, and timely processing of the sample. To further delineate the type of lymphoma suspected, adjunctive modalities have been developed, based on the experience with systemic lymphomas. If a T-cell lymphoma is suspected, then in conjunction with cytopathology, critical adjunctive studies may include flow cytometry, immunophenotyping and molecular analyses. Immunocytochemical studies can be performed on the vitreous specimens or on the chorioretinal tissues. The primary antibodies for a T-cell lymphoma usually include CD3, CD4, CD5 and CD8.31 Immunocytological stains are utilized, to detect antigens expressed by T-cells. To add to the diagnostic dilemma, there is reportedly a paucity of immunohistochemical markers for T-cell monoclonality.11 In flow cytometry a fluorescence-activated cell sorter (FACS) replaces microscopy.14 We and others have reported the utilization of microdissection and polymerase chain reaction (PCR) as a useful adjunct for the diagnosis of PIOL.5,11,39,47 This technique allows for the selection and molecular analysis of malignant or atypical cells after pathologic analysis. In the cases reviewed, the most common diagnostic procedures performed were histopathology and cytology, both utilized in 46% of cases; immunohistochemistry/immunophenotyping 30% of the cases and flow cytometry and molecular analysis utilized in less than 25% of the cases analyzed.
UNIQUE FEATURES OF THIS CASE
There are a number of distinctive features in this case. Firstly, we have demonstrated that adjunctive studies, such as molecular analysis and immuno-phenotyping are critical in identifying a T-cell lymphoma, and making molecular comparisons to the previous tumor. Immunophenotyping of the vitreous specimen showed the atypical cells were CD3+, CD20−, with both κ and λ positive cells, flow cytometry demonstrated an aberrant CD3+ T-cell immunophenotype, microdissection and PCR, revealed no IgH rearrangement, but detected clonal TCR-γ rearrangement with a similar band compared to the previous skin biopsy. These pathological findings confirmed the diagnosis of metastatic intraocular T-cell lymphoma. Secondly, this patient had no detectable clinical signs of concurrent systemic disease, at presentation. From published cases, concurrent systemic disease is found in most cases and only a few cases are limited to the eye.22 In the current review 17/29 cases had concurrent systemic and/or CNS disease and in 5 cases it was not reported. However our patient did show cytological finding of leptomeningeal involvement, 15 weeks after his initial presentation. Thirdly, this local recurrence, created a therapeutic dilemma, the choice of systemic versus local therapy. This patient initially received local skin irradiation. He was disease free, without extracutaneous involvement for 3 years. His recurrence presented intraocular, as an isolated PIOL. The decision was made, in conjunction with the hematology/oncologists to treat with systemic and periocular corticosteroid and intravitreal methotrexate.15,44 Of the cases reviewed, there were no prior reports of T-cell intraocular lymphoma treated with intravitreal methotrexate.
TREATMENT AND PROGNOSIS
Therapeutic modalities for intraocular T-cell lymphomas are limited. The reports analyzed in the current review identified the following modalities, whole brain and/or globe irradiation (16/29), systemic chemotherapy (11/29), intrathecal chemotherapy (5/29) and intravitreal chemotherapy (1/29). The most commonly used modality was globe irradiation. The reason for the choice of modality was not delineated. Radiotherapy was first choice of treatment, in earlier published studies, as lymphoma cells are highly sensitive to radiation. The most common complications of radiotherapy are cataract and keratoconjunctivitis sicca. The more serious complications of radiation retinopathy and optic atrophy were less frequently seen.1,2 24
When evaluating patients with PCPTCL, there are certain prognostic factors that can be considered, when deciding on a therapeutic approach. PCPTCL unspecified, irrespective of the presence or absence of extracutaneous disease; cell size, and immunophenotype; initial presentation in the skin, have an unfavorable prognosis, with a 5-year survival rate of less than 20%. The recommendation is usually to treat patients with multi-agent chemotherapy.43 The result of this case, intraocular metastatic T-cell lymphoma, demonstrates poor prognosis for this malignancy and that as a single modality, intravitreal methotrexate does not appear effective, compared to published results in B-cell PIOL cases.44
Intraocular lymphoma has two distinct clinically recognized patterns. The first is the B-cell PIOL, which is typically a vitreoretinal involvement. The second is a uveal involvement, due to a metastatic systemic lymphoma. The vitreoretinal pattern is considered a multicentric PCNSL, while the uveal pattern is typically considered a hematogeneous spread.37 The cases reviewed in this series indicated that the metastatic T-cell lymphomas, presented predominantly with vitreoretinal involvement. This is in contrast, to metastatic B-cell intraocular lymphoma, which usually presents with uveal involvement. The T-cell metastatic lymphoma closely mirrors the clinical pattern of B-cell PIOL, and this clinical pearl, has not been previously reported. Histopathologic reports support this distinction. In B-cell PIOL, chorioretinal biopsy shows the PIOL cells are located between the retinal pigment epithelium and the Bruch’s membrane and vitreous biopsy identifies PIOL cells in the vitreous.21,37 In metastatic intraocular lymphoma, the preferred site is the choroid, followed by the optic nerve.42 Ophthalmoscopy typically reveals creamy choroidal infiltrates that are more commonly unilateral, compared to PIOL (bilateral).
In summary, metastatic T-cell lymphomas, unlike metastatic B-cell lymphomas can masquerade as an intraocular inflammmatory disorder and invade primarily the retina and vitreous. This case demonstrates the utility of critical adjunctive studies in the diagnosis of a metastatic T-cell PIOL. Further clinical trials are necessary, in conjunction with basic science research, to delineate tumor biology and develop targeted therapeutic modalities, as the appropriate management algorithm for this rare tumor remains elusive.
Method of Literature Search
Medline was searched via the PubMed interface, which also included Index Medicus records back to 1950 at the time of our search. MeSH terms were selected to retrieve leukemia-lymphoma, t-cell, acute, HTLV-1-associated and lymphoma, t-cell; the taxonomy’s ‘explode’ feature was utilized to retrieve more specific terms throughout the search. Natural language terms to retrieve additional references from pre-indexed PubMed content and those references which were not indexed by these MeSH terms included “t-cell lymphoma*,” “t-cell leukem*,” “mycosis fungoides.” This retrieval was refined by specifying cutaneous or systemic aspects. Metast* or secondary or “neoplasm metastasis”[MeSH] further defined the secondary and metastatic retrieval. Finally, the ocular focus of the search was specified by “eye neoplasms”[MeSH] or eye[MeSH] or “eye diseases”[MeSH] or natural language (ocular or eye[tw] or intraocul* or vitreous or vitritis or intravitr* or vitrectom*). Indexing of the retrieved references was reviewed for additional terms. Embase.com, which includes citations from Embase from 1974 to the present as well as Medline, was searched using the same strategy. Web of Knowledge from 1955 to the present was searched with the identical strategy, with MeSH terms converted to natural language phrases as appropriate; cited references in papers identified with this strategy were reviewed for additional references. The Cochrane Library (Wiley) and Cumulative Index to Nursing and Allied Health Literature (OVID) were also searched Monographs on ocular neoplasms in the National Institutes of Health Library were reviewed, including their cited references. Sir Stewart Duke-Elder’s System of Ophthalmology, vol. 11 (Diseases of the Uveal Tract) was consulted.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Berenbom A, Davila RM, Lin HS, et al. Treatment outcomes for primary intraocular lymphoma: implications for external beam radiotherapy. Eye. 2007;21:1198–1201. doi: 10.1038/sj.eye.6702437. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S, Paulino AC, Buatti JM, et al. Curative radiotherapy for primary orbital lymphoma. Int J Radiat Oncol Biol Phys. 2002;54:818–823. doi: 10.1016/s0360-3016(02)02966-8. [DOI] [PubMed] [Google Scholar]
- 3.Buggage RR. Ocular manifestations of human T-cell lymphotropic virus type 1 infection. Curr Opin Ophthalmol. 2003;14:420–425. doi: 10.1097/00055735-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Carlton WC, Hutchinson AK, Grossniklaus HE. Ocular tumors in animals and humans. Ames: Iowa State Press; 2002. Ocular lymphoid proliferations; pp. 379–413. [Google Scholar]
- 5.Chan CC, Shen D, Nussenblatt RB, et al. Detection of molecular changes in primary intraocular lymphoma by microdissection and polymerase chain reaction. Diagn Mol Pathol. 1998;7:63–64. doi: 10.1097/00019606-199802000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Chan CC, Wallace DJ. Intraocular lymphoma: update on diagnosis and management. Cancer Control. 2004;11:285–295. doi: 10.1177/107327480401100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Char DH. Clinical ocular oncology. 2 ed. Philadelphia: Lippincott-Raven; 1997. pp. 192–202. [Google Scholar]
- 8.Char DH. Intraocular Lymphoid and Myeloid lesions. In: Ginsberg RJ, editor. American Cancer Society Atlas of Clinical Oncology. Philadelphia, BC: Decker Inc; 2001. [Google Scholar]
- 9.Char DH, Ljung BM, Deschenes J, et al. Intraocular lymphoma: immunological and cytological analysis. Br J Ophthalmol. 1988;72:905–911. doi: 10.1136/bjo.72.12.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook BE, Jr, Bartley GB, Pittelkow MR. Ophthalmic abnormalities in patients with cutaneous T-cell lymphoma. Ophthalmology. 1999;106:1339–1344. doi: 10.1016/S0161-6420(99)00721-6. [DOI] [PubMed] [Google Scholar]
- 11.Coupland SE, Anastassiou G, Bornfeld N, et al. Primary intraocular lymphoma of T-cell type: report of a case and review of the literature. Graefes Arch Clin Exp Ophthalmol. 2005;243:189–197. doi: 10.1007/s00417-004-0890-2. [DOI] [PubMed] [Google Scholar]
- 12.Coupland SE, Foss HD, Assaf C, et al. T-cell and T/natural killer-cell lymphomas involving ocular and ocular adnexal tissues: a clinicopathologic, immunohistochemical, and molecular study of seven cases. Ophthalmology. 1999;106:2109–2120. doi: 10.1016/S0161-6420(99)90492-X. [DOI] [PubMed] [Google Scholar]
- 13.Damato B. Ocular tumors: diagnosis and treatment. Liverpool: Butterworth Heinemann; 2000. pp. 186–196. [Google Scholar]
- 14.Davis JL, Viciana AL, Ruiz P. Diagnosis of intraocular lymphoma by flow cytometry. Am J Ophthalmol. 1997;124:362–372. doi: 10.1016/s0002-9394(14)70828-1. [DOI] [PubMed] [Google Scholar]
- 15.de Smet MD, Vancs VS, Kohler D, et al. Intravitreal chemotherapy for the treatment of recurrent intraocular lymphoma. Br J Ophthalmol. 1999;83:448–451. doi: 10.1136/bjo.83.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duke-Elder S, Perkins ES. The Reticuloses. In: Duke-Elder S, Perkins ES, editors. System of Ophthalmology: Diseases of the uveal tract. London: Henry Kimpton; 1966. pp. 813–821. [Google Scholar]
- 17.Erny BC, Egbert PR, Peat IM, et al. Intraocular involvement with subretinal pigment epithelium infiltrates by mycosis fungoides. Br J Ophthalmol. 1991;75:698–701. doi: 10.1136/bjo.75.11.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foerster HC. Mycosis fungoides with intraocular involvement. Trans Am Acad Ophthalmol Otolaryngol. 1960;64:308–313. [PubMed] [Google Scholar]
- 19.Goeminne JC, Brouillard A, Jaumain P, et al. Bilateral granulomatous panuveitis as initial presentation of diffuse systemic T cell lymphoma. Ophthalmologica. 1999;213:323–326. doi: 10.1159/000027447. [DOI] [PubMed] [Google Scholar]
- 20.Goldey SH, Stern GA, Oblon DJ, et al. Immunophenotypic characterization of an unusual T-cell lymphoma presenting as anterior uveitis. A clinicopathologic case report. Arch Ophthalmol. 1989;107:1349–1353. doi: 10.1001/archopht.1989.01070020419047. [DOI] [PubMed] [Google Scholar]
- 21.Gonzales JA, Chan CC. Biopsy techniques and yields in diagnosing primary intraocular lymphoma. Int Ophthalmol. 2007;27:241–250. doi: 10.1007/s10792-007-9065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman PM, McKelvie P, Hall AJ, et al. Intraocular lymphoma: a series of 14 patients with clinicopathological features and treatment outcomes. Eye. 2003;17:513–521. doi: 10.1038/sj.eye.6700378. [DOI] [PubMed] [Google Scholar]
- 23.Hunyor AP, Harper CA, O'Day J, et al. Ocular-central nervous system lymphoma mimicking posterior scleritis with exudative retinal detachment. Ophthalmology. 2000;107:1955–1959. doi: 10.1016/s0161-6420(00)00342-0. [DOI] [PubMed] [Google Scholar]
- 24.Isobe K, Ejima Y, Tokumaru S, et al. Treatment of primary intraocular lymphoma with radiation therapy: a multi-institutional survey in Japan. Leuk Lymphoma. 2006;47:1800–1805. doi: 10.1080/10428190600632881. [DOI] [PubMed] [Google Scholar]
- 25.Jensen OA, Johansen S, Kiss K. Intraocular T-cell lymphoma mimicking a ring melanoma. First manifestation of systemic disease. Report of a case and survey of the literature. Graefes Arch Clin Exp Ophthalmol. 1994;232:148–152. doi: 10.1007/BF00176784. [DOI] [PubMed] [Google Scholar]
- 26.Keltner JL, Fritsch E, Cykiert RC, et al. Mycosis fungoides. Intraocular and central nervous system involvement. Arch Ophthalmol. 1977;95:645–650. doi: 10.1001/archopht.1977.04450040111017. [DOI] [PubMed] [Google Scholar]
- 27.Kumar SR, Gill PS, Wagner DG, et al. Human T-cell lymphotropic virus type I-associated retinal lymphoma. A clinicopathologic report. Arch Ophthalmol. 1994;112:954–959. doi: 10.1001/archopht.1994.01090190102028. [DOI] [PubMed] [Google Scholar]
- 28.Leib ML, Lester H, Braunstein RE, et al. Ocular findings in cutaneous T-cell lymphoma. Ann Ophthalmol. 1991;23:182–186. [PubMed] [Google Scholar]
- 29.Leitch RJ, Rennie IG, Parsons MA. Ocular involvement in mycosis fungoides. Br J Ophthalmol. 1993;77:126–127. doi: 10.1136/bjo.77.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy-Clarke GA, Buggage RR, Shen D, et al. Human T-cell lymphotropic virus type-1 associated t-cell leukemia/lymphoma masquerading as necrotizing retinal vasculitis. Ophthalmology. 2002;109:1717–1722. doi: 10.1016/s0161-6420(02)01132-6. [DOI] [PubMed] [Google Scholar]
- 31.Levy-Clarke GA, Byrnes GA, Buggage RR, et al. Primary intraocular lymphoma diagnosed by fine needle aspiration biopsy of a subretinal lesion. Retina. 2001;21:281–284. doi: 10.1097/00006982-200106000-00023. [DOI] [PubMed] [Google Scholar]
- 32.Levy-Clarke GA, Chan CC, Nussenblatt RB. Diagnosis and management of primary intraocular lymphoma. Hematol Oncol Clin North Am. 2005;19:739–749. doi: 10.1016/j.hoc.2005.05.011. viii. [DOI] [PubMed] [Google Scholar]
- 33.Lobo A, Larkin G, Clark BJ, et al. Pseudo-hypopyon as the presenting feature in B-cell and T-cell intraocular lymphoma. Clin Experiment Ophthalmol. 2003;31:155–158. doi: 10.1046/j.1442-9071.2003.00624.x. [DOI] [PubMed] [Google Scholar]
- 34.Lois N, Hiscott PS, Nash J, et al. Immunophenotypic shift in a case of mycosis fungoides with vitreous invasion. Arch Ophthalmol. 2000;118:1692–1694. doi: 10.1001/archopht.118.12.1692. [DOI] [PubMed] [Google Scholar]
- 35.Mochizuki M, Watanabe T, Yamaguchi K, et al. Human T-lymphotropic virus, Type I associated disease. In: Holland GN, Wilhelmus KR, editors. Ocular Infection and Immunity. St. Louis: Mosby; 1996. pp. 1366–1385. [Google Scholar]
- 36.Ohba N, Matsumoto M, Sameshima M, et al. Ocular manifestations in patients infected with human T-lymphotropic virus type I. Jpn J Ophthalmol. 1989;33:1–12. [PubMed] [Google Scholar]
- 37.Ozcan AA, Paydas S, Soylu M, et al. Bilateral choroidal infiltration from indolent non-Hodgkin'a: a rapid course with poor prognosis. Leuk Lymphoma. 2005;46:615–617. doi: 10.1080/10428190500032554. [DOI] [PubMed] [Google Scholar]
- 38.Saga T, Ohno S, Matsuda H, et al. Ocular involvement by a peripheral T-cell lymphoma. Arch Ophthalmol. 1984;102:399–402. doi: 10.1001/archopht.1984.01040030317027. [DOI] [PubMed] [Google Scholar]
- 39.Shen DF, Zhuang Z, LeHoang P, et al. Utility of microdissection and polymerase chain reaction for the detection of immunoglobulin gene rearrangement and translocation in primary intraocular lymphoma. Ophthalmology. 1998;105:1664–1669. doi: 10.1016/S0161-6420(98)99036-4. [DOI] [PubMed] [Google Scholar]
- 40.Shibata K, Shimamoto Y, Nishimura T, et al. Ocular manifestations in adult T-cell leukemia/lymphoma. Ann Hematol. 1997;74:163–168. doi: 10.1007/s002770050276. [DOI] [PubMed] [Google Scholar]
- 41.Shields JA, Shields CA. Atlas of intraocular tumors. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 324–333. [Google Scholar]
- 42.Shields JA, Stephens RF, Augsburger JJ. Intraocular tumors: International symposium under the auspices of the European Ophthalmological Society. Berlin: Springer-Verlag; 1983. pp. 433–444. [Google Scholar]
- 43.Slater DN. The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants. Br J Dermatol. 2005;153:874–880. doi: 10.1111/j.1365-2133.2005.06905.x. [DOI] [PubMed] [Google Scholar]
- 44.Smith JR, Rosenbaum JT, Wilson DJ, et al. Role of intravitreal methotrexate in the management of primary central nervous system lymphoma with ocular involvement. Ophthalmology. 2002;109:1709–1716. doi: 10.1016/s0161-6420(02)01125-9. [DOI] [PubMed] [Google Scholar]
- 45.Stenson S, Ramsay DL. Ocular findings in mycosis fungoides. Arch Ophthalmol. 1981;99:272–277. doi: 10.1001/archopht.1981.03930010274010. [DOI] [PubMed] [Google Scholar]
- 46.Whitcup SM, de Smet MD, Rubin BI, et al. Intraocular lymphoma. Clinical and histopathologic diagnosis. Ophthalmology. 1993;100:1399–1406. doi: 10.1016/s0161-6420(93)31469-7. [DOI] [PubMed] [Google Scholar]
- 47.White VA, Gascoyne RD, Paton KE. Use of the polymerase chain reaction to detect B- and T-cell gene rearrangements in vitreous specimens from patients with intraocular lymphoma. Arch Ophthalmol. 1999;117:761–765. doi: 10.1001/archopht.117.6.761. [DOI] [PubMed] [Google Scholar]
- 48.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 49.Williams GC, Holz E, Lee AG, et al. T-cell lymphoproliferative disorder of vitreous associated with mycosis fungoides. Arch Ophthalmol. 2000;118:278–280. doi: 10.1001/archopht.118.2.278. [DOI] [PubMed] [Google Scholar]
- 50.Wylen EL, Williams RB, Nanda A. Cutaneous T-cell lymphoma with intracerebral and bilateral intraocular spread. Neurol Res. 1998;20:307–312. doi: 10.1080/01616412.1998.11740523. [DOI] [PubMed] [Google Scholar]
- 51.Yahalom C, Cohen Y, Averbukh E, et al. Bilateral iridociliary T-cell lymphoma. Arch Ophthalmol. 2002;120:204–207. [PubMed] [Google Scholar]
- 52.Yeh KH, Lien HC, Hsu SM, et al. Quiescent nasal T/NK cell lymphoma manifested as primary central nervous system lymphoma. Am J Hematol. 1999;60:161–163. doi: 10.1002/(sici)1096-8652(199902)60:2<161::aid-ajh15>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]


