Abstract
BACKGROUND
Atrial fibrillation (AF) may be due to an inherited trait, particularly in lone AF patients. A family history of AF in lone AF patients has not previously been compared to those with AF and established risk factors (non-lone AF).
OBJECTIVE
To compare the frequency of having a first-degree relative with AF in lone and non-lone AF patients.
METHODS
We performed a case control study of consecutive subjects presenting to a single electrophysiology laboratory. A convenience sample of subjects with no known arrhythmias was also enrolled.
RESULTS
429 subjects were enrolled: 136 with AF (54 with lone AF), 84 with atrial flutter, 158 with other supraventricular arrhythmias, and 51 with no known arrhythmias. Significantly more subjects with AF reported a first degree family history of AF than the remainder of the cohort (25% versus 5%, p<0.001). In multivariable analysis adjusting for potential confounders, AF patients had a 6 fold greater odds of having a family member with AF (95% CI 2.93–12.7, p<0.001). Lone AF patients had a first degree family member with AF substantially more often than those with non-lone AF (41% versus 14%, p<0.001). After adjusting for potential confounders, lone AF patients remained significantly more likely than other AF patients to have a first degree relative with AF (OR 7.2, 95% CI 2.1–24.7, p=0.002).
CONCLUSION
Lone AF patients have a first degree family member with AF substantially more often than other AF patients. This suggests that an inherited trait may be particularly important in this subgroup of patients.
Keywords: Family history, Familial, Atrial Fibrillation, Lone atrial fibrillation
Introduction
Atrial fibrillation (AF) is the most common arrhythmia, responsible for significant morbidity and mortality.1, 2 Although several risk factors for the development of AF have been identified, up to 30% of all patients with the disease have no known risk factors (so called “lone” AF patients).2 Recent data suggest that the etiology of AF may be genetic,3–6 but the proportion of AF patients with an inherited genetic trait is unknown.
A recent study showed that significantly more lone AF patients have first or second degree relatives with AF than historical controls,7 but a comparison to other patients or controls in the same study has not yet been performed. While data from the Framingham cohort showed that a family history of AF is an important risk factor,8 it is unknown if a family history is more important in lone AF patients compared to AF patients with established risk factors (non-lone AF patients).
Because AF is likely multifactorial in origin (it might be due in part to any of the established risk factors such as age, hypertension, or heart failure in the majority of patients with non-lone AF),1 we hypothesized that those with lone AF would more often report a family history of AF than those with non-lone AF. In other words, if inherited genetic traits are important in AF, those with lone AF would more often carry genes specific to AF itself as opposed to non-lone AF patients who may carry genes related to the risk factors themselves (such as hypertension), which may or may not lead to AF in other family members. In order to minimize recall bias, we limited our analysis to those with and without first degree relatives with AF.
Methods
Study Design and Subjects
We performed a prospective observational study of consecutive adult patients presenting for ablation or cardioversion of AF to a university medical center over a 2 year period. Over the same time period, consecutive patients presenting to the same electrophysiology laboratory for ablations of atrial flutter, paroxysmal supraventricular tachycardia (SVT), including AV nodal reentrant tachycardia, atrioventricular reciprocating tachycardia, and focal atrial tachycardia, were enrolled as controls. A second control group of normal subjects with no known arrhythmias derived from a convenience sample of health care workers and administrative staff at a university medical center was also enrolled.
Data Collection
In addition to obtaining a medical history from chart review and patient interviews, each patient underwent a structured interview that included a standard question regarding a family history of AF. All subjects were asked if they had a first degree family member (a biologically related parent, sibling, or child) with a known history of AF. If subjects were not certain of the answer, the answer was coded as negative. Patient report of family history was validated by contacting a proportion of the first degree family members. The diagnosis of lone AF required the following criteria: age ≤ 60 years of age, no known history or current evidence of hypertension, diabetes, coronary artery disease, or heart failure.
After the initial findings of the study regarding first degree relatives was analyzed, an effort was made to re-contact all subjects with a family history of AF by phone for more detailed information regarding age of diagnosis and exact family members with the diagnosis. These data were analyzed for all family members as well as a restricted sample to only include those over age 30 (as children and siblings of subjects younger than age 30 were likely at a particularly low risk for AF).
All patients provided witnessed and informed consent. The study was approved by the UCSF Committee on Human Research.
Statistical Analysis
Normally distributed continuous variables are expressed as means ± SD. Bivariate analyses of normally distributed continuous variables were assessed using t-tests or analysis of variance as appropriate, and categorical variables were compared using the χ2 test. Multivariable analysis was performed with logistic regression analysis, and covariates/ potential confounders were selected based on both important demographics (eg, age, race, and gender) and those covariates significantly associated with both the predictors and outcomes of interest with p values <0.10 or covariates that substantially changed the regression coefficient (>20%). A general linear model for over-dispersed binomial outcomes was used to examine statistical differences in proportions of family members with AF between groups. Stata version 9.0 (College Station, Texas) was used for statistical computations. Two-tailed p values < 0.05 were considered statistically significant.
Results
A total of 429 patients were enrolled: 136 with AF (54 with lone AF), 84 with atrial flutter (36 [42%] of whom had a history of AF), 158 with SVT (14 [9%] of whom had at least one previous documented episode of AF), and 51 with no known arrhythmias. Baseline characteristics of those with and without a family history of AF for each group are shown in Table 1. Compared to the control groups (SVT and subjects with no known arrhythmias), the AF patients were generally older, more often white, and more often had a history of hypertension, coronary artery disease, and heart failure.
Table 1.
Baseline characteristics of subjects in each group.
| Atrial Fibrillation | Atrial Flutter | p value | SVT | p value | Normal Controls | p value | |
|---|---|---|---|---|---|---|---|
| (n=136) | (n=84) | (n=158) | (n=51) | ||||
| Age (years) | 59 ± 11 | 61 ± 14 | 0.17 | 46 ± 17 | <0.001 | 44 ± 14 | <0.001 |
| Male | 101 (73%) | 60 (71%) | 0.21 | 65 (41%) | <0.001 | 29 (55%) | 0.009 |
| White | 105 (80%) | 61 (73%) | 102 (65%) | 33 (62%) | |||
| Black | 3 (2%) | 3 (4%) | 6 (4%) | 2 (4%) | |||
| Asian | 14 (11%) | 12 (15%) | 20 (13%) | 11 (21%) | |||
| Latino | 6 (5%) | 4 (5%) | 16 (10%) | 3 (6%) | |||
| 0.73 | 0.098 | 0.007† | |||||
| Body mass index (kg/m2) | 28 ± 6 | 28 ± 6 | 0.92 | 27 ± 7 | 0.075 | 28 ± 17 | 0.69 |
| Hypertension | 49 (36%) | 34 (40%) | 0.51 | 32 (20%) | 0.003 | 4 (7%) | <0.001 |
| Type II diabetes | 10 (7%) | 13 (16%) | 0.056 | 10 (6%) | 0.73 | 3 (6%) | 0.64 |
| Coronary artery disease | 11 (8%) | 14 (17%) | 0.051 | 7 (4%) | 0.19 | 0 | 0.03 |
| Ejection Fraction (%)* | 58 ± 10 | 58 ± 11 | 0.99 | 62 ± 6 | 0.010 | -- | -- |
| History of congestive heart Failure | 9 (7%) | 8 (10%) | 0.43 | 0 | 0.001 | 0 | 0.051 |
P values are for comparisons to subjects with atrial fibrillation
Echocardiograms were available for 163 patients (66 with atrial fibrillation, 42 with atrial flutter, 55 with SVT, and none of the normal controls).
This difference was driven primarily by more white patients in the atrial fibrillation group SVT denotes supraventricular tachycardia
SVT denotes supraventricular tachycardia
A validation sample of first degree family members reported to have AF was contacted (family members of 5 lone AF subjects, 4 non-lone AF subjects, 2 SVT subjects, 1 atrial flutter subject, and 1 subject with no known arrhythmias), and 100% of the family members confirmed the diagnosis of AF.
Patients with AF more frequently had a first degree family member with AF (Figure 1). The baseline characteristics of the AF subjects with and without a family history of AF are shown in Table 2. Of those with lone AF, 41% reported a first degree family member as having AF: in bivariate analysis, this proportion was significantly greater than the non-lone AF subjects and than all of the other subjects combined. Although approximately only one fourth of AF and atrial flutter subjects reported a family history of AF, each more commonly had a family history than did the control subjects. The atrial flutter patients with a previous history of AF more often had a first degree family member with AF than those without a prior history of AF (14% and 8%, respectively), but this was not statistically significantly (p=0.42). Lone atrial flutter patients (n=21) more often had a first degree relative with AF (19%) than the non-lone atrial flutter patients (8%), but this finding was not statistically significant (p=0.15).
Figure 1.
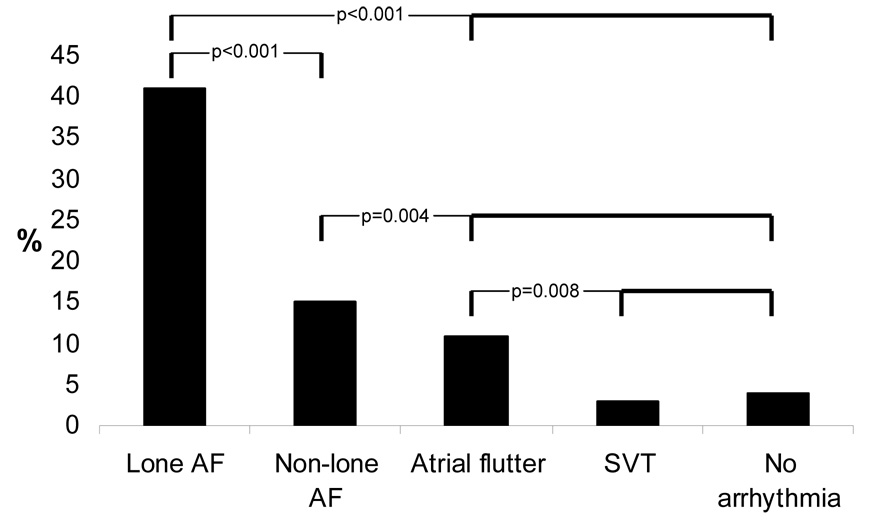
Proportions of patients in each group that reported having at least one first degree family member with atrial fibrillation. P values represent comparisons denoted by brackets in bold.
Table 2.
Baseline characteristics of atrial fibrillation patients with and without a first degree relative with atrial fibrillation.
| First degree relative with atrial fibrillation | |||
|---|---|---|---|
| Yes (n=34) | No (n=102) | p value | |
| Age (years) | 56 ± 10 | 60 ± 12 | 0.16 |
| Male | 26 (76%) | 75 (74%) | 0.73 |
| White | 27 (82%) | 78 (80%) | |
| Black | 2 (6%) | 1 (1%) | |
| Asian | 3 (9%) | 11 (11%) | |
| Latino | 1 (3%) | 5 (5%) | 0.39 |
| Body mass index (kg/m2) | 27 ± 26 | 28 ± 27 | 0.65 |
| Hypertension | 8 (24%) | 41 (40%) | 0.08 |
| Type II diabetes | 1 (3%) | 9 (9%) | 0.26 |
| Coronary artery disease | 0 | 11 (11%) | 0.046 |
| Ejection Fraction (%)* | 59 ± 10 | 58 ± 11 | 0.65 |
| Heart Failure | 0 | 9 (9%) | 0.073 |
Echocardiograms were available on 66 patients (22 with a family history and 44 without a family history of atrial fibrillation).
The ultimate diagnosis made during electrophysiology study in the SVT group resulted in a primary diagnosis of typical AV nodal reentrant tachycardia in 68 (43%), atrioventricular reciprocating tachycardia in 43 (26%), focal atrial tachycardia in 23 (15%), atypical AV nodal reentrant tachycardia in 6 (4%), reentrant atrial tachycardia in 1 (1%), junctional tachycardia in 1 (1%) and no inducible arrhythmia in 15 (10%, 2 with ventricular preexcitation). There was no difference in reported family history of AF between these groups, nor between those SVT patients with a history of AF and SVT subjects without a history of AF.
After logistic regression analysis adjusting for age, gender, race, hypertension, coronary artery disease, heart failure, and body mass index, those with AF had a 6 fold greater odds of reporting a first degree family member than the remainder of the subjects (95% confidence interval [CI] 2.93–12.7, p<0.001). Because, by definition, the lone AF patients did not have hypertension, coronary disease, or congestive heart failure, a separate logistic regression model was examined controlling for age, race, gender, and BMI (Figure 2): after this adjustment, lone AF, non-lone AF, and atrial flutter patients each exhibited significantly greater odds of reporting a first degree family member with AF.
Figure 2.
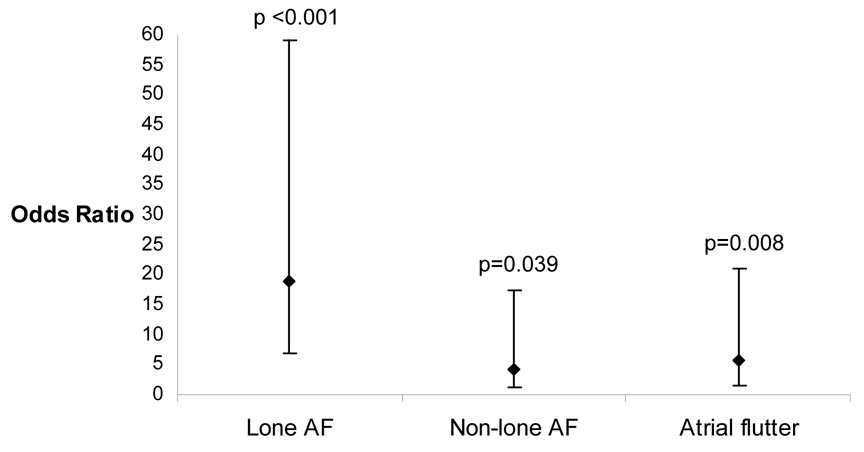
Odds ratios of having at least one first degree relative with AF in the lone AF, non-lone AF, and atrial flutter patients compared to controls after adjusting for age, race, gender, and body mass index. Error bars denote 95% confidence intervals.
Finally, after adjusting for these same potential confounders, lone AF patients had a 7 fold greater odds (95% CI 2.1–24.7, p=0.002) of reporting a first degree relative with AF than non-lone AF patients.
An attempt to re-contact the 49 subjects with a first degree family history of AF was made a mean 759 ± 295 days after the initial interview: 3 patients could not be contacted because they had moved out of the area and/or had changed phone numbers, 1 was deceased, and 13 declined to participate. Of the 31 that underwent a more detailed interview, 13 had lone AF, 8 had non-lone AF, 5 had AFL (1 with lone AFL), 3 had SVT, and 2 were normal controls. Three (23%) lone AF patients, 2 (25%) non-lone AF patients, and 1 (20%) AFL patient had 2 family members with AF. Only 2 subjects reported having 3 family members with AF: 1 with lone AF and 1 with lone AFL. The mean age at the first diagnosis of AF in the lone AF subjects was 59 ± 11 years old. Although not statistically significant, the mean age was older for the family members of the rest of the cohort (65 ± 11 years old, p=0.11) and for the family members of the non-lone AF subjects alone (68 ± 11 years old, p=0.074). The types of family members with AF are shown in Table 3; of note, the total number of siblings, total number of children, and the total number of children over age 30 was not significantly different for those with lone AF versus the other groups. Although these findings did not reach statistical significance, of all subjects with at least one family member with AF who provided a detailed family history, the proportion of all family members with AF was greater for those with lone AF (25%) versus the remainder of the cohort (20%, p=0.52) and versus those with non-lone AF (19%, p=0.36); an analysis including only family members older than age 30 yielded similar results. Finally, only 2 subjects reported having 3 consecutive generations with AF, and both were lone AF subjects: a 47 year old white male with both parents and a son with AF and a 56 year old white male with a father and a son with AF.
Table 3.
Of the 31 subjects with complete family history data, the number of subjects with at least one family member of the type described in each row is shown.
| Family Member Type (at least one) | Lone Atrial Fibrillation (n=13) | All other subjects without lone AF*(n=18) | p value | Non-Lone Atrial Fibrillation (n=8) | p value |
|---|---|---|---|---|---|
| Parent | 11 (85%) | 12 (67%) | 0.26 | 5 (62%) | 0.25 |
| Father | 7 (53%) | 6 (33%) | 0.25 | 1 (13%) | 0.058 |
| Sibling† | 4 (31%) | 6 (35%) | 0.79 | 3 (38%) | 0.59 |
| Children† | 2 (15%) | 1 (6%) | 0.36 | 0 | 0.24 |
| Aunt or Uncle | 1 (8%) | 4 (22%) | 0.28 | 1 (13%) | 0.72 |
| Grandparent | 0 | 1 (6%) | 0.39 | 0 | |
| Male family members | 10 (77%) | 11 (61%) | 0.35 | 4 (50%) | 0.20 |
| Female family members | 6 (46%) | 12 (67%) | 0.25 | 6 (75%) | 0.20 |
This group consists of 8 non-lone atrial fibrillation subjects, 5 atrial flutter subjects, 3 supraventricular tachycardia subjects, and 2 normal controls.
All of these relatives were male
Discussion
We found that patients with lone AF report a first degree relative with AF substantially more frequently than those with AF associated with established risk factors. This finding persisted after adjustment for multiple potential confounders and, importantly, was evident despite the fact that the non-lone AF patients more often reported a first degree family history of AF than controls.
Parental AF has been shown to be a risk factor for AF,8 and one previous study found that 38% of lone AF patients had a first or second degree family member with a history of AF.7 That 41% of lone AF patients reported a first degree relative with AF in our study validates this previous study, and our finding that this proportion far exceeds that observed in non-lone AF patients further supports the notion that the genetic component in lone AF patients involves a mechanism unique to AF itself (rather than an indirect link via modifying risk factors for AF). We of course can not exclude the possibility that there remain other inherited as yet unknown modifying risk factors. Of note, it is highly unlikely that our findings reflect an error due to an insufficient sample size: even the lowest estimate (from the 95% confidence interval of the adjusted analysis) suggests that the “worst case scenario” weakest relationship would be a 2 fold greater odds of having a first degree family member in those with lone AF compared to those with non-lone AF.
Although various genetic mutations have been described in certain rare families with AF,9, 10 the genes responsible for the majority AF are likely more common because AF is a relatively common disease. It is also likely that AF does not represent a manifestation of a single genetic mutation,11 but rather represents a final common pathway and/ or a manifestation of more complex combinations of genetic polymorphisms: for example, tissue specific genetic mutations in the atria of AF patients have been described,12 and single nucleotide polymorphisms (SNPs) in genes related to electrophysiologic properties9, 13 and the myocardial substrate5, 6 have previously been shown to be important in AF. Given known risk factors for AF (such as hypertension, valvular disease, thyrotoxicosis, a post-operative state),1 the etiology may often also commonly involve environmental factors. Therefore, while the genetic studies have been helpful in determining what genes may be statistically more often found in AF patients, it is equally important to characterize the proportion of AF patients that might actually have an inherited trait as the primary etiology of their arrhythmia. Finally, it is important to emphasize that, while a family history is suggestive of an inherited genetic trait, environmental factors may also be more common within families and therefore responsible for clustering of a disease within a family.
Of interest, we found that patients with atrial flutter more often had a first degree family member with AF. This may reflect the fact that those with atrial flutter often also have AF (and that they are inter-related),14 but the proportion with a family history did not significantly differ between the atrial flutter patients with and without a known history of AF. Consistent with our overall findings (although only nearing statistical significance [p=0.15]), those with “lone” atrial flutter more often reported a family history of AF than the remainder of the atrial flutter patients.
Although the number of subjects providing a full detailed family history after being re-contacted was relatively small (n=31), likely contributing to a lack of statistically significant findings, several discoveries remain noteworthy: as the differences in gender did not significantly differ between groups, the male predominance appears to reflect the generally greater risk of AF in men rather than any particular sex-linked mode of inheritance in one group versus another. First degree family members of lone AF subjects with AF were generally younger and made up a larger proportion of all family members, and more than 2 family members with AF (as well as more than one generation with AF) were only observed in lone AF or lone AFL subjects, all supporting the notion that this group more likely has an important inherited component to their disease.
This study has several potential limitations. First, detailed family histories were not obtained in all subjects. However, previous important studies regarding family history of arrhythmias have also been limited to obtaining a dichotomous (yes/ no) answer regarding a first degree relative.15, 16 While this may limit the details of potential analyses, it does not negate our positive findings. Second, we cannot exclude the possibility of recall bias- for example, those with lone AF may be more motivated to learn about their family history and potentially more quick to ascribe a diagnosis of AF to a family member with an arrhythmia. We attempted to minimize recall bias in 4 ways: 1) Only those who reported a definitive and specific family history of AF were counted as positive 2) By limiting the primary analysis to first degree relatives, subjects were likely more certain of the history (ie, limited only to parents, children, or siblings) 3) Our control subjects also had motivation to seek out a potential family history of arrhythmia (the non-lone AF patients, the atrial flutter patients and the SVT patients) or would likely be sufficiently knowledgeable to recognize or be aware of the diagnosis in the family (in the case of the controls without arrhythmias, the majority of which worked at a medical center) 4) A validation sample of first degree family members contacted revealed that all (100%) confirmed the diagnosis, suggesting that the family histories were likely accurate. Of note, excluding those who were uncertain of a family history of AF heightened the specificity of the outcome, potentially sacrificing sensitivity; however, this strategy would bias our findings towards the null hypothesis and therefore does not negate the positive findings described. Finally, the sample studied arose from consecutive subjects presenting to an electrophysiology laboratory rather than a selection from a broader population (such as those presenting to a cardiology or general medicine clinic); while this may limit extrapolation to the general population with lone AF, it should not bias the internal validity of the study. For example, we cannot exclude the possibility that these particular lone AF patients presenting for ablation were especially drug resistant and/or symptomatic, potentially reflecting a unique subset of lone AF patients.
In conclusion, a very large proportion of patients with lone AF have a first degree family member with AF, suggesting that a genetic etiology specific to atrial pathology may be particularly important in this subgroup of AF patients. As those with AF associated with established risk factors reported a first degree relative with AF substantially less often, etiologies in that group may more often reflect environmental factors and/ or inherited traits related to the risk factors (eg, hypertension) themselves rather than atrial abnormalities per se. Future studies aimed at characterizing phenotypic differences in AF patients with and without a family history of AF (such as differences in mechanisms of initiation of the arrhythmia or differences in left atrial/pulmonary vein anatomy) can help elucidate the more exact inherited processes involved, potentially helping to imply candidate genes or gene regions.
Acknowledgments
Funding Sources
This work was made possible by Grant Number K12 RR024130 (G.M.M.) from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research, and the American Heart Association Western States Affiliate Beginning Grant-in-Aid Award (G. M. M.).
Abbreviations
- AF
Atrial fibrillation
- SVT
Supraventricular tachycardia
- CI
Confidence interval
- BMI
Body mass index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author disclosures/ potential conflicts of interest: None
References
- 1.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48(4):854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Blackshear JL, Shen WK, et al. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37(2):371–378. doi: 10.1016/s0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 3.Gudbjartsson DF, Arnar DO, Helgadottir A, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448(7151):353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 4.Ellinor PT, Shin JT, Moore RK, et al. Locus for atrial fibrillation maps to chromosome 6q14–16. Circulation. 2003;107(23):2880–2883. doi: 10.1161/01.CIR.0000077910.80718.49. [DOI] [PubMed] [Google Scholar]
- 5.Firouzi M, Ramanna H, Kok B, et al. Association of human connexin40 gene polymorphisms with atrial vulnerability as a risk factor for idiopathic atrial fibrillation. Circ Res. 2004;95(4):e29–e33. doi: 10.1161/01.RES.0000141134.64811.0a. [DOI] [PubMed] [Google Scholar]
- 6.Tsai CT, Lai LP, Lin JL, et al. Renin-angiotensin system gene polymorphisms and atrial fibrillation. Circulation. 2004;109(13):1640–1646. doi: 10.1161/01.CIR.0000124487.36586.26. [DOI] [PubMed] [Google Scholar]
- 7.Ellinor PT, Yoerger DM, Ruskin JN, et al. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118(2):179–184. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, Parise H, D'Agostino RB, Sr., et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291(23):2851–2855. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 9.Chen YH, Xu SJ, Bendahhou S, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299(5604):251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 10.Brugada R, Tapscott T, Czernuszewicz GZ, et al. Identification of a genetic locus for familial atrial fibrillation. N Engl J Med. 1997;336(13):905–911. doi: 10.1056/NEJM199703273361302. [DOI] [PubMed] [Google Scholar]
- 11.Darbar D, Herron KJ, Ballew JD, et al. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol. 2003;41(12):2185–2192. doi: 10.1016/s0735-1097(03)00465-0. [DOI] [PubMed] [Google Scholar]
- 12.Gollob MH, Jones DL, Krahn AD, et al. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med. 2006;354(25):2677–2688. doi: 10.1056/NEJMoa052800. [DOI] [PubMed] [Google Scholar]
- 13.Lai LP, Su MJ, Yeh HM, et al. Association of the human minK gene 38G allele with atrial fibrillation: evidence of possible genetic control on the pathogenesis of atrial fibrillation. Am Heart J. 2002;144(3):485–490. doi: 10.1067/mhj.2002.123573. [DOI] [PubMed] [Google Scholar]
- 14.Waldo AL. The interrelationship between atrial fibrillation and atrial flutter. Prog Cardiovas Dis. 2005;48(1):41–56. doi: 10.1016/j.pcad.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Jouven X, Desnos M, Guerot C, et al. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99(15):1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 16.Friedlander Y, Siscovick DS, Arbogast P, et al. Sudden death and myocardial infarction in first degree relatives as predictors of primary cardiac arrest. Atherosclerosis. 2002;162(1):211–216. doi: 10.1016/s0021-9150(01)00701-8. [DOI] [PubMed] [Google Scholar]


