Abstract
The ventral striatum mediates goal-directed behaviors based, in part, on inputs from the amygdala. However, striatal areas caudal to the ventral striatum also receive inputs from the amygdala. In primates, the amygdala projects to the central ventral putamen, lateral amygdalostriatal area, and caudal ventral putamen, suggesting that these regions are also “limbic-related.” The anterior insula, which integrates sensory and amygdaloid inputs, projects to the classic ventral striatum. We used retrograde and anterograde tract tracing techniques to determine the extent to which specific subdivisions of the insula influence the caudal ventral striatum in the primate. The anterior (agranular and rostral dysgranular) insula has significant inputs to caudal ventral striatal regions that receive projections from the amygdala. In contrast, the posterior (granular) insula has sparse projections. Within the agranular insula, the posteromedial agranular (Iapm), lateral agranular (Ial), and posterolateral agranular (Iapl) subdivisions have the strongest inputs. These subdivisions mediate olfactory, gustatory, and visceral information processing (Carmichael and Price JL [1996b] J. Comp. Neurol. 363:642–640). In contrast, the intermediate agranular subdivision (Iai) is relatively devoid of visceral/gustatory inputs and has few inputs. In summary, caudal ventral striatal areas that receive amygdaloid inputs also receive significant innervation by agranular and dysgranular insula subdivisions that are themselves connected with the amygdala. Within this projection, the Ial, Iapm, and Iapl make the strongest contribution, suggesting that highly processed visceral/autonomic information, taste, and olfaction influence behavioral responses mediated by the caudal ventral striatum.
Keywords: limbic, gustatory, caudate, putamen, amygdalostriatal area, amygdala, tract tracing
The rostral ventral striatum is considered a substrate for goal-directed behaviors based on its inputs from multiple brain regions mediating motivation and reward (Mogenson et al., 1980; Nauta, 1986; Ferry et al., 2000; Haber et al., 2000). However, it is increasingly recognized that caudal ventromedial striatal regions also receive inputs from several “limbic” brain regions, including the amygdala and anterior cingulate cortex (Russchen et al., 1985; Selemon and Goldman-Rakic, 1985; Nauta, 1986; McDonald et al., 1999; Ferry et al., 2000; Shammah-Lagnado et al., 2001; Fudge et al., 2004). We have previously defined the primate “caudal ventral striatum” based on amygdaloid afferent inputs and histochemical markers typical of the ventral striatum (Fudge and Haber, 2002; Fudge et al., 2004). Based on these criteria, the caudal ventral striatum includes the ventromedial putamen surrounding, and caudal to, the decussation of the anterior commissure, the lateral amygdalostriatal area, and the medial tail of the caudate nucleus.
The anterior insula is involved in emotional and multisensory processing (Lane et al., 1997; Phillips et al., 1998; Chua et al., 1999; Small et al., 2001; Krolak-Salmon et al., 2003) and has prominent inputs to the classic ventral striatum (Chikama et al., 1997; Ferry et al., 2000). The anterior half of the insular cortex also has strong interconnections with the amygdala (Aggleton et al., 1980; Mufson et al., 1981; Carmichael and Price, 1996a) as well as an array of inputs from sensory and sensory association regions (Carmichael and Price, 1996b). Consistent with these afferent connections, the anterior insula is metabolically active during a variety of emotional experiences including disgust (Phillips et al., 1998; Krolak-Salmon et al., 2003; Heining et al., 2003; Wicker et al., 2003), fear (Schienle et al., 2002), and sadness (Lane et al., 1997; Damasio et al., 2000; Liotti et al., 2002). Early classic studies in monkeys and humans showed that stimulation of the anterior insula generates gustatory, autonomic, and visceral responses (Kaada et al., 1949; Penfield and Faulk, 1955; Hoffman and Rasmussen, 1953), suggesting that the insula links emotional states with internal responses (see reviews by Augustine, 1996; Mesulam, 2000).
The anterior insula is composed of the agranular cortex, including the posterior orbital surface, and dysgranular cortex (Id) (Rose, 1928; Mesulam and Mufson, 1982; Carmichael and Price, 1994). More recently, the agranular insula has been subdivided into multiple territories based on unique cytoarchitectural, histochemical, and connectional properties (Carmichael and Price, 1994). These agranular insular subdivisions have differential connections to the specific subregions of the sensory cortex and thalamus (Carmichael and Price, 1996b). In contrast to the anterior insula, the posterior insula has a granular organization (Ig) and receives few inputs from the amygdala (Mesulam and Mufson, 1982; Aggleton et al., 1980; Mufson et al., 1981). Although previous studies show that both the agranular and dysgranular regions of the anterior insula have significant inputs to the classic ventral striatum in primates (Chikama et al., 1997; Ferry et al., 2000), there is little information on specific insular projections to caudal aspects of the “limbic-related” striatum.
To investigate the extent to which the caudal ventral striatum is influenced by specific subdivisions of the insula, we charted the distribution of retrogradely labeled cells in the insular subdivisions following small injections of retrograde tracers into several caudal ventral striatal regions. Injection sites were classified as being in the “limbic-related” striatum if they resulted in labeled cells in the amygdala based on our previous analysis (Fudge et al., 2004). Anterograde tracers then were placed into the anterior insula to verify the data from retrograde studies.
MATERIALS AND METHODS
Injection sites
We placed injections of horseradish peroxidase conjugated to wheat germ agglutinin (HRP-WGA; 10%, Sigma, St. Louis, MO), Lucifer Yellow conjugated to dextran amine (LY; 10%, Molecular Probes, Eugene, OR), fluorescein conjugated to dextran amine (FS; 10%, Molecular Probes), or Fluoro Ruby conjugated to dextran amine (tetramethylrhodamine; FR; 4%, Molecular Probes) into the amygdalostriatal area and caudal ventral striatum as previously described (Fudge et al., 2004). One striatal injection in the caudal dorsomedial striatum, which resulted in no labeled cells in the amygdala, served as a control (case J11WGA; see Results). Several injection sites were also placed in the central amygdaloid nucleus for comparison. Anterograde injections of LY, FR, FS, or tritiated amino acids (AA; equal amounts of tritiated proline and leucine; New England Nuclear, Boston, MA) were then placed into the rostral insula.
Surgeries
Eight Old World monkeys (Macaque nemestrina and Macaque fascicularis) weighing between 3.0 and 4.5 kg were used in these studies (Labs of Virginia, Yemassee, SC and Three Springs Laboratories, Pekaski, PA). All animals were male except cases J7 and J8. All experiments were carried out in accordance with National Institutes of Health guidelines. Experimental design and techniques were aimed at minimizing animal use and suffering and were reviewed and approved by the University of Rochester Committee on Animal Research. Initial anesthesia was administered by an intramuscular injection of ketamine hydrochloride (10 mg/kg), and a deep anesthesia was induced by intravenous injection of pentobarbital (initial dose 20 mg/kg IV and maintained as needed). A craniotomy was then performed, and the bone flap was removed to visualize the cortical surface. Electrophysiological mapping was performed to locate appropriate injection sites. The location of neurons encountered in a series of penetrations was used to prepare a map indicating the boundaries of different subcortical structures and the fiber tracts surrounding them. Striatal regions were identified by the characteristic discharge patterns of tonically active neurons (Aosaki et al., l995). The stereotaxic coordinates for the anterior commissure were determined first. The striatal regions of interest were then located.
After stereotaxic coordinates for striatal targets were determined, small deposits of retrograde tracers (40–50 nL) were pressure-injected over 10 minutes into discrete regions of the amygdalostriatal area and caudal striatum using a 0.5-µL Hamilton syringe. Following each injection, the syringe remained in situ for 20 minutes to prevent leakage up the needle track. Additional injections of FR, LY, FS (40–50 nL), and tritiated amino acids (150–200 nL) were pressure-injected in the insula, using a 0.5-µL Hamilton syringe, after electrophysiologic localization. Following injections, the bone flap was replaced, and the overlying musculature and skin were sutured. Animals were treated with routine prophylactic antibiotics and analgesics for 7 to 10 days following surgery.
Ten to 13 days after surgery, animals were deeply anesthetized and killed by perfusion through the heart with 0.9% saline containing 0.5 mL heparin sulfate, followed by cold 4% paraformaldehyde solution in 0.1 M phosphate buffer (pH 7.4). The brains were then removed and cryoprotected in increasing gradients of sucrose (10, 20, and 30%). The entire brain was cut into serial sections of 50 µm on a freezing microtome and placed into 0.1 M phosphate buffer or cryoprotectant solution. Combinations of adjacent sections through the insula were selected for labeling of tracer and histochemical markers of the agranular insula subdivisions: calbindin-D28K (CaBP), parvalbumin, cresyl violet, acetylcholinesterase (AChE), and SMI-32 (nonphosphorylated neurofilament protein) (Carmichael and Price, 1994). Additional compartments were labeled for tracer and counterstained with cresyl violet or AChE.
Immunohistochemistry
Tracers
Sections were first thoroughly rinsed in 0.1 M phosphate buffer (pH 7.2) with 0.3% Triton X-100 (PB-T). Following treatment with endogenous peroxidase inhibitor, and more PB-T rinses, tissue was preincubated in 10% normal goat serum (NGS) diluted with PB-T (NGS-PB-T) for 30 minutes. Tissue was then placed in primary antisera to HRP-WGA (1:10,000; Sigma, rabbit), LY (1:2,000; Molecular Probes, rabbit), FS (1:2,000; Molecular Probes, rabbit), or FR (1:1,000; Molecular Probes, rabbit) for approximately 96 hours at 4°C. Following rinses with PB-T, the tracers were visualized by using the avidinbiotin complex reaction (Vectastain ABC kit, Vector, Burlingame, CA). Staining was enhanced by incubating sections for 1–3 minutes in 3,3′-diaminobenzidine tetrahydrochloride and 0.03% hydrogen peroxide intensified with 1% cobalt chloride and 1% nickel ammonium sulfate. Additional compartments from each case were processed for tracer and counterstained with cresyl violet or AChE (Geneser-Jensen and Blackstad, 1971) to determine the distribution of labeled cells within specific cytoarchitectonic subdivisions of the insula and the relative placement of the injection site within the striatal area.
Parvalbumin, SMI-32, tyrosine hydroxylase (TH), and CaBP immunostaining
Control studies in which the primary antibodies were omitted showed no labeling for any of the markers. Sections were processed in the manner described above, using a monoclonal antibody to human parvalbumin at 1:10,000 dilution (mouse monoclonal, Swant, Bellinzona, Switzerland), SMI-32 at 1:5,000 dilution (mouse monoclonal, Sternberger Monoclonals, Lutherville, MD), TH at 1:10,000 (mouse monoclonal, Chemicon, Temecula, CA), or CaBP at 1:10,000 (mouse monoclonal, Sigma).
Autoradiography
Sections for autoradiography were mounted on chrome-alum gelatin-coated slides and defatted into xylene for 48 hours. Slides were dipped in Kodak NTB2 photographic emulsion (Kodak, Rochester, NY) and exposed for 4–6 months at −20°C in a light-tight box. The slides were then developed in cold Kodak D19 (15°C) for 2.5 minutes, fixed, washed, and counterstained with cresyl violet.
Analysis
Retrograde injection sites were classified as being in the central amygdaloid nucleus or caudal ventral striatum based on their placement into the AChE-poor (central nucleus/medial amygdalostriatal area) or AChE-positive zone (lateral amygdalostriatal area and conventional striatum). Injection sites determined to be within the striatum were classified as being in the ventral (“limbic-related”) caudal striatum based on the presence of retrogradely labeled cells in the amygdala.
Cases were then examined for labeled cells in the insula, which were hand-charted by using a drawing tube attached to the microscope under brightfield illumination (10× objective). The boundaries of the insula were hand-traced from adjacent sections counterstained for cresyl violet and/or AChE and from adjacent sections processed for parvalbumin, CaBP, or SMI-32 immunoreactivity. Hand-drawn charts of retrogradely labeled cells were entered into the computer by using a drawing tablet (Wacom Technology, Vancouver, BC, Canada) in conjunction with the program Canvas 5.0 (Deneba Systems, Vancouver, BC, Canada). The boundaries of the insula were also entered into the computer by using the drawing tablet and were aligned over charts of retrogradely labeled neurons, matching landmarks such as blood vessels and fiber tracts. For anterograde cases, the distribution of anterogradely labeled fibers in the striatum and amygdalostriatal area was charted by hand with the drawing tube. Charts were prepared under bright- and dark.eld illumination using a 25× objective. Hand-drawn charts of labeled fibers were then scanned into the computer with Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA) and an Epson 3200 Perfection scanner (300 dpi). Photographs were captured digitally by using an Optronics Microfire color CCD and converted to grayscale. Contrast and brightness enhancement were used to optimize the visibility of the relevant structures.
RESULTS
Defining the caudal ventral striatum
We define the caudal ventral striatum based on amygdaloid inputs, as well as cytoarchitectural and histochemical features found in the “classic ventral” striatum (Fudge and Haber, 2002; Fudge et al., 2004) (Fig. 1). Although both the caudal ventral striatum and closely apposed central amygdaloid nucleus received amygdaloid inputs, the caudal ventral striatum contained moderate AChE activity and strong TH immunoreactivity, both of which are traditional striatal markers across species (Sarnat and Netsky, 1981) (Fig. 1A,D, C,F). The medial amygdalostriatal area is excluded from the caudal ventral striatum based on a lack of AChE and TH (Heimer et al., 1999; Fudge and Haber, 2002) (Fig. 1A,D, C,F). The lateral amygdalostriatal area and contiguous caudal ventral putamen and tail of the caudate nucleus are thus considered part of the ventral (limbic-related) striatum, based on the presence of striatal markers, and inputs from the amygdala (Sarnat and Netsky, 1981; Fudge et al., 2004). The lateral amygdalostriatal area is further distinguished by low CaBP immunoreactivity compared with the rest of the caudoventral striatum, reminiscent of the “shell” subterritory of the rostral ventral striatum (Fudge and Haber, 2002) (Fig. 1B,E, dashed line).
Fig. 1.
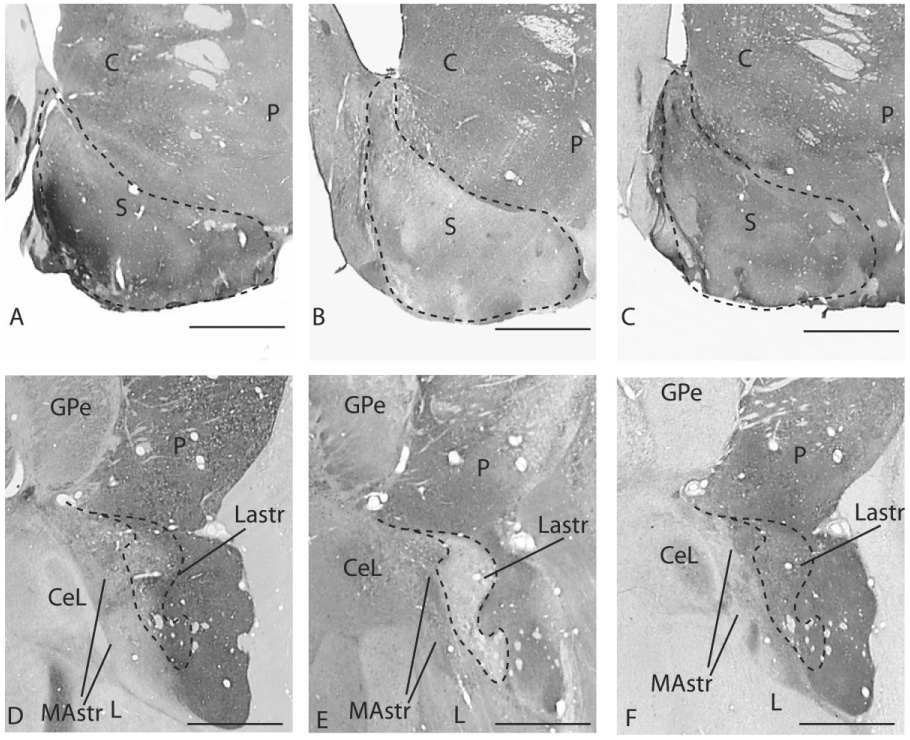
Photomicrographs of sections through the rostral (A–C) and caudal (D–F) ventral striatum stained for AChE (A,D), CaBP (B,E), and TH (C,F). Intermediate to high AChE activity is found in the ventral striatum both rostrally and caudally (A,D) Low CaBP immunoreactivity is found in the shell of the rostral ventral striatum (B) and lateral amygdalostriatal area (E), overlapping areas of intermediate to high AChE staining. Dotted line depicts the CaBP-poor shell and lateral amygdalostriatal area. The medial amygdalostriatal area contains very low to absent AChE activity (D) and TH immunostaining (F) and high CaBP immunoreactivity (E). For abbreviations, see list. Scale bars = 1 mm in A–F.
Insular subdivisions
We use the criteria put forth by Carmichael and Price (1994) to describe the cytoarchitectonic subdivisions of the agranular insula in the primate. The agranular insula is defined by its lack of granule cell layers in layers II and IV and its association with the claustrum (Fig. 2A–C). Based on this definition, the agranular insula extends rostrally from the lateral fissure onto the posterior orbital surface. It is bordered anteriorly by area 13 and the caudal part of area 12 (12o), laterally by the gustatory cortex, and caudally and medially by the primary olfactory cortex (the anterior olfactory nucleus [AON] and the piriform cortex). The rostral agranular insula is divided into five subdivisions based on cytoarchitectonic and histochemical criteria: the medial (Iam), intermediate (Iai), lateral (Ial), posteromedial (Iapm), and posterolateral (Iapl) agranular insula (Carmichael and Price, 1994). In Nissl-stained sections, the medial subdivisions, Iam and Iapm, are less differentiated and lack a sublaminated layer V (Fig. 2A). The lateral subdivisions, in contrast, have five layers and a sublaminated layer V. The gustatory cortex is in the superior limiting sulcus (SLS), laterally adjacent to the Ial and Iapl on the caudal orbital surface and is identified by its dense parvalbumin-immunoreactive neuropil in layers III/IV (Carmichael and Price, 1994) (Fig. 2A,D).
Fig. 2.
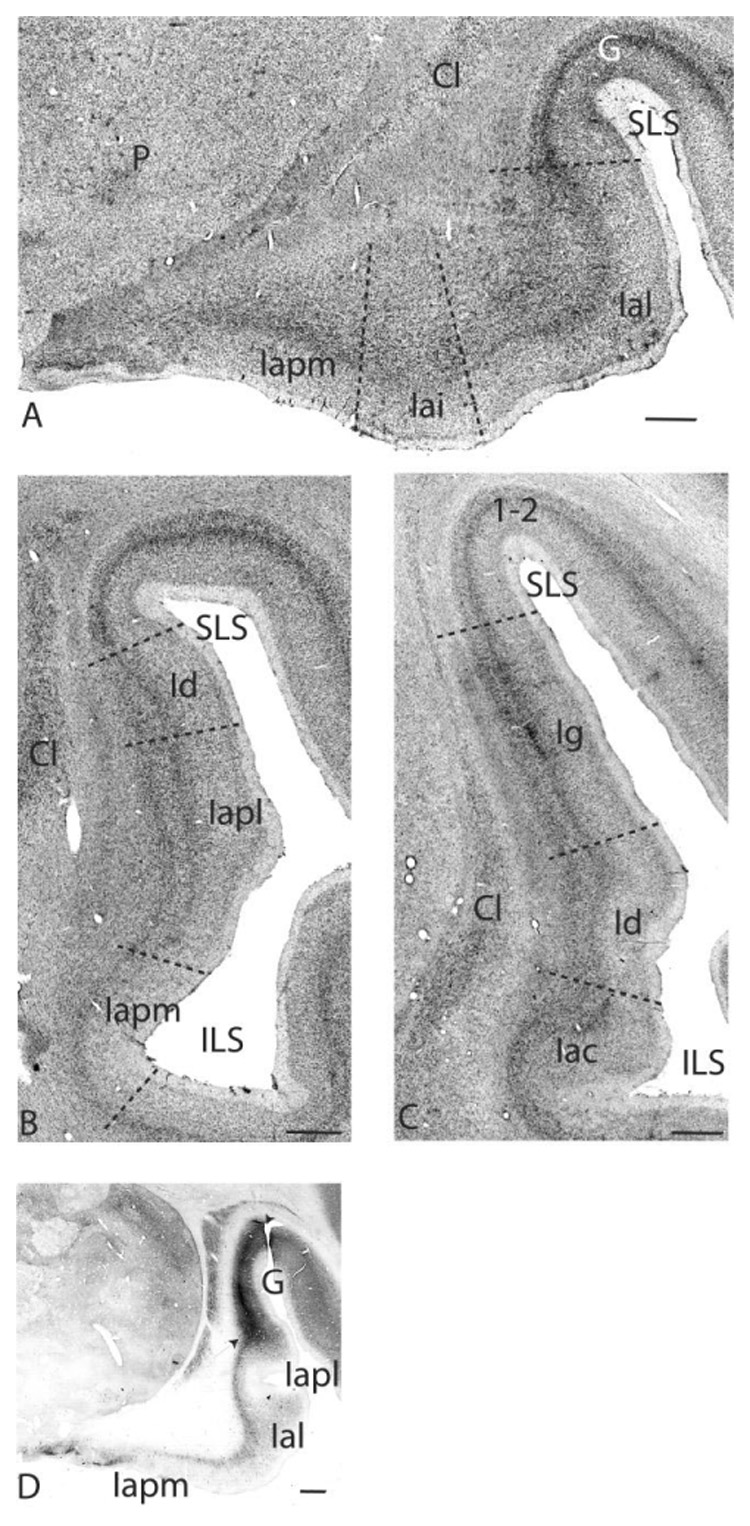
Nissl-stained sections at several rostrocaudal levels of the insula. A: The rostral agranular insula is found on the caudal orbital surface and is bordered laterally by the gustatory cortex. The Iapm has three rudimentary layers, and the Ial has five layers including a sublaminated layer V. Iai has a partially sublaminated layer V. B: Further caudally, the Iapm and Iapl extend into the sylvian fissure. They are bordered dorsally by the Id. C: The Iac, Id, and Ig at the level of the decussation of the anterior commissure. D: Dense parvalbumin immunoreactivity in layers III/IV distinguishes the gustatory cortex. Iapm is distinguished by low parvalbumin staining. Photographs were digitally captured and tiled by using ImagePro software. For abbreviations, see list. Scale bars = 1 mm in A–D.
Viewed rostrocaudally, the Iam is the rostralmost subdivision. Iai is located lateral to it. Iapm, Ial, and Iapl are the more posteriorly located subdivisions, with Iapm and Iapl continuing caudally into the lateral fissure to form the rostral medial bank of the inferior limiting sulcus (ILS; Fig. 2B). There is a caudal agranular insular area (Iac), which forms the caudalmost reaches of the agranular insula, posterior to the Iapm and Iapl (Fig. 2C) (Carmichael and Price, 1996b). The dysgranular cortex is dorsally and caudally adjacent to the Iapl and Iac in the lateral fissure. It is a transition zone that has poorly formed granule layers in both layers II and IV (Fig. 2B,C) (Mesulam and Mufson, 1982). The granular insula lies caudal and dorsal to the dysgranular region and has five to six well-differentiated layers, including granular layers II and IV (Fig. 2C).
Location of retrograde injection sites
There were a total of ten retrograde injection sites (Fig. 3A–C). Of these, seven injections were located in the caudal ventral striatum (Fig. 3 A–C, gray sites), one injection was outside the caudal ventral striatum (Fig. 3C, white site), and two injections were in the central nucleus (Fig. 3C, black sites).
Fig. 3.
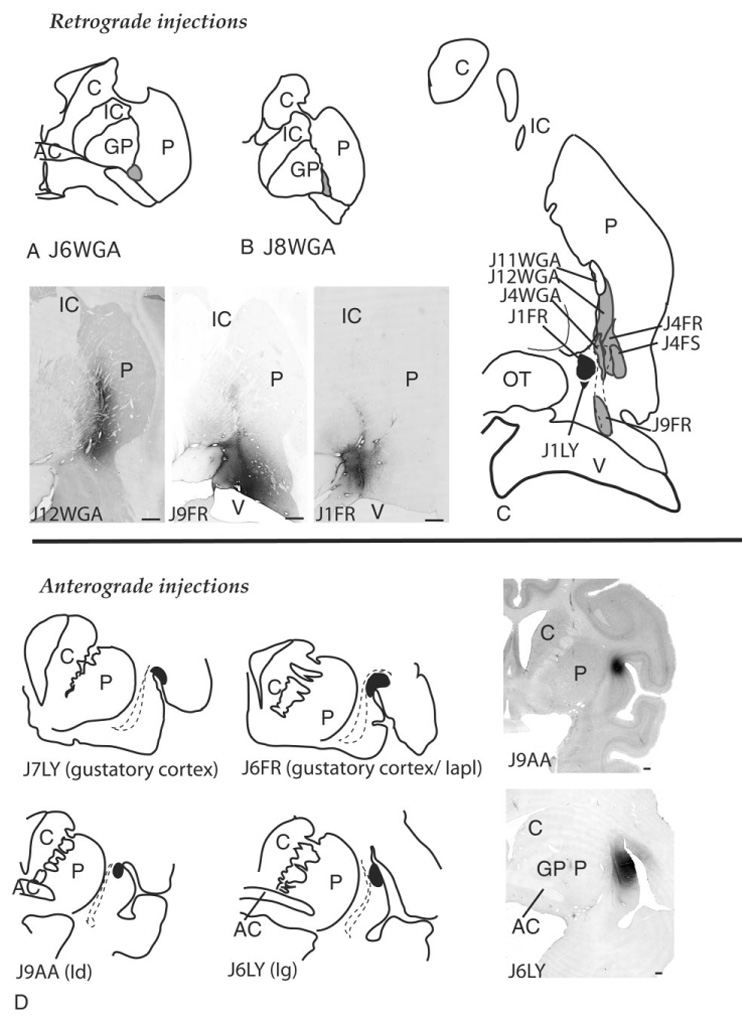
Schematic of retrograde injection sites in the caudal striatum and central nucleus (A–C, top) and anterograde injection sites in the insula and gustatory cortex (D, bottom). Photomicrographs of several injection sites are shown for each series. For abbreviations, see list. Scale bars = 1 mm in A,B,D.
In the caudal ventral striatum, two injections were located in the ventral putamen at the level of the crossing of the anterior commissure (cases J6WGA, J8WGA; Fig. 3A,B), and five injection sites were located in the ventromedial striatum posterior to the decussation of the anterior commissure (cases J12WGA, J4WGA, J9FR, J4FR, J4FS; Fig. 3C). The injection site in case J9FR was placed caudally in the CaBP-poor zone of the emerging tail of the caudate nucleus and included a small area of the medial amygdalostriatal area.
The two injection sites in the central amygdaloid nucleus were centered in the lateral central core subdivision (cases J1LY, J1FR; Fig. 3C, black sites). The injection site in J1LY was smaller than the site in J1FR and was confined to the lateral core subdivision. The injection site in J1FR was in the lateral core at the same rostrocaudal level but encroached into the medial subdivision of the central nucleus and medial amygdalostriatal area.
Location of anterograde injection sites
There were four anterograde injection sites (Fig. 3D). The injection site in J7LY was located in the gustatory cortex. The injection site in J6FR was caudal to that in J7LY and included the gustatory cortex and adjacent Iapl. Both injections resulted in retrogradely labeled cells in the ventroposteromedial nucleus (parvicellular division) (VPMpc), the thalamic taste center (Pritchard et al., 1986) (not shown). Case J9AA contained a tritiated amino acid injection in the rostral dysgranular insula. Case J6 LY was situated in the rostral granular insula.
Retrograde cases
Injections into the caudal ventral striatum
Case J6WGA (not shown) and case J8WGA (Fig. 4)
Fig. 4.

A–F: Schematic of retrogradely labeled cells in the insula following an injection of WGA-HRP into the central ventromedial putamen (case J8WGA). Boxed area in C depicts the area photographed in Figure 5. For abbreviations, see list. Each dot = 1 cell.
These two injection sites were placed in the ventromedial putamen at the level of the crossing of the anterior commissure. The injection site in J6WGA was slightly rostral to that in J8WGA. The distribution of labeled cells in each case was similar, and they are therefore described together. Retrogradely labeled cells extended rostrocaudally from the agranular insula on the orbital surface to the caudal dysgranular insula. There were relatively few labeled cells in the posterior (granular) insula. At rostral levels, labeled cells were mainly concentrated in layer V of the Ial (Fig. 4A). Clusters of labeled cells were also seen in the Iapm, with scattered labeled cells in the Iai. Labeled cells were most densely concentrated at levels slightly caudal to this region, with the majority of labeled cells in the Ial, moderate numbers of labeled cells in the Iapm, and relatively few labeled cells in the Iai (Fig. 4B). Further caudally, labeled cells were highly concentrated in the Iapm and Iapl (Fig. 4C, Fig. 5A,B). Labeled cells in the Iapl continued dorsally into layer V of the gustatory cortex, where they were less densely concentrated. At the level of the anterior commissure, the majority of labeled cells were in the Iapl and the adjacent rostral dysgranular insula (Fig. 4D). There were few labeled cells in the Iapm at this level. Posterior to the anterior commissure, the density of labeled cells declined. There was a moderate density of labeled cells in the dysgranular cortex and few to no labeled cells in the granular insular cortex (Fig. 4E,F).
Fig. 5.

Photomicrographs of retrogradely labeled cells in Iapl in case J8WGA. A: Macroscopic view of labeled cells in the Iapl (boxed area in Fig. 4C). The photograph was digitally captured using a 4× objective and tiled into a montage by using ImagePro software. B: Higher power views of labeled cells in boxed area in A. For abbreviations, see list. Scale bars = 1 mm in A; 100 µm in B.
Case J12WGA (Fig. 6; injection site shown in Fig. 3)
Fig. 6.

A–F: Schematic of retrogradely labeled cells in the insula following an injection of WGA-HRP into the caudal ventromedial putamen (case J12WGA). For abbreviations, see list. Each dot = 1 cell.
This injection site was in the ventromedial putamen posterior to the decussation of the anterior commissure. The rostrocaudal extent of the projection was similar to that in cases J6WGA and J8WGA above. In the rostral insula, the preponderance of labeled cells was also in the Ial, especially along the medial bank of the emerging SLS (Fig. 6A,B). The Iam and Iai contained relatively low numbers of labeled cells, similar to cases J6 and J8 above. At slightly caudal levels, HRP-positive cells predominated in the Iapm, with moderate concentrations of labeled cells in the Iapl that extended into the gustatory cortex (Fig. 6C). At the level of the crossing of the anterior commissure, the Iapm and Iapl also contained many labeled cells, which continued into the dysgranular insula (Fig. 6D). Caudal to the crossing of the anterior commissure, there were many labeled cells in the Iac and rostral dysgranular insula (Fig. 6E). A light distribution of HRP-immunoreactive cells was found in the rostral granular insula. At caudal levels, there were small clusters of labeled cells in the dysgranular insula and few to no labeled cells in the granular insula (Fig. 6F).
Case J4 WGA (Fig. 7)
Fig. 7.
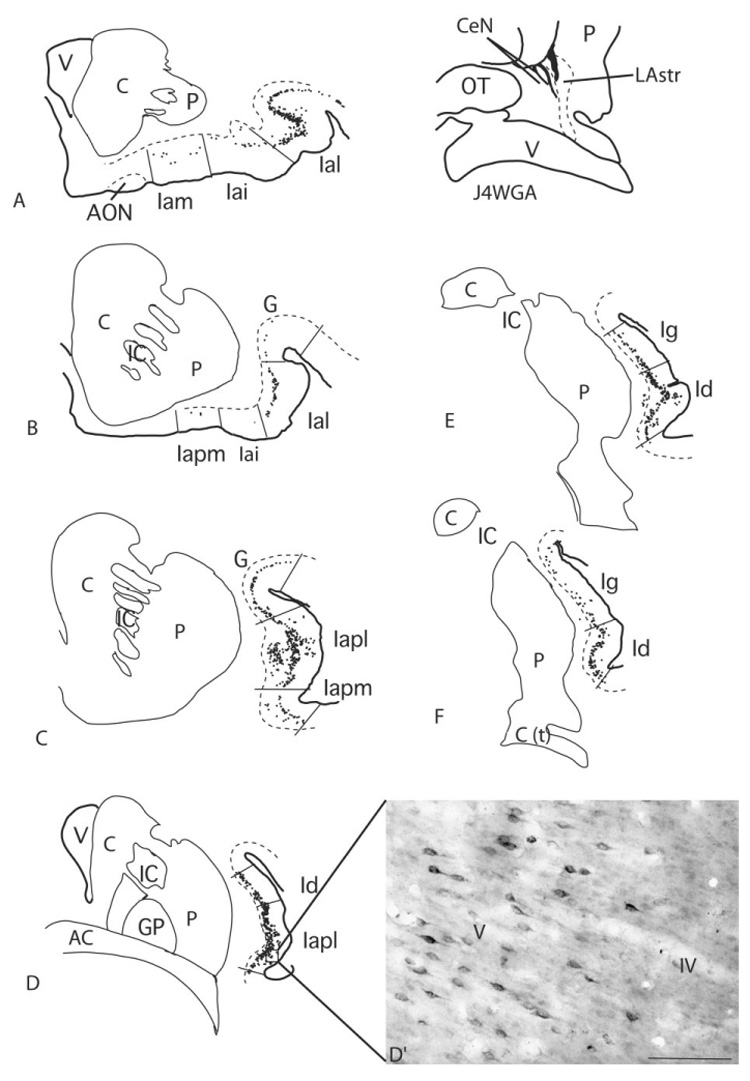
A–F: Schematic of WGA-HRP-positive cells in the insula in case J4WGA. D′: Labeled cells in layer V (box in D). The CaBP-poor lateral amygdalostriatal area is illustrated with a dotted line. Each dot = 1 cell. For abbreviations, see list. Scale bar = 100 µm in D′
The injection site in this case was placed in the lateral amygdalostriatal area (CaBP-poor zone of the caudoventral striatum) but included the CaBP-positive ventromedial putamen. Retrogradely labeled cells extended rostrocaudally from the rostral agranular insula, throughout the dysgranular insula, and into the rostral granular insula. As in cases J6WGA, J8WGA, and J12WGA (above), at rostral levels, the Ial contained the preponderance of labeled cells (Fig. 7A,B). However, small clusters of labeled cells were also found in the Iam and Iai. At slightly more caudal levels, the Iapm and Iapl contained very high concentrations of labeled cells, again similar to the distribution seen in the previous cases (Fig. 7C). WGA-HRP-labeled cells in the Iapl flowed into the gustatory cortical area, where they were more moderately concentrated. At the level of the decussation of the anterior commissure, labeled cells were densely distributed in the Iapl and dysgranular insular cortex. The Ig contained a moderate distribution of labeled cells. Posteriorly, the concentration of labeled cells declined, as in the previous cases (Fig. 7E,F). A moderate concentration of labeled cells in the caudal dysgranular insula continued for a short distance into the rostral granular insula. There were no labeled cells in the granular cortex caudal to this level.
Case J9FR (Fig. 8A–C; see injection site in Fig. 3)
Fig. 8.
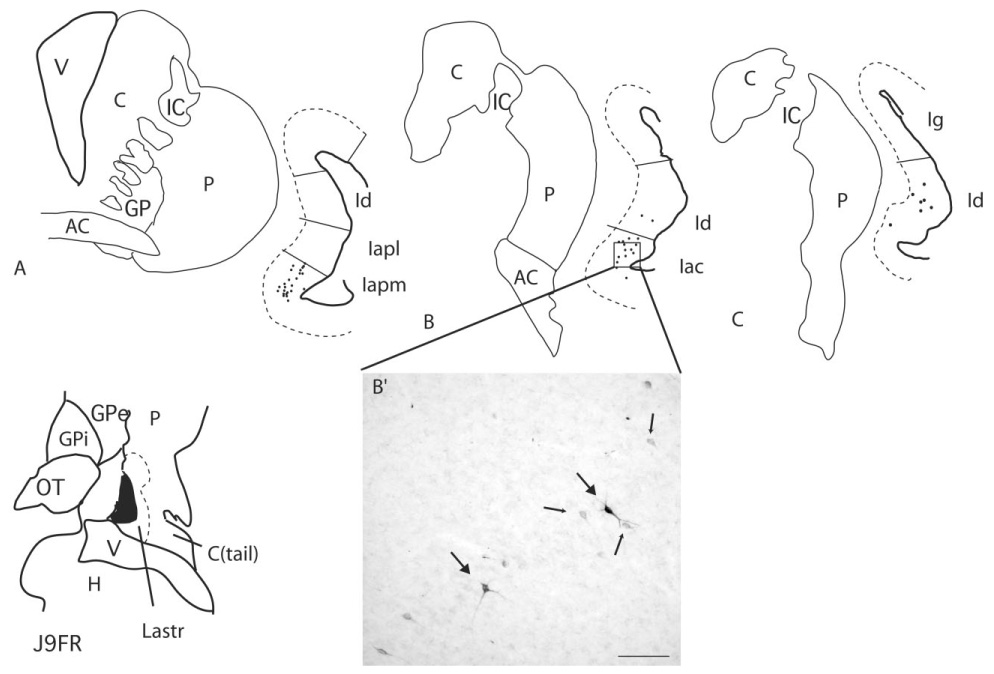
A–C: Retrogradely labeled cells in the insula following an FR injection into the lateral amygdalostriatal area near the emerging tail of the caudate nucleus (injection site shown in Fig. 3). The CaBP-poor lateral amygdalostriatal area is illustrated with a dotted line. Each dot = 1 cell. B′: FR-labeled cells in the Iac (boxed area in B). Lightly labeled cells (small arrows) are seen in addition to darkly staining cells (large arrows). For abbreviations, see list. Scale bar = 100 µm in B′.
This injection site was located in the CaBP-poor zone of the ventromedial striatum, near the emerging tail of the caudate nucleus. It encroached slightly on the medial amygdalostriatal (AChE poor and TH-poor) area. In general, the rostrocaudal extent of the projection was more confined and was caudally located compared with the previous cases. Labeled cells were clustered in the Iapm and Iac at the level of the anterior commissure (Fig. 8A,B,B′). There were only scattered labeled cells in the rostral dysgranular insula at these levels. The caudal extent of the projection was located in the dysgranular insula posterior to the crossing of the anterior commissure, where there was a light distribution of labeled cells (Fig. 8C). There were no labeled cells in the granular insula.
Case J4FR (Fig. 9)
Fig. 9.

A–E: Schematic of retrogradely labeled cells in case J4FR, following an injection into the ventromedial caudal striatum. The CaBP-poor lateral amygdalostriatal area is illustrated with a dotted line. Each dot = 1 cell. D′: Labeled cells in layer V of the dysgranular insula (boxed area in D). For abbreviations, see list. Scale bar = 100 µm in D′.
The injection site in this case was caudal to the injection in J4WGA and placed slightly dorsolateral to it, in the conventional (CaBP-positive) striatum. Labeled cells in the insula were somewhat less broadly distributed compared with J4WGA. In particular, there were only scattered labeled cells in the Ial, in contrast to the abundant labeled cells in case J4WGA (Fig. 9A). Similar to J4WGA, there were only scattered labeled cells in the Iai. The majority of labeled cells were in the Iapm and Iac within the sylvian fissure and were continuous with less densely concentrated labeled cells in the adjacent dysgranular insula (Fig. 9B–D,D′). At the caudal aspect of the projection, FR-positive cells were scattered in the caudal dysgranular cortex, and there were occasional labeled cells in the granular insula (Fig. 9E).
Case J4FS (not shown)
The injection site was at the same rostrocaudal level of the ventral putamen as the injection in J4FR but was more laterally placed in the caudal central, ventral putamen. Of the cases classified within the “limbic” striatum, this case had the fewest labeled cells in the insula. There were no labeled cells in the agranular cortex. Scattered labeled cells were seen in the caudal dysgranular and rostral granular insula posterior to the crossing of the anterior commissure.
Injections outside the caudal, ventral striatum
Case J11WGA (not shown)
This injection site was located in the caudal dorsomedial putamen and was the most caudally placed injection site. There were only occasional labeled cells, which were scattered in the caudal dysgranular insula. There were no labeled cells in the agranular insula, rostral dysgranular insula, or granular insula.
Injections into the central nucleus/medial amygdalostriatal area
Case J1FR (Fig. 10A–D; see injection site in Fig. 3)
Fig. 10.

Schematic showing retrogradely labeled cells after injections into the central nucleus: case J1FR (left, A–D) and case J1 LY (right, E). The CaBP-poor lateral amygdalostriatal area is illustrated with a dotted line. Each dot = 1 cell. C′: Retrogradely labeled cells in the Iapl in case J1FR (boxed area in C). For abbreviations, see list. Scale bar = 1 mm in C′.
The injection site in this case was situated in the lateral core of the central nucleus and included a small area of the medial subdivision of the central nucleus and the medial amygdalostriatal area. The projection field was diffusely distributed compared with a more confined injection into the lateral core of the central nucleus (case J1LY, below). Labeled cells were lightly distributed in the rostral agranular insula (Fig. 10A,B), mainly in the Iai and Ial. There were no labeled cells in the gustatory cortex. Labeled cells were most concentrated in the Iapm and Iapl within the sylvian fissure (Fig. 10C). At the level of the crossing of the anterior commissure, there was a moderate distribution of labeled cells in the rostral dysgranular insula and scattered labeled cells in the Iac region (Fig 10D).
Case J1LY (Fig 10E)
This small injection was confined to the lateral core of the central nucleus at its most caudal levels. The majority of labeled cells were confined to Iapm and Iapl at the level of the temporal poles (Fig. 10E). Only a few scattered LY-positive cells were seen in the agranular insula rostral to this (not shown). There were no labeled cells in the dysgranular or granular subregions of the insula. The gustatory cortex was devoid of labeled cells.
Anterograde cases
Case J9AA (Fig. 11; injection site pictured in Fig. 3D)
Fig. 11.

A–E: Schematic of the distribution of silver grains in the striatum following a tritiated amino acid injection into the rostral dysgranular insula (case J9AA). The injection site is pictured in Figure 3. For abbreviations, see list.
This tritiated amino acid injection site was situated in the rostral, dorsal dysgranular insula. Anterogradely labeled fibers extended from the rostral striatum to the caudal striatum at the level of the caudal amygdala/rostral hippocampus. In the rostral striatum, small patches of labeled fibers were distributed in a diagonal band-like arrangement in the central putamen (Fig. 11A). There were no labeled fibers in the rostral ventral striatum, the caudate nucleus, or the dorsolateral putamen. At the level of the rostral anterior commissure and emerging globus pallidus, labeled fibers were very densely concentrated in the ventromedial putamen, which bordered the anterior commissure dorsally and laterally (Fig. 11B). Surrounding this dense concentration of labeling, silver grains were more lightly concentrated in the ventral, central putamen. There were no labeled fibers in the caudate nucleus or in the central or dorsal putamen. At the level of the posterior limb of the anterior commissure, dense patches of labeled fibers were confined to the medial edge of the putamen where they formed a near-continuous strip (Fig. 11C). There were few to no labeled fibers in the rest of the putamen, or in the caudate nucleus.
In the caudal striatum, labeled fibers were most densely concentrated in the ventromedial putamen (Fig. 11D). Small patches of labeled fibers also extended two-thirds of the way up the ventromedial edge of the putamen. A small patch of labeled fibers also occupied the ventrolateral edge of the putamen and ventral body of the caudate nucleus. The central and dorsal putamen and the emerging tail of the caudate nucleus were relatively devoid of labeled fibers. There were no labeled fibers in the central nucleus or medial amygdalostriatal area. At the caudal end of the projection, labeled fibers were confined to several patches in the ventromedial putamen (Fig. 11E). There were few to no labeled fibers in the central or dorsal putamen, or in the body or tail of the caudate nucleus.
Case J7LY (not shown) and Case J6FR (Fig. 12)
Fig. 12.

A–G: Schematic of labeled fibers in the striatum following an FR injection into the gustatory cortex/Iapl (case J6FR). For abbreviations, see list.
Both injection sites were in the gustatory cortex in the rostral SLS. The injection in case J6FR was slightly larger and encompassed a small area of the Iapl. The distribution of labeled fibers was similar for each case, and they are therefore described together. In general, labeled fibers in both cases were distributed in areas containing dense deposits of silver grains in case J9AA (above). However, the distribution of labeled fibers was broader. In the rostral striatum, patches of labeled fibers were sparsely distributed in rostral, central putamen, with some fibers extending into the central, lateral caudate nucleus near the internal capsule (Fig. 12A,B).
At the level of the emerging decussation of the anterior commissure, patches of labeled fibers occupied the ventromedial, ventrolateral, and central putamen (Fig. 12C). There were also patches of labeled fibers that extended from the ventral putamen along the striatal bridge linking the caudate nucleus and putamen. However, there were few labeled fibers in the caudate nucleus proper. At the level of posterior limb of the anterior commissure, labeled fibers were densely concentrated along the medial edge of the putamen (Fig. 12D). More moderate densities of labeled fibers were found in the ventral central putamen and in the ventrolateral putamen. At the caudal limit of the projection, a densely concentrated patch of LY-positive fibers occupied the ventromedial putamen (Fig. 12E–G). The central and lateral ventral putamen contained a moderate distribution of labeled fibers, and scattered labeled fibers occupied the lateral amygdalostriatal area. There were no labeled fibers in the tail of the caudate nucleus or in the central amygdaloid nucleus.
Case J6LY (not shown)
The injection site was in the rostral granular cortex at the level of the crossing of the anterior commissure (Fig. 3D). There were relatively few labeled fibers in the striatum compared with the previous cases. Labeled fibers were confined to the caudal striatum at the level of the amygdalohippocampal junction. The ventromedial and ventral central putamen at this level received a light distribution of labeled fibers. There were no LY-positive fibers in the CaBP-negative ventromedial striatum, in the dorsolateral putamen, in the body or tail of the caudate nucleus, or in the central nucleus.
DISCUSSION
Insular inputs to the caudoventral striatum: overview
The caudal ventral striatum, which we have operationally defined based on its amygdaloid input, receives robust inputs from the insula that originate mainly in its agranular and dysgranular subregions. The agranular insula has relatively stronger inputs compared with the dysgranular insula. However, there is not a clear segregation of agranular and dysgranular insular projections to a given striatal region. Agranular insular projections are frequently accompanied by more moderate inputs from adjacent dysgranular insula or gustatory cortex. In contrast, the granular insula has relatively few inputs to the caudal ventral striatal region.
The rostral agranular insula, along with other sectors of the orbital and medial prefrontal cortex (OMPFC), has recently been divided into multiple subdivisions based on a variety of architectonic, histochemical, and connectional features(Carmichael and Price, 1994). Within this organizational scheme, the Iam, Iapm, Ial, and Iapl comprise the “posterior orbital network,” which receives specific sensory inputs from brain regions mediating gustatory, olfactory, and visceral functions. The Iai, although situated among the other subdivisions, receives few inputs from regions mediating these sensory modalities (Carmichael et al., 1994; Carmichael and Price, 1996b). The amygdala sends projections across all agranular insular subdivisions, as does the entorhinal and perirhinal cortex (Porrino et al., 1981; Amaral and Price, 1984; Carmichael and Price, 1996a). The present results show that injections into the caudoventral striatum result in a preponderance of labeled cells in insular subdivisions mediating visceral, olfactory, and gustatory information; there were relatively few labeled cells in the Iai following any of our retrograde injections. Within the posterior orbital insula, the Iapm and Iapl are the most consistently labeled subdivisions (Table 1).
TABLE 1.
Relative Densities of Labeled Cells in Insular Subdivisions Following Tracer Injections into the Caudal Striatum and Central Nucleus1
| Iam | Iai | Ial | Iapl | Iapm | Iac | Dysgran | Gran | |
|---|---|---|---|---|---|---|---|---|
| Caudal striatum | ||||||||
| J6 and J8WGA | − | + | ++++ | ++++ | ++++ | ++ | +++ | − |
| J12WGA | − | + | ++++ | ++++ | +++ | ++ | +++ | + |
| J4WGA | + | + | ++++ | ++++ | +++ | +++ | ++++ | ++ |
| J9FR | − | − | − | − | ++ | ++ | + | − |
| J4FR | − | − | − | − | + | ++ | + | − |
| J4FS | − | − | − | − | − | − | ++ | + |
| J11WGA2 | − | − | − | − | − | + | + | |
| Central nucleus | ||||||||
| J1FR | − | + | + | ++ | + | − | ++ | − |
| J1LY | − | − | − | + | + | − | − | − |
Cases are arranged in rostral to caudal order, with J6/J8WGA being most rostral. Insular subdivisions are arranged in rostral-caudal order from left to right. Iapm, Iapl, and Iac extend caudally to form the medial wall of the lateral fissure. −, scattered or no labeled cells; +, light distribution of labeled cells; ++, moderate distribution of labeled cells; +++, moderately heavy distribution of labeled cells; ++++, very heavy distribution of labeled cells. For abbreviations, see list.
No amygdaloid inputs.
Within this general scheme, afferent projections to the caudal ventral striatum follow a rostral-caudal topography (Table 1). Agranular insular subdivisions on the caudal orbital surface (mainly the Iam, Ial, Iapm, and Iapl) project to the ventromedial putamen in the region of the anterior commissure and just posterior to it (cases J6WGA, J8WGA, J12WGA, J4WGA). The Ial, Iapm, and Iapl have strong inputs to the caudal ventral striatum in these cases, whereas the more rostrally located Iam has relatively weaker inputs, which mainly target the central ventral putamen (cases J6WGA and J8WGA) and dissipate caudally. In contrast, the caudalmost aspects of the ventral striatum (cases J4FR and J9FR) receive the majority of inputs from the Iapm and Iapl, and from the Iac; these are located within the lateral fissure. These caudal striatal areas receive few to no inputs from subdivisions of the agranular insula that occupy the orbital surface (the Iam, Ial, and rostral Iapm and Iapl).
The caudal agranular insula forms the main input to the central nucleus
Based on our two retrograde injections, insular inputs to the central amygdaloid nucleus derive mainly from the caudal agranular insula (the Iapl) near the ILS. Labeled cells were more broadly dispersed in case J1FR, which included some of the medial subdivision of the central nucleus in the injection site. The Iapm and Iapl at the level of the rostral temporal lobe contained the majority of labeled cells in both cases. Because both injection sites were at relatively caudal levels of the central nucleus, we cannot rule out projections from other sectors of the insula to more rostral regions of the central nucleus. However, our anterograde injections, which were placed in various sites outside the Iapm and Iapl, resulted in no labeled fibers in the central nucleus. The present retrograde data are positively supported by anterograde studies in which tritiated amino acid injections into the caudal agranular insula resulted in specific, dense labeling of the central nucleus (Mufson et al., 1981; Stefanacci and Amaral, 2002).
Projections of the gustatory cortex
The gustatory cortex is considered a “paralimbic” structure because it forms a transitional zone between the periallocortex (agranular cortex) and the isocortex (Mesulam, 2000). Anatomic and physiologic studies indicate that inputs from the “thalamic taste center” (the VPMpc) are found along the trajectory from the anterior insula into the gustatory cortex (Bagshaw and Pribram, 1953; Benjamin and Burton, 1968; Mufson and Mesulam, 1984; Pritchard et al., 1986; Yaxley et al., 1990). VPMpc cells also project to the Iapm and Ial but are located ventrolateral to those projecting to the gustatory cortex (Carmichael and Price, 1996b). As described by Carmichael and Price (1996b), Iapm- and Ial-projecting neurons are positioned to receive inputs from the caudal vagalrecipient area of the nucleus of the solitary tract, rather than its rostral gustatory part. These subdivisions are thus hypothesized to be “visceral cortex” that may mediate sensations such as satiety. The Ial also receives direct inputs from the gustatory cortex, suggesting that gustatory and visceral information converge in this region (Carmichael and Price, 1996b). In our material, retrogradely labeled cells in the Ial (and Iapl) extend without interruption into the gustatory cortex after injections into the caudal ventral putamen (cases J6WGA, J8WGA, J12WGA, J4WGA), suggesting further convergence of gustatory and visceral information at the level of the caudal ventral striatum.
Anterograde results show that the gustatory cortex projects to the ventral striatal territories and also extends projections to the central putamen and ventrolateral putamen (Fig. 12). The latter are somatosensory-associated areas, based on physiologic and anatomic studies in the monkey (Kemp and Powell, 1970; Alexander and DeLong, 1985; Selemon and Goldman-Rakic, 1985; Flaherty and Graybiel, 1991). The ventrolateral putamen at the level of the commissure, an area labeled in cases J7LY and J6FR, is linked to movements and sensations of the tongue, mouth, and face, indicating that there are direct links between gustatory sensory cortex and striatal domains that are probably involved in coordinating eating movements (Flaherty and Graybiel, 1991, 1994).
Comparison with previous studies in the primate
Previous studies in the primate indicate that insular inputs to the striatum follow the cytoarchitectural gradient of the insula, with relatively less differentiated (agranular) areas projecting mainly to the ventral striatum and more differentiated (granular) regions projecting to the dorsolateral striatum (Chikama et al., 1997). The shell, medial ventral striatum, and core of the ventral striatum receive the majority of inputs from the agranular insula and modest inputs from the dysgranular insula. The present data complement these studies because both the agranular and adjacent dysgranular insula project to caudal striatal regions that, like the “classic” ventral striatum, receive strong amygdaloid inputs. A more recent study examining corticostriatal inputs from specific OMPFC subdivisions shows that the ventrolateral shell and adjacent ventromedial putamen are innervated by the Iam, Ial, Iapm, and Iapl and receive relatively few inputs from the Iai (Ferry et al., 2000). This distribution is similar to the pattern seen after injections into the caudal ventral striatum surrounding the commissure (present study, cases J6, J8, J12, J4WGA), with the exception that the caudal striatal sites result in relatively fewer labeled cells in the Iam.
Comparison with rodent studies
Although a precise homology has not been demonstrated, the caudoventral striatum in primates may at least partly correspond to the region known as the interstitial nucleus of the posterior limb of the anterior commissure (IPAC) in the rodent (deOlmos and Ingram, 1972; Heimer et al., 1997). The IPAC has traditionally been referred to as the “fundus striati” and is proposed to be a caudal extension of the ventral striatum (Nauta et al., 1978). The IPAC encompasses the striatum surrounding the posterior limb of the decussating anterior commissure, and its lateral aspect has connectional and histochemical features found in the ventral striatum, including amygdaloid inputs (Shammah-Lagnado et al., 2001). The IPAC receives strong inputs from the anterior dysgranular insula (which corresponds to the “gustatory” insula; Kosar et al., 1986) and the posterior agranular insula, but few inputs from the rostral agranular insula, which projects to the rostral ventral striatum (Brog et al., 1993; Shi and Cassell, 1998; McDonald et al., 1999; Shammah-Lagnado et al., 2001). This overall organization corresponds to our findings in the primate: the agranular insula projects to the subregions of the ventromedial striatum that receive amygdaloid inputs and does so along a general rostrocaudal gradient (present results; Chikama et al., 1997). Furthermore, the anterior dysgranular (gustatory) insula projects to the IPAC, consistent with our finding that the gustatory cortex (and adjacent insular regions) innervates the central ventromedial putamen (cases J6 and J8 WGA), which is situated in a similar location.
Functional considerations
Classic experiments show that the anterior insula and adjacent gustatory cortex respond to various properties of food. Subsets of neurons are “tuned” to specific taste qualities (such as sweetness, saltiness, and bitterness), textural properties, and fat content (Benjamin and Burton, 1968; Yaxley et al., 1990; Smith-Swintosky et al., 1991; Plata-Salaman et al., 1993; Kinomura et al., 1994; Rolls et al., 1999; Scott and Plata-Salaman, 1999; Small et al., 2001; Rolls et al., 2003; De Araujo and Rolls, 2004). A large literature also implicates this area in mediating the rewarding properties of gustatory and olfactory stimuli (Tremblay and Schultz, 2000; O’Doherty et al., 2001; Small et al., 2001; Anderson et al., 2003; Small et al., 2003). Subregions of the anterior insula, including the posterior orbital cortex, are also selectively activated by the anticipation of a rewarding or aversive stimulus, suggesting a role in the evaluation of hedonic properties prior to the actual sensory experience (Chua et al., 1999; Montague and Berns, 2002; O’Doherty et al., 2002). Similarly, the anterior insula is activated by self-induced mood states, independent of an external sensory stimulus (Lane et al., 1997; Mayberg et al., 1999; Damasio et al., 2000; Liotti et al., 2002; Krolak-Salmon et al., 2003; Wicker et al., 2003; Wright et al., 2003). Limbic inputs from the amygdala, polysensory inputs from the entorhinal and perirhinal cortices, and visceral inputs place the posterolateral agranular insula in an ideal position to assess the hedonic value of sensory stimuli, either actual or anticipated. Afferents from the viscera may play an especially important role in setting the hedonic value of a stimulus because internal sensations associated with fear, hunger, and satiety help determine the biologic value of an external cue.
The classic ventral striatum is a key substrate for guiding goal-directed behavior (Wise and Bozarth, 1985; Mogenson et al., 1993; Robbins and Everitt, 1996), and it mediates the ingestion of highly palatable, high-energy foods(Zhang and Kelley, 2000; Kelley et al., 2002). The insula probably provides important information that shapes such behavior. Insular lesions attenuate the devaluation of food rewards following satiety and extinction, suggesting that the insula encodes representations of the incentive value of taste under specific conditions (Butter, 1969; Balleine and Dickinson, 2000). Lesions of the insula also attenuate conditioned taste avoidance, so that food that has been previously paired with a sickening agent is no longer avoided (Dunn and Everitt, 1988; Bermudez-Rattoni and McGaugh, 1991). Whereas insular projections to the classic ventral striatum are well established, the current results show that the caudal ventral striatum is also a key target of the agranular and dysgranular insula. In particular, insular subdivisions that occupy the posterolateral orbital surface have a strong afferent influence on these more caudal, but “limbic-related” striatal territories. These insular subregions (the Ial, Iapm, and Iapl) are characterized by relays from the visceral thalamus and cortex, suggesting that visceral responses to both positive and negative stimuli may influence behaviors mediated by the caudal ventral striatum.
ACKNOWLEDGEMENTS
We gratefully acknowledge the technical advice and photography assistance of Ms. Tracy Bubel.
Grant sponsor: National Institutes of Health/National Institute of Mental Health; Grant number: R01 MH63291 (to J.L.F.).
Abbreviations
- AA
tritiated amino acids
- AC
anterior commissure
- AON
anterior olfactory nucleus
- C
caudate nucleus
- C (tail)
caudate nucleus, tail
- CeL
central amygdaloid nucleus, lateral core subdivision
- CeN
central amygdaloid nucleus
- Cl
claustrum
- FR
Fluoro Ruby (tetramethylrhodamine)
- FS
fluorescein
- G
gustatory cortex
- GP
globus pallidus
- GPe
globus pallidus, pars externa
- GPi
globus pallidus, pars interna
- H
hippocampus
- Iac
caudal agranular insular area
- Iai
intermediate agranular insular area
- Ial
lateral agranular insular area
- Iam
medial agranular insular area
- Iapl
posterolateral insular area
- Iapm
posteromedial insular area
- IC
internal capsule
- Id
dysgranular insula
- Ig
granular insula
- ILS
inferior limiting sulcus
- L
lateral nucleus, amygdala
- LAstr
lateral amygdalostriatal area
- LY
Lucifer yellow
- MAstr
medial amygdalostriatal area
- OT
optic tract
- P
putamen
- S
shell
- SLS
superior limiting sulcus
- V
ventricle
- WGA
wheat germ horseradish peroxidase
LITERATURE CITED
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR. Microstimulation of the primate neostriatum. II. Somatotopic organization of striatal microexcitable zones and their relation to neuronal response properties. J Neurophysiol. 1985;53:1417–1430. doi: 10.1152/jn.1985.53.6.1417. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. Dissociated neural representations of intensity and valence in human olfaction.[see comment] Nat Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J Neurophysiol. 1995;73:1234–1252. doi: 10.1152/jn.1995.73.3.1234. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Bagshaw MH, Pribram KH. Cortical organization in gustation. J Neurophysiol. 1953;16:499–508. doi: 10.1152/jn.1953.16.5.499. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. The effect of lesions of the insular cortex on instrumental conditioning: evidence for a role in incentive memory. J Neurosci. 2000;20:8954–8964. doi: 10.1523/JNEUROSCI.20-23-08954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin RM, Burton H. Projection of taste nerve afferents to anterior opercular-insular cortex in squirrel monkey. Brain Res. 1968;7:221–231. doi: 10.1016/0006-8993(68)90100-5. [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F, McGaugh JL. Insular cortex and amygdala lesions differentially affect acquisition on inhibitory avoidance and conditioned taste aversion. Brain Res. 1991;549:165–170. doi: 10.1016/0006-8993(91)90616-4. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported Fluoro-Gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Butter CM. Perseveration in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta. Physiol Behav. 1969;4:163–171. [Google Scholar]
- Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. J Comp Neurol. 1994;346:403–434. doi: 10.1002/cne.903460306. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1996a;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996b;363:642–640. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- Chikama M, McFarland N, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- De Araujo IE, Rolls ET. Representation in the human brain of food texture and oral fat. J Neurosci. 2004;24:3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deOlmos JS, Ingram WR. The projection field of the stria terminalis in the rat brain. J Comp Neurol. 1972;146:303–333. doi: 10.1002/cne.901460303. [DOI] [PubMed] [Google Scholar]
- Dunn LT, Everitt BJ. Double dissociations of the effects of amygdala and insular cortex lesions on conditioned taste aversion, passive avoidance, and neophobia in the rat using the excitotoxin ibotenic acid. Behav Neurosci. 1988;102:3–23. doi: 10.1037//0735-7044.102.1.3. [DOI] [PubMed] [Google Scholar]
- Ferry AT, Ongur D, An X, Price JL. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol. 2000;425:447–470. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. Cortical transformations in the primate somatosensory system. Projections from physiologically mapped body-part representations. J Neurophysiol. 1991;66:1249–1263. doi: 10.1152/jn.1991.66.4.1249. [DOI] [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. Input-output organization of the sensorimotor striatum in the squirrel monkey. J Neurosci. 1994;14:599–610. doi: 10.1523/JNEUROSCI.14-02-00599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge J, Haber S. Defining the caudoventral striatum in primates: cytoarchitectural and histochemical features. J Neurosci. 2002;22:10078–10082. doi: 10.1523/JNEUROSCI.22-23-10078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, McClain C. Amygdaloid inputs define a caudal component of the ventral striatum in primates. J Comp Neurol. 2004;476:330–347. doi: 10.1002/cne.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geneser-Jensen FA, Blackstad TW. Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. Z Zellforsch. 1971;114:460–481. doi: 10.1007/BF00325634. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland N. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The acumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- Heimer L, De Olmos JS, Alheid GF, Pearson J, Sakamoto N, Shinoda K, Marksteiner J, Switzer RC. The human basal forebrain. Part II. In: Bloom FE, Bjorkland A, Hokfelt T, editors. Handbook of chemical neuroanatomy. Amsterdam: Elsevier; 1999. pp. 57–226. [Google Scholar]
- Heining M, Young AW, Ioannou G, Andrew CM, Brammer MJ, Gray JA, Phillips ML. Disgusting smells activate human anterior insula and ventral striatum. Ann N Y Acad Sci. 2003;1000:380–384. doi: 10.1196/annals.1280.035. [DOI] [PubMed] [Google Scholar]
- Hoffman BL, Rasmussen T. Stimulation studies of insular cortex of Macaca mulatta. J Neurophysiol. 1953;16:343–351. doi: 10.1152/jn.1953.16.4.343. [DOI] [PubMed] [Google Scholar]
- Kaada BR, Pribram KH, Epstein JA. Respiratory and vascular responses in monkeys from temporal pole, insula, orbital surface and cingulate gyrus. J Neurophysiol. 1949;12:348–356. doi: 10.1152/jn.1949.12.5.347. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum.[see comment] Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TPS. The cortico-striate projection in the monkey. Brain. 1970;93:525–546. doi: 10.1093/brain/93.3.525. [DOI] [PubMed] [Google Scholar]
- Kinomura S, Kawashima R, Yamada K, Ono S, Itoh M, Yoshioka S, Yamaguchi T, Matsui H, Miyazawa H, Itoh H. Functional anatomy of taste perception in the human brain studied with positron emission tomography. Brain Res. 1994;659:263–266. doi: 10.1016/0006-8993(94)90890-7. [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Res. 1986;379:329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- Krolak-Salmon P, Henaff MA, Isnard J, Tallon-Baudry C, Guenot M, Vighetto A, Bertrand O, Mauguiere F. An attention modulated response to disgust in human ventral anterior insula.[see comment] Ann Neurol. 2003;53:446–453. doi: 10.1002/ana.10502. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychol. 1997;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression.[see comment] Am J Psychiatr. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatr. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann N Y Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Principles of behavioral and cognitive neurology. Oxford: Oxford University Press; 2000. [Google Scholar]
- Mesulam M-M, Mufson EJ. Insula of the Old World monkey. I: Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Brudzynski SM, Wu M, Yang CR, Yim CCY. From motivation to action: a review of dopaminergic regulation of limbic-nucleus accumbens-pedunculopontine nucleus circuitries involved in limbic-motor integration. In: Kalivas PW, Barnes CD, editors. Limbic motor circuits and neuropsychiatry. Boca Raton: CRC Press; 1993. pp. 193–236. [Google Scholar]
- Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J Comp Neurol. 1984;227:109–120. doi: 10.1002/cne.902270112. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM, Pandya DN. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience. 1981;6:1231–1248. doi: 10.1016/0306-4522(81)90184-6. [DOI] [PubMed] [Google Scholar]
- Nauta HJW. A simplified perspective on the basal ganglia and their relation to the limbic system. In: Doane BK, Livingston KE, editors. Limbic system: functional organization and clinical disorders. New York: Raven Press; 1986. pp. 67–77. [Google Scholar]
- Nauta WJH, Smith GP, Faull RLM, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex.[see comment] Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward.[see comment] Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Penfield W, Faulk ME. The insula: further observations on its function. Brain. 1955;78:445–470. doi: 10.1093/brain/78.4.445. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Williams SCR, Gray JA, David AS. Specific neural substrates for disgust and fear in normal and clinical groups. Biol Psychiatr. 1998;43:73S. [Google Scholar]
- Plata-Salaman CR, Scott TR, Smith-Swintosky VL. Gustatory neural coding in the monkey cortex: the quality of sweetness. J Neurophysiol. 1993;69:482–493. doi: 10.1152/jn.1993.69.2.482. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol. 1981;198:121–136. doi: 10.1002/cne.901980111. [DOI] [PubMed] [Google Scholar]
- Pritchard TC, Hamilton RB, Morse JR, Norgren R. Projections of thalamic gustatory and lingual areas in the monkey. J Comp Neurol. 1986;244:213–228. doi: 10.1002/cne.902440208. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Browning AS, Hernadi I, Lenard L. Responses to the sensory properties of fat of neurons in the primate orbitofrontal cortex. J Neurosci. 1999;19:1532–1540. doi: 10.1523/JNEUROSCI.19-04-01532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Verhagen JV, Kadohisa M. Representations of the texture of food in the primate orbitofrontal cortex: neurons responding to viscosity, grittiness, and capsaicin. J Neurophysiol. 2003;90:3711–3724. doi: 10.1152/jn.00515.2003. [DOI] [PubMed] [Google Scholar]
- Rose M. Die inselrinde des menschen und der tiere. J Psychol Neurol. 1928;37:467–624. [Google Scholar]
- Russchen FT, Bakst I, Amaral DG, Price JL. The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res. 1985;329:241–257. doi: 10.1016/0006-8993(85)90530-x. [DOI] [PubMed] [Google Scholar]
- Sarnat H, Netsky M. Evolution of the nervous system. New York: Oxford University Press; 1981. Telencephalon; pp. 321–378. [Google Scholar]
- Schienle A, Stark R, Walter B, Blecker C, Ott U, Kirsch P, Sammer G, Vaitl D. The insula is not specifically involved in disgust processing: an fMRI study. Neuroreport. 2002;13:2023–2026. doi: 10.1097/00001756-200211150-00006. [DOI] [PubMed] [Google Scholar]
- Scott TR, Plata-Salaman CR. Taste in the monkey cortex. Physiol Behav. 1999;67:489–511. doi: 10.1016/s0031-9384(99)00115-8. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Striatal and central extended amygdala parts of the interstitial nucleus of the posterior limb of the anterior commissure: evidence from tract-tracing techniques in the rat. J Comp Neurol. 2001;439:104–126. doi: 10.1002/cne.1999. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998;399:440–468. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation.[see comment] Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Smith-Swintosky VL, Plata-Salaman CR, Scott TR. Gustatory neural coding in the monkey cortex: stimulus quality. J Neurophysiol. 1991;66:1156–1165. doi: 10.1152/jn.1991.66.4.1156. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Modifications of reward expectation-related neuronal activity during learning in primate orbitofrontal cortex. J Neurophysiol. 2000;83:1877–1885. doi: 10.1152/jn.2000.83.4.1877. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psych Med. 1985;3:445–460. [PubMed] [Google Scholar]
- Wright CI, Martis B, McMullin K, Shin LM, Rauch SL. Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biol Psychiatr. 2003;54:1067–1076. doi: 10.1016/s0006-3223(03)00548-1. [DOI] [PubMed] [Google Scholar]
- Yaxley S, Rolls ET, Sienkiewicz ZJ. Gustatory responses of single neurons in the insula of the macaque monkey. J Neurophysiol. 1990;63:689–700. doi: 10.1152/jn.1990.63.4.689. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]


