Abstract
More than a century after the discovery of the complex life cycle of its causative agent, malaria remains a major health problem. Understanding mosquito–malaria interactions could lead to breakthroughs in malaria control. Novel strategies, such as the design of transgenic mosquitoes refractory to Plasmodium, or design of human vaccines emulating mosquito resistance to the parasite, require extensive knowledge of processes involved in immune responses and of microevolutionary mechanisms that create and maintain variation in immune responses in wild vector populations. The recent realization of how intimately and specifically mosquitoes and Plasmodium co-evolve in Nature is driving vector molecular biologists and evolutionary ecologists to move closer to the natural setting under the common umbrella of ‘Ecological immunology’.
Natural vector–parasite interactions in context
A key to interrupting human malaria transmission lies in unravelling the physiological and molecular mechanisms characterizing Plasmodium-infected mosquitoes. In the past decade, research on the mosquito immune response has been particularly dynamic, focusing on identifying mosquito genes giving resistance to Plasmodium infection using gene mapping, and also focusing on characterizing immune defence mechanisms through gene expression analyses. This area of research benefited tremendously from the completion of the Anopheles gambiae genome and from advances in genomics and transcriptomics, such as the development of microarray technology and RNAi gene silencing, resulting in a rapidly expanding body of literature.
Research has also focused on evaluating the fitness costs incurred by mosquito hosts as a direct consequence of infection by Plasmodium parasites and the costs and benefits of mounting an immune response to such an infection. From an evolutionary point of view, strong immune defence responses might not always be advantageous to malaria vectors and understanding why some mosquitoes develop an infection whereas others do not could be as important to our understanding of malaria transmission as describing the immune defence mechanisms themselves (Box 1).
Box 1. Distribution of infections among mosquito hosts.
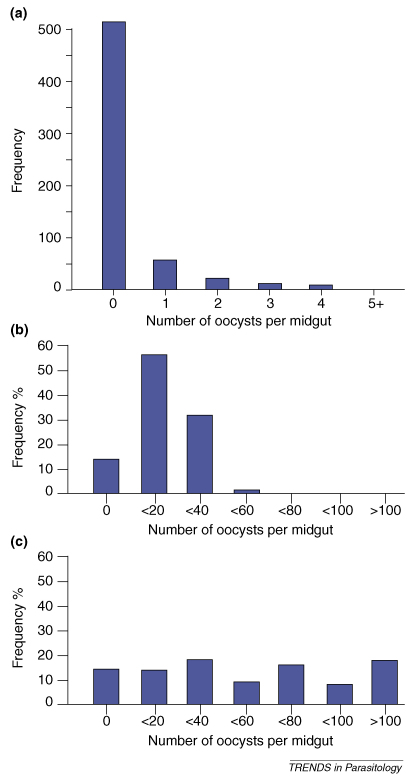
As is true for many parasites, in natural populations Plasmodium infections are usually binomially distributed among mosquito hosts. Figure Ia shows the distribution of oocysts in a large dataset of wild Anopheles gambiae females collected during the rainy season, during high malaria transmission in Northeast Tanzania [61]. Most female mosquitoes were either uninfected or carried few parasites, whereas a few females carried more than five parasites. Although there is no single and simple explanation for this distribution, infective gametocytes in bloodmeals could already be aggregated [62]. Thereafter, heterogeneities in the mosquito and Plasmodium population linked to variation in genetic and environmental factors shape the course of Plasmodium infections in individual mosquitoes and could account for Plasmodium aggregation (Box 2). Individual mosquitoes might also differ in the number of infected bloodmeals they ingest and could acquire one or multiple parasite genotypes, thus further complicating the picture. Mosquito–Plasmodium model systems used in laboratory settings feature few of the factors determining patterns of infections in wild populations. Figure Ib and c show contrasted distributions of P. yoelii nigeriensis oocysts in experimentally infected females of the ZAN-U and KIL An. gambiae strains, two strains that have been maintained in the laboratory for decades and exhibit high susceptibility to infection [50]. For some time model systems involving non-natural mosquito–Plasmodium associations were the only option for the experimental study of mosquito–Plasmodium interactions. The lack of genetic and environmental variation of experimental systems could explain the uniform distribution of infection observed in the KIL strain. Although model systems facilitated some experiments, they offer limited insights on the processes that create and maintain variation in mosquito immune responses in natural populations, hence current research favours studies conducted closer to the field giving more realistic experimental systems.
For some time the fields of mosquito immunology and evolutionary ecology have relied on laboratory vector–parasite model systems often only remotely similar to natural Anopheles–Plasmodium associations but easier to experiment with. However, current research is leaning strongly towards natural vector–parasite systems. There is good reason for this ’return to the field’. For example, more than two decades of research on infection-induced fitness costs using laboratory models have yielded precious few convincing reports and much contradictory data [1]. Gene expression studies of immune responses have suffered a somewhat similar fate, with different mosquito–Plasmodium model systems revealing their unique facets rather than suggesting a unified anti-Plasmodium response or defence mechanism [2–4]. Thus, results from both specialties emphasize how specifically and intimately mosquito and Plasmodium traits co-evolve and underscore the need to work closer to the natural setting [5–8].
Ecological immunology
The answer to these concerns lies in ‘ecological immunology’, a field of research that focuses on mechanisms and function of immune responses in their ecological and evolutionary context [9,10]. The term ‘ecological immunology’ was first coined in 1996 [9] to describe a growing body of literature produced by evolutionary ecologists focusing on the impact of parasites on host life-history traits, sexual selection and population dynamics. At the core of ecological immunology is the notion that mounting an immune defence is energetically expensive and that individuals must trade-off energy devoted to immunological functions against energy devoted to other life-history activities, such as growth, reproduction and survival [9].
The ecological immunology of mosquito–parasite interactions is a growing area of research that should help us understand how ecological factors affect interactions between mosquito vectors and the malaria parasite to create and maintain variation in host immune defence mechanisms and Plasmodium virulence in natural populations. This discipline is benefiting directly from the continuing improvement of research infrastructures in countries endemic for malaria and from a worldwide increase in the number of facilities dedicated to culturing human malaria and experimentally infecting its natural vectors. Here we review what is currently known about genetic and environmental factors affecting mosquito–Plasmodium interactions as inferred mostly from experimental laboratory systems and some field studies. This literature is further discussed in relation to population genetic and theoretical studies to delineate promising avenues for future research.
Parasite and immunity-mediated fitness costs
By their very nature, parasitic infections affect their hosts in adverse ways, which promote the selection of counter measures. However, there is a growing realization that immune systems are themselves costly and that their evolution and successful deployment depends on how they impact life-history traits, such as fecundity and survival, in infected and non-infected organisms [9,10]. Thus, the adaptive advantage of investing resources in immune defences will be related to the virulence of the infection (i.e. the potential extent of parasite-mediated costs) and the risk of exposure in a given population.
Parasite-mediated costs
Experimental infections of Anopheles stephensi with Plasmodium falciparum (a natural mosquito–Plasmodium association) have shown that parasite-mediated costs begin to be incurred within hours of infection, when nitric oxide synthase (NOS) transcription is upregulated in the midgut epithelium [11]. The transit of ookinetes through midgut epithelial cells induces apoptosis/necrosis in invaded cells, which are subsequently extruded into the gut lumen and replaced by regenerative cells [12]. This model of midgut repair, although likely to be costly, would prevent the development of perforations that could result in increased susceptibility to bacterial infection as suggested by experimental infections of An. stephensi with Plasmodium berghei, a non-natural host–parasite combination [13]. Midgut invasion was shown to coincide with the transcriptional upregulation of several aspects of the immune system, the downregulation of vitellogenin production in the fat body and resorption of developing ovarian follicles in the non-natural model system An. gambiae/Plasmodium yoelii nigeriensis [14,15]. There is also a suggestion that some free amino acids are depleted following infection [16,17]. Different studies based on natural [18] and non-natural associations [19,20] showed that, at a later stage, the presence of sporozoites in the salivary glands increases feeding persistence and probing behaviour, which can result in increased mortality through increased host contacts [21].
Most of these predicted costs have not been directly related to the fate of Plasmodium-infected mosquitoes, but several studies have shown that infection sometimes affects survivorship and fecundity (reviewed in [1,22]). Effects of Plasmodium infection on mosquito longevity are more likely to be found in unnatural vector–parasite associations and in studies that follow mortality until sporozoite invasion of salivary glands occurs [1]. Although this suggests that longevity could be favoured over egg production in infected mosquitoes (reviewed in [23]), recent studies have shown that infection also curtails reproduction in several mosquito–Plasmodium interactions (reviewed in [22]; see also [24,25]). However, most of these laboratory studies were again based on vector–parasite model systems that do not occur in nature (e.g. [24,25]) and thus some of these findings remain to be validated using natural model systems.
Immunity-mediated costs
Immune responses against pathogens are broadly divided into two categories that differ in their roles, modes of action and costs. (i) Constitutive immune defences are a first line of defence directed against a broad array of pathogens and are constantly activated. (ii) Inducible defences are specific and are switched on in response to a particular threat [26]. The physiological and genetic pathways involved in both types of immune responses are probably also involved in other functions (see, for example, [27]). As a result, an evolutionary change in immune responses might be associated with changes in other traits with which they are genetically correlated. Importantly, if the correlation is negative an increase in immune defences translates into a decrease in other fitness-related traits resulting in an evolutionary trade-off.
Trade-offs are a major cause of constitutive and inducible immune defence costs and, in their simplest form, arise when energetically costly immune responses compete with resources or with a particular gene product required for other important functions [9]. In practice, trade-offs can be driven by complex mechanisms and describing their functions can be challenging. A good description of such trade-off comes from work on Drosophila melanogaster in which selection for increased encapsulation ability against parasitoids resulted in constitutive costs in the form of reduced larval competitive ability and lower survival rate [28]. Resistant flies had twice as many haemocytes as susceptible ones, suggesting that resistance depends on investment in haemocytes [29]. Further work led to the identification of two of the major genes underlying this resistance (Rlb and Rat) that could be driving the trade-off between immunity and competitive feeding ability [30]. In Aedes aegypti, selection for early and late pupation resulted in correlated changes in body size and melanization response, revealing trade-offs between those traits [31].
In Anophelines, microarray studies have shown that the invasion of the mosquito midgut and salivary glands by Plasmodium parasites induces the transcription of several immune-related molecules [3] (reviewed in [32–34]). The production of these molecules is expected to be energetically costly and to divert resources away from growth and maintenance [1]. Nitric oxide (NO) is one such molecule that is part of a peroxidase induction cascade that initiates apoptosis/necrosis in mosquito midgut cells as the ookinetes move through them [35], a process that has been shown to directly or indirectly kill parasites in An. stephensi infected with P. berghei [36,37]. In this case, inducible costs are expected because NO synthesis requires arginine, a key component of other metabolic pathways such as egg production and sperm maturation in insects that can only be obtained through the insect diet [38]. Another potential cost of NO induction is the autoimmune response it can induce owing to its high toxicity and wide spectrum of action [38].
Similarly, the phenoloxidase cascade responsible for the melanotic encapsulation of parasites in insects produces phenol intermediates that are cytotoxic to the individual [27]. In An. gambiae, artificial stimulation of the phenoloxidase cascade and antimicrobial peptide production results in a reduction in egg production owing to the induction of apoptosis in cells of the follicular epithelium and subsequent resorption of eggs [39,40]. However, the exact mechanisms underlying this trade-off are not well understood.
Most of the advances discussed above were obtained through studies based on laboratory models of malaria infection. Consequently, the upregulation of immune genes that these studies describe might not always accurately mirror the upregulation operating in natural associations [2]. Furthermore, these studies do not take into account potentially important genetic variation in response to infection in natural populations. As an example, the strong melanization response of selected An. gambiae refractory strains has no match in nature. By contrast, melanization of P. falciparum is rarely observed in East African mosquito populations [41] and is observed moderately frequently in West African populations [8].
Effect of genetic and environmental factors on mosquito fitness and infection
Our understanding of mosquito immunity and fitness costs comes mainly from laboratory studies, but the situation in the field, where environmental stresses will be operating against a background of varied host and parasite genotypes, is probably very different. Here, the outcome of an infection will depend on the mosquito and Plasmodium genotypes, on the environment and, importantly, on the way that their genotype responds to changes in the environment; a phenomenon known as phenotypic plasticity (Box 2). For example, even minor changes in environmental temperature can lead to different responses among individuals and, hence, greatly alter the effect of infection [42].
Box 2. Determinants of mosquito–Plasmodium infections.
Mosquito–Plasmodium interactions are as simple or complex as the fundamental genetic mechanisms underlying them. In simple quantitative genetics terms, phenotypic variation in infected mosquitoes (e.g. survival, immune response level and body condition) can be described as:
| (Equation 1) |
And similarly, the phenotypic variance of the infecting Plasmodium parasites (survival, multiplication, rate of development, etc.) can be summarized as:
| (Equation 2) |
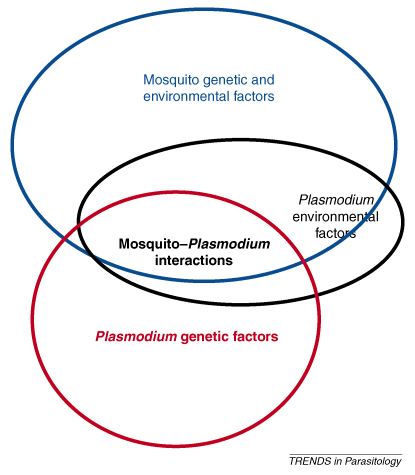
Where VP is the phenotypic variance among individuals, VG the genetic variance, VE the variance owing to the direct effect of the environment and VG*E the genotype-by-environment interaction, which occurs when the effect of the environment differs among genotypes. Thus, the phenotypic variance observed among hosts and parasites in natural populations is due to genetic and environmental factors and the interactions between the two. Variation in the outcome of an infection is thus a particularly complex phenotype in that it is determined by factors from both host and parasite equations (Figure I, Table 1). Both host and parasite strongly influence infection and their respective phenotypic variances can be considered an essential component of each other's ‘environment’ that strongly affect their VE and VG*E terms.
Certain environmental factors (Box 2, Table 1) might influence the outcome of a mosquito feeding on an infective bloodmeal either directly (Box 3), or indirectly, as a result of genotype-by-environment interactions (Box 4). Some of these might alter the expression and success of the immune response directed against the parasite, others will affect the distribution of resources within the mosquito and eventually influence its survival during the parasite developmental period. Although studies of the effect of different environmental qualities on mosquito–Plasmodium interactions are not common, Plasmodium-induced mortality varies with the temperature [43], diet [44] and density at which adult mosquitoes are kept [45]. However, in a meta-analytic study of several Anopheles–Plasmodium natural and unnatural interactions, diet and humidity seemed to have an effect on survival in infected mosquitoes wherease temperature did not [1]. In An. stephensi infected with P. berghei, concomitant bacterial infection [13] also affected Plasmodium infections. Finally, in adults of a refractory An. gambiae strain, competition at the larval stage strongly influenced melanization response [46] (Box 3). The ability to melanize beads was also affected by adult nutrition [47] and age, with all adults melanizing beads immediately after eclosion but only 23% by the time they were 7 days old [48].
Table 1.
Genetic and environmental factors directly or indirectly affecting mosquito–Plasmodium infections
| Factors | Possible mechanisms | Expected effect | Refsa |
|---|---|---|---|
| Mosquito genetic factors | |||
| Genetic determinants of susceptibility and resistance | Immune surveillance molecules (PRRs) immune effector molecules | Parasite survival and development | [34] |
| Genetic determinants of adult body quality | Adult size and longevity | Parasite development | |
| Mosquito environmental factors | |||
| Larval environment | Temperature, habitat quality, food, larval density | Adult quality, adult longevity, adult immune system, resistance to malaria | [46,63] |
| Adult environment during infection | Dietary components, temperature, humidity, adult density | Mosquito immune response, survival, parasite development | [1,43,45,47,64] |
| Interaction between genetic and environmental factors | |||
| Interactions with larval environment | Food, temperature, habitat quality, larval density | Adult quality, resistance to malaria | [1] |
| Interactions with adult environment during infection | Sugar feeding, temperature, humidity | Resistance to malaria and vector fitness | [49,50] |
| Plasmodiumgenetic factors | |||
| Genetic determinants of susceptibility and resistance | Virulence genes, ligands for surveillance molecules (PAMPs), ability to suppress host immune response | Vector survival and fecundity and mosquito immune response | [44,65,66] |
| Plasmodiumenvironmental factors | |||
| Mosquito genetic and environmental factors | See mosquito environmental factors within this table and associated references | Parasite survival and development | [1,43,45–47,63,64] |
| Environmental factors independent of the vector | Temperature and parasite density | Parasite survival and development | [67] |
| Interaction between genetic and environmental factors | |||
| Interactions between Plasmodium, mosquito and environmental factors | Plasmodium virulence and sugar feeding | Parasite survival and development | [44] |
Where possible, references have been selected to support the concept that genetic and environmental factors will affect infection. Abbreviations: PAMPs, pathogen-associated molecular pattern; PPRs, host pattern recognition receptor.
Box 3. Mosquito environmental factors.
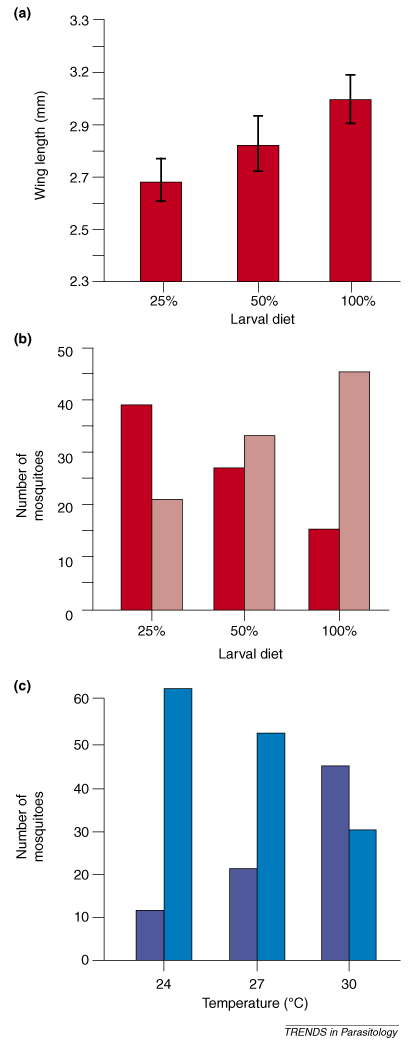
Factors such as food availability, temperature and humidity or rainfall affect mosquito development at the larval stage and their body condition at the adult stage. Environmental factors largely determine the condition of mosquito hosts when they are infected and probably have a crucial role in determining the outcome of Plasmodium infections in nature. Evidence from laboratory models suggests a strong link between environmental factors and the mosquito immune system. For example, Suwanchaichinda and Paskewitz [46] showed that the amount of resources available for larval development affects adult body size and condition, using a selected line of An. gambiae refractory to the rodent malaria P. berghei. This phenotypic response subsequently negatively affected melanization of foreign particles, the main determinant of infection in the mosquito strain selected for strong constitutive immune response (Figure Ia,b). Ambient temperature also had a strong effect on mosquito melanization response (Figure Ic) [46]. Thus, in natural populations environmental variability probably strongly affects interactions between mosquitoes and their parasites through its influence on host body condition and immune system function. Note that changes in environmental conditions generally affect all mosquitoes, but that particular genotypes could respond more or less strongly to varying environmental conditions. Such interactions are essential for maintaining genetic variation in mosquito immune responses in wild populations (Box 4).
Box 4. Genetic factors and interactions with the environment.
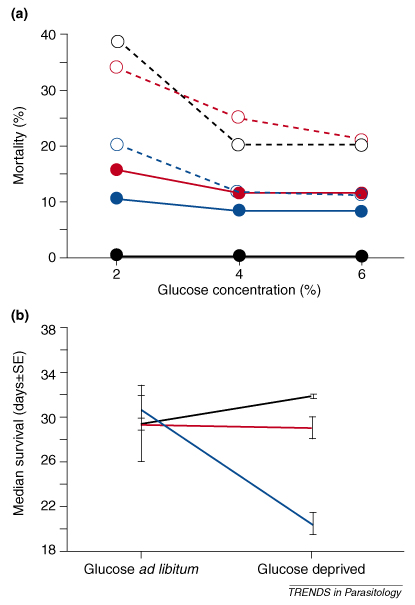
Whether an infected mosquito clears an infection, survives with a given parasite load or succumbs to infection depends largely on mosquito and Plasmodium genetic factors, as well as interactions between genetic factors and the environment. Figure Ia shows the negative effects of glucose deprivation in three Anopheles stephensi lines either non-infected or infected with the rodent malaria parasite P. yoelii. In this particular study there was a strong effect of mosquito genotype on likelihood to survive, but no significant interaction between mosquito genotype and the environment [49]. Figure Ib shows strikingly different effects of two lines of the rodent malaria P. chabaudi on the survival of An. stephensi females in response to glucose deprivation [44]. Such contrasting mosquito survival rates suggest that the virulence of different parasite genotypes could vary greatly in response to environmental factors. Generally speaking, genetic-by-environmental interactions indicate that different genotypes have higher fitness under different sets of environmental conditions and thus provide a simple explanation for the creation and maintenance of genetic variation in mosquito and Plasmodium populations.
The environment might also influence the expression of resistance if the phenotype is determined by genotype-by-environment interactions, that is if different genotypes respond differently to variation of environmental conditions [42,49] (Box 4). Such an interaction was found in An. stephensi mosquitoes infected with Plasmodium chabaudi, a non-natural association in which the effect of sugar-water deprivation on infection strongly depended on the genotype of the parasite [44]. However, in another study differences in the concentration of sugar solutions (2%, 4% and 6%) fed to eight isofemale lines of An. stephensi infected with Plasmodium yoelii yoelii affected infection intensity but did not interact with mosquito genotype [49].
Laboratory experiments are usually designed to test the effect of one stressor, but in the field situation mosquitoes will be exposed simultaneously to multiple stresses that could compound their effects and add an extra dimension to the outcome of exposure to infection. This might be particularly true for parasites that are not highly virulent, for which subtle effects can simply be compensated for by the host, for example by enhanced food intake. In such cases, the major effects of parasitism will only appear when the host is under multiple stresses. For example, immune cost was seen in a refractory line of An. gambiae infected with P. yoelii nigeriensis when simultaneously subjected to the stresses of induced flight, food deprivation and low temperature [50]. Although one study based on the An. stephensi and P. yoelii yoelii model system suggests environmental stress negatively affects mosquito survival and infection intensity [49], no study has reported on the effects of environmental stress on fitness cost of P. falciparum infection in An. gambiae s.s. or An. stephensi in the laboratory or in field situations.
Maintenance of resistance and susceptibility in natural populations
The costs associated with constitutive and (perhaps to a lesser extent) inducible immunity are thought to be the main reason for the maintenance of susceptibility in natural populations [51]. Although alleles for strong constitutive defences might be selected for in laboratory experiments, it is unlikely that these could occur at high frequencies in wild populations. Constitutive defences are thought to be rapid-acting and generally less specific than inducible responses [26], therefore theory predicts that such defences should be favoured when pathogens are highly prevalent in populations and have high growth rates [52]. Inducible defences are expected to be more specific and comparatively less costly and therefore could occur at higher frequency in natural populations [10]. However, this might not always be the case. For example, Armitage et al. [53] showed that constitutive investment in prophylactic cuticular melanization in Tenebrio molitor fed ad libitum did not carry fitness costs, but that an induced encapsulation response affected longevity. One of the obvious challenges of ecological immunology is to identify the ecological factors and genetic mechanisms that determine the investment in constitutive and inducible immune defences and generate variation in these defences among individuals in natural populations.
Sinden et al. [5] argue that all mosquitoes should maintain defence mechanisms that have sustainable evolutionary costs and hence they should all be refractory to infection to a certain degree. There is good evidence from laboratory studies suggesting that mosquitoes have baseline defence mechanisms that limit parasite development such that only a small fraction of malaria parasites develop to the sporozoite stage [36,54]. Recent advances also suggest that some wild mosquito genotypes might be resistant to most infections [55,56]. Using mosquito families raised from wild caught females and fed on blood from locally infected patients to ensure a study system similar to the natural system, Riehle et al. [56] genetically mapped an ‘island of resistance’ responsible for most of the variation in oocyst number. Genes responsible for melanization were also identified, but melanization only affected a fraction of oocysts in infected females and thus could not account for refractoriness. Instead, a gene encoding for an Anopheles Plasmodium-responsive leucine-rich repeat 1 protein (APL1) was responsible for this strong inducible defence against Plasmodium [56]. Because refractory and susceptible alleles were found in this study, the important question remains as to what factors determine their frequencies in natural populations.
The environment in which wild mosquito populations live is highly variable in time and in space. There is strong evidence from laboratory systems that genetic and environmental factors interact to determine the fitness of infected and non-infected individuals such that different genotypes might have higher fitness in different environmental conditions (Box 4). Environmental conditions vary spatially and temporally and selection regimes differ markedly, particularly in subtropical and tropical climates typically associated with strong seasonality. For example, large areas of Africa are characterized by dry seasons with low mosquito densities and low malaria transmission, during which the majority of mosquitoes will rarely feed on infected blood [57]. Under such conditions, individuals with lower levels of immunity to Plasmodium should be fitter than their resistant high malaria season counterpart, creating seasonal cycles in the frequency of resistance alleles. To date few field studies have attempted to correlate variation in environmental characteristics with variation in immune responses [58].
Another source of variation in immune defence that leads to cycles in allelic frequencies is frequency-dependent selection. Laboratory studies support the idea that the outcome of infection depends on the host and Plasmodium genotypes (Box 2, Box 4). Strong genetic interactions between different An. gambiae families and natural mixed-infection P. falciparum isolates suggest that such interactions could have an important role in nature [59]. In this scenario, parasite genotypes that are most able to invade the most common host genotypes increase in frequency until they become so frequent that rare host genotypes become favoured, resulting in a continual negative frequency-dependent selection process. Theory predicts that under this type of selection regime no single resistance allele should reach fixation [60], hence frequency-dependence could have a fundamental role in promoting and maintaining variation in the immune system.
Concluding remarks
Despite years of research, relatively little is known about microevolutionary processes that create and maintain variation in immune responses in wild mosquito populations. The ecological immunology of mosquito–Plasmodium interactions remains in its infancy and this review should serve as a strong incentive for re-examining the effects of genetic and environmental factors on Plasmodium infections using controlled laboratory experiments based on natural mosquito–Plasmodium associations. Future transcriptomic and metabolomic studies based on similar experimental designs will further bolster our understanding of the underlying mechanisms leading to variation in immune responses in natural populations. The challenge of the field of ecological immunology is to design tractable field studies that incorporate the multiple ecological factors that are essential for determining the course of an infection. The complexity of the infected mosquito phenotype can easily turn experiments into exercises in confounding factors and correlative interpretations. However, despite these difficulties, describing population-level processes occurring between Plasmodium and mosquito hosts remains a priority and a key component in our quest to resolve the malaria problem.
Figure I.
Distribution of Plasmodium infections among mosquito hosts. (a) Binomial distribution of P. falciparum oocysts in a natural population of An. gambiae in Northeast Tanzania. (b) A normal distribution of oocysts in the ZAN-U strain of An. gambiae experimentally infected with P. yoelii nigeriensis. (c) Uniform distribution of oocysts in the KIL strain of An. gambiae infected with P. yoelii nigeriensis. The two laboratory strains have been maintained in the laboratory for decades and exhibit high susceptibility to infection. Adapted with permission from [50,61].
Figure I.
Mosquito–Plasmodium interactions as a complex phenotype. Variation in the outcome of an infection is a particularly complex phenotype in that both host and parasite genetic and environmental factors can be considered essential components of each other's ‘environment’ and can strongly influence infection (intersection between blue, red and black ellipses). As an example, the environmental factors affecting Plasmodium development inside mosquitoes (black ellipse) are almost entirely determined by mosquito genetic and environmental determinants (blue ellipse).
Figure I.
Environmental effects on host body condition and the immune system. (a) Phenotypic changes in body size of adult An. gambiae females in response to the amounts of food available to the larvae (represented by the percentage of powder food provided). Adult body size is strongly negatively affected by decreasing amounts of resources available at the larval stage. (b) Larval food deprivation also affects the intensity of an immune response of adult mosquitoes, as evaluated by the amount of melanization of sephadex beads inoculated in the thorax. Light red bars indicate the number of females with bead melanization ≥50%, dark red bars females with melanization <50%. This is probably because starved larvae give rise to small imagines that are in poor body condition. (c) Temperature is another environmental factor that has a strong effect on the immune response to sephadex beads. Light blue bars are number of females with bead melanization ≥50%, dark blue bars females with melanization <50%. Adapted with permission from [46].
Figure I.
Interaction between genotypes and the environment. (a) Effect of food availability (glucose solution) on the survival of different lines (black, red and blue) of An. stephensi infected with P. yoelii yoelii (dashed lines) or uninfected (solid lines) showing a strong effect of genetic factors on survivorship. (b) Changes in the survival of An. stephensi infected with different strains of P. chabaudi and provided with high and low amounts of food (glucose solution). Infection with two different strains (blue and black lines) results in contrasted norms of reaction to glucose deprivation. Interestingly and predictably, a combined infection with the two strains (red line) gives a response intermediate to the two individual ones. Adapted with permission from [44,49].
Acknowledgements
We thank three anonymous referees for comments on the manuscript.
References
- 1.Ferguson H.M., Read A.F. Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol. 2002;18:256–261. doi: 10.1016/s1471-4922(02)02281-x. [DOI] [PubMed] [Google Scholar]
- 2.Cohuet A. Anopheles and Plasmodium: from laboratory models to natural systems in the field. EMBO Rep. 2006;7:1285–1289. doi: 10.1038/sj.embor.7400831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong Y. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michel K. Increased melanizing activity in Anopheles gambiae does not affect development of Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16858–16863. doi: 10.1073/pnas.0608033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinden R.E. Mosquito–malaria interactions: a reappraisal of the concepts of susceptibility and refractoriness. Insect Biochem. Mol. Biol. 2004;34:625–629. doi: 10.1016/j.ibmb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar R. Anopheles infection responses: laboratory models versus field malaria transmission systems. Acta Trop. 2005;95:285–291. doi: 10.1016/j.actatropica.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Boete C. Malaria parasites in mosquitoes: laboratory models, evolutionary temptation and the real world. Trends Parasitol. 2005;21:445–447. doi: 10.1016/j.pt.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Vernick K.D. Molecular genetics of mosquito resistance to malaria parasites. Curr. Top. Microbiol. Immunol. 2005;295:383–415. doi: 10.1007/3-540-29088-5_15. [DOI] [PubMed] [Google Scholar]
- 9.Sheldon B.C., Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- 10.Rolff J., Siva-Jothy M.T. Invertebrate ecological immunology. Science. 2003;301:472–475. doi: 10.1126/science.1080623. [DOI] [PubMed] [Google Scholar]
- 11.Lim J. Induction of nitric oxide synthase in Anopheles stephensi by Plasmodium falciparum: mechanism of signaling and the role of parasite glycosylphosphatidylinositols. Infect. Immun. 2005;73:2778–2789. doi: 10.1128/IAI.73.5.2778-2789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baton L.A., Randford-Cartwright L.C. Morphological evidence for proliferative regeneration of the Anopheles stephensi midgut epithelium following Plasmodium falciparum ookinete invasion. J. Invertebr. Pathol. 2007;96:244–254. doi: 10.1016/j.jip.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Seitz H.M. Concomitant infections of Anopheles stephensi with Plasmodium berghei and Serratia marcescens: additive detrimental effects. Zentralbl. Bakteriol. Mikrobiol. Hyg. [A] 1987;266:155–166. doi: 10.1016/s0176-6724(87)80029-9. [DOI] [PubMed] [Google Scholar]
- 14.Hopwood J.A. Malaria-induced apoptosis in mosquito ovaries: a mechanism to control vector egg production. J. Exp. Biol. 2001;204:2773–2780. doi: 10.1242/jeb.204.16.2773. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed A.M. Effects of malaria infection on vitellogenesis in Anopheles gambiae during two gonotrophic cycles. Insect Mol. Biol. 2001;10:347–356. doi: 10.1046/j.0962-1075.2001.00273.x. [DOI] [PubMed] [Google Scholar]
- 16.Beier J.C. Malaria parasite development in mosquitoes. Annu. Rev. Entomol. 1998;43:519–543. doi: 10.1146/annurev.ento.43.1.519. [DOI] [PubMed] [Google Scholar]
- 17.Hurd H. Interactions between bloodfeeding, fecundity and infection in mosquitoes. Parasitol. Today. 1995;11:411–416. [Google Scholar]
- 18.Wekesa J.W. Effect of Plasmodium falciparum blood feeding behaviour of naturally infected Anopheles mosquitoes in western Kenya. Am. J. Trop. Med. Hyg. 1992;47:484–488. doi: 10.4269/ajtmh.1992.47.484. [DOI] [PubMed] [Google Scholar]
- 19.Rossignol P.A. Increased intradermal probing time in sporozoite-infected mosquitoes. Am. J. Trop. Med. Hyg. 1984;33:17–20. doi: 10.4269/ajtmh.1984.33.17. [DOI] [PubMed] [Google Scholar]
- 20.Anderson R.A. The effect of Plasmodium yoelii nigeriensis infection on the feeding persistence of Anopheles stephensi Liston throughout the sporogenic cycle. Proc. Biol. Sci. 1999;266:1729–1733. doi: 10.1098/rspb.1999.0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson R.A. Plasmodium falciparum sporozoites increase feeding-associated mortality of their mosquito hosts Anopheles gambiae s.l. Parasitology. 2000;120:329–333. doi: 10.1017/s0031182099005570. [DOI] [PubMed] [Google Scholar]
- 22.Hurd H. Manipulation of medically important insect vectors by their parasites. Annu. Rev. Entomol. 2003;48:141–161. doi: 10.1146/annurev.ento.48.091801.112722. [DOI] [PubMed] [Google Scholar]
- 23.Hurd H. Host fecundity reduction: a damage limitation strategy? Trends Parasitol. 2001;17:363–368. doi: 10.1016/s1471-4922(01)01927-4. [DOI] [PubMed] [Google Scholar]
- 24.Gray E.M., Bradley T.J. Malarial infection in Aedes aegypti: effects on feeding, fecundity and metabolic rate. Parasitology. 2006;132:169–176. doi: 10.1017/S0031182005008966. [DOI] [PubMed] [Google Scholar]
- 25.Marrelli M.T. Transgenic malaria-resistant mosquitoes have a fitness advantage when feeding on Plasmodium-infected blood. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5580–5583. doi: 10.1073/pnas.0609809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmid-Hempel P. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 2005;50:529–551. doi: 10.1146/annurev.ento.50.071803.130420. [DOI] [PubMed] [Google Scholar]
- 27.Sugumaran M. Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res. 2002;15:2–9. doi: 10.1034/j.1600-0749.2002.00056.x. [DOI] [PubMed] [Google Scholar]
- 28.Fellowes M.D.E. Trade-off associated with selection for increased ability to resist parasitoid attack in Drosophila melanogaster. Proc. Biol. Sci. 1998;265:1553–1558. doi: 10.1098/rspb.1998.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraaijeveld A.R. Basis of the trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Proc. Biol. Sci. 2001;268:259–261. doi: 10.1098/rspb.2000.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poirie M. Drosophila resistance genes to parasitoids: chromosomal location and linkage analysis. Proc. Biol. Sci. 2000;267:1417–1421. doi: 10.1098/rspb.2000.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koella J.C., Boëte C. A genetic correlation between age at pupation and melanization immune response of the yellow fever mosquito Aedes aegypti. Evolution Int. J. Org. Evolution. 2002;56:1074–1079. doi: 10.1111/j.0014-3820.2002.tb01419.x. [DOI] [PubMed] [Google Scholar]
- 32.Vlachou D., Kafatos F.C. The complex interplay between mosquito positive and negative regulators of Plasmodium development. Curr. Opin. Microbiol. 2005;8:415–421. doi: 10.1016/j.mib.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Barillas-Mury C., Kumar S. Plasmodium–mosquito interactions: a tale of dangerous liaisons. Cell. Microbiol. 2005;7:1539–1545. doi: 10.1111/j.1462-5822.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- 34.Michel K., Kafatos F.C. Mosquito immunity against Plasmodium. Insect Biochem. Mol. Biol. 2005;35:677–689. doi: 10.1016/j.ibmb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S. Inducible peroxidases mediate nitration of Anopheles midgut cells undergoing apoptosis in response to Plasmodium invasion. J. Biol. Chem. 2004;279:53475–53482. doi: 10.1074/jbc.M409905200. [DOI] [PubMed] [Google Scholar]
- 36.Luckhart S. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han Y.S. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. EMBO J. 2000;19:6030–6040. doi: 10.1093/emboj/19.22.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivero A. Nitric oxide: an antiparasitic molecule of invertebrates. Trends Parasitol. 2006;22:219–225. doi: 10.1016/j.pt.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz A., Koella J.C. The cost of immunity in the yellow fever mosquito, Aedes aegypti depends on immune activation. J. Evol. Biol. 2004;17:834–840. doi: 10.1111/j.1420-9101.2004.00720.x. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed A.M., Hurd H. Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiae via follicle apoptosis. Microbes Infect. 2005;8:308–315. doi: 10.1016/j.micinf.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz A., Koella J.C. Melanization of Plasmodium falciparum and C-25 sephadex beads by field-caught Anopheles gambiae (Diptera: Culicidae) from southern Tanzania. J. Med. Entomol. 2002;39:84–88. doi: 10.1603/0022-2585-39.1.84. [DOI] [PubMed] [Google Scholar]
- 42.Thomas M.B., Blanford S. Thermal biology in insect–parasite interactions. Trends Ecol. Evol. 2003;18:344–350. [Google Scholar]
- 43.Gad A.M. Pathology of Anopheles stephensi after infection with Plasmodium berghei berghei. I. Mortality rate. Z. Parasitenkd. 1979;60:249–261. doi: 10.1007/BF00929172. [DOI] [PubMed] [Google Scholar]
- 44.Ferguson H.M., Read A.F. Genetic and environmental determinants of malaria parasite virulence in mosquitoes. Proc. Biol. Sci. 2002;269:1217–1224. doi: 10.1098/rspb.2002.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maier W.A. Mortality of Culex pipiens fatigans following infection with Plasmodium cathemerium. Z. Parasitenkd. 1973;41:11–28. doi: 10.1007/BF00329627. [DOI] [PubMed] [Google Scholar]
- 46.Suwanchaichinda C., Paskewitz S.M. Effects of larval nutrition, adult body size, and adult temperature on the ability of Anopheles gambiae (Diptera: Culicidae) to melanize sephadex beads. J. Med. Entomol. 1998;35:157–161. doi: 10.1093/jmedent/35.2.157. [DOI] [PubMed] [Google Scholar]
- 47.Koella J.C., Sorense F.L. Effect of adult nutrition on the melanization immune response of the malaria vector Anopheles stephensi. Med. Vet. Entomol. 2002;16:316–320. doi: 10.1046/j.1365-2915.2002.00381.x. [DOI] [PubMed] [Google Scholar]
- 48.Chun J. Effect of mosquito age and reproductive status on melanization of sephadex beads in Plasmodium-refractory and -susceptible strains of Anopheles gambiae. J. Invertebr. Pathol. 1995;66:11–17. doi: 10.1006/jipa.1995.1054. [DOI] [PubMed] [Google Scholar]
- 49.Lambrechts L. Environmental influence on the genetic basis of mosquito resistance to malaria parasites. Proc. Biol. Sci. 2006;273:1501–1506. doi: 10.1098/rspb.2006.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurd H. Evaluating the costs of mosquito resistance to malaria parasites. Evolution Int. J. Org. Evolution. 2005;59:2560–2572. [PMC free article] [PubMed] [Google Scholar]
- 51.Boete C., Koella J.C. Evolutionary ideas about genetically manipulated mosquitoes and malaria control. Trends Parasitol. 2003;19:32–38. doi: 10.1016/s1471-4922(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 52.Shudo E., Iwasa Y. Inducible defense against pathogens and parasites: optimal choice among multiple options. J. Theor. Biol. 2001;209:233–247. doi: 10.1006/jtbi.2000.2259. [DOI] [PubMed] [Google Scholar]
- 53.Armitage S.A. Examining costs of induced and constitutive immune investment in Tenebrio molitor. J. Evol. Biol. 2003;16:1038–1044. doi: 10.1046/j.1420-9101.2003.00551.x. [DOI] [PubMed] [Google Scholar]
- 54.Gouagna L.C. The early sporogonic cycle of Plasmodium falciparum in laboratory-infected Anopheles gambiae: an estimation of parasite efficacy. Trop. Med. Int. Health. 1998;3:21–28. doi: 10.1046/j.1365-3156.1998.00156.x. [DOI] [PubMed] [Google Scholar]
- 55.Riehle M.M. A major genetic locus controlling natural Plasmodium falciparum infection is shared by East and West African Anopheles gambiae. Malar. J. 2007;6:87. doi: 10.1186/1475-2875-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riehle M.M. Natural malaria infection in Anopheles gambiae is regulated by a single genomic control region. Science. 2006;312:577–579. doi: 10.1126/science.1124153. [DOI] [PubMed] [Google Scholar]
- 57.Drakeley C. The epidemiology of Plasmodium falciparum gametocytes: weapons of mass dispersion. Trends Parasitol. 2006;22:424–430. doi: 10.1016/j.pt.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Luckhart S. Anopheles gambiae immune gene variants associated with natural Plasmodium infection. Mol. Biochem. Parasitol. 2003;128:83–86. doi: 10.1016/s0166-6851(03)00016-1. [DOI] [PubMed] [Google Scholar]
- 59.Lambrechts L. Host genotype by parasite genotype interactions underlying the resistance of anopheline mosquitoes to Plasmodium falciparum. Malar. J. 2005;4:3. doi: 10.1186/1475-2875-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agrawal A., Lively C.M. Infection genetics: gene-for-gene versus matching-alleles models and all points in between. Evol. Ecol. Res. 2002;4:79–90. [Google Scholar]
- 61.Hogg J.C., Hurd H. The effects of natural Plasmodium falciparum infection on the fecundity and mortality of Anopheles gambiae s.l. in north east Tanzania. Parasitology. 1997;114:325–331. doi: 10.1017/s0031182096008542. [DOI] [PubMed] [Google Scholar]
- 62.Paul R.E. Aggregation in malaria parasites places limits on mosquito infection rates. Infect. Genet. Evol. 2007;7:577–586. doi: 10.1016/j.meegid.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 63.Okech B.A. Larval habitats of Anopheles gambiae s.s. (Diptera: Culicidae) influences vector competence to Plasmodium falciparum parasites. Malar. J. 2007;6:50. doi: 10.1186/1475-2875-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beier M.S. Effects of para-aminobenzoic acid, insulin, and gentamicin on Plasmodium falciparum development in anopheline mosquitoes (Diptera: Culicidae) J. Med. Entomol. 1994;31:561–565. doi: 10.1093/jmedent/31.4.561. [DOI] [PubMed] [Google Scholar]
- 65.Ferguson H.M. Mosquito mortality and the evolution of malaria virulence. Evolution Int. J. Org. Evolution. 2003;57:2792–2804. doi: 10.1111/j.0014-3820.2003.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 66.Boete C. Reduced efficacy of the immune melanization response in mosquitoes infected by malaria parasites. Parasitology. 2002;125:93–98. doi: 10.1017/s0031182002001944. [DOI] [PubMed] [Google Scholar]
- 67.Noden B.H. The impact of variations in temperature on early Plasmodium falciparum development in Anopheles stephensi. Parasitology. 1995;111:539–545. doi: 10.1017/s0031182000077003. [DOI] [PubMed] [Google Scholar]