Abstract
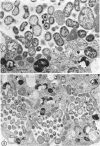
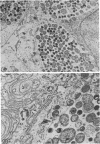
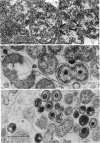
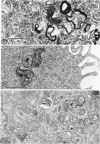
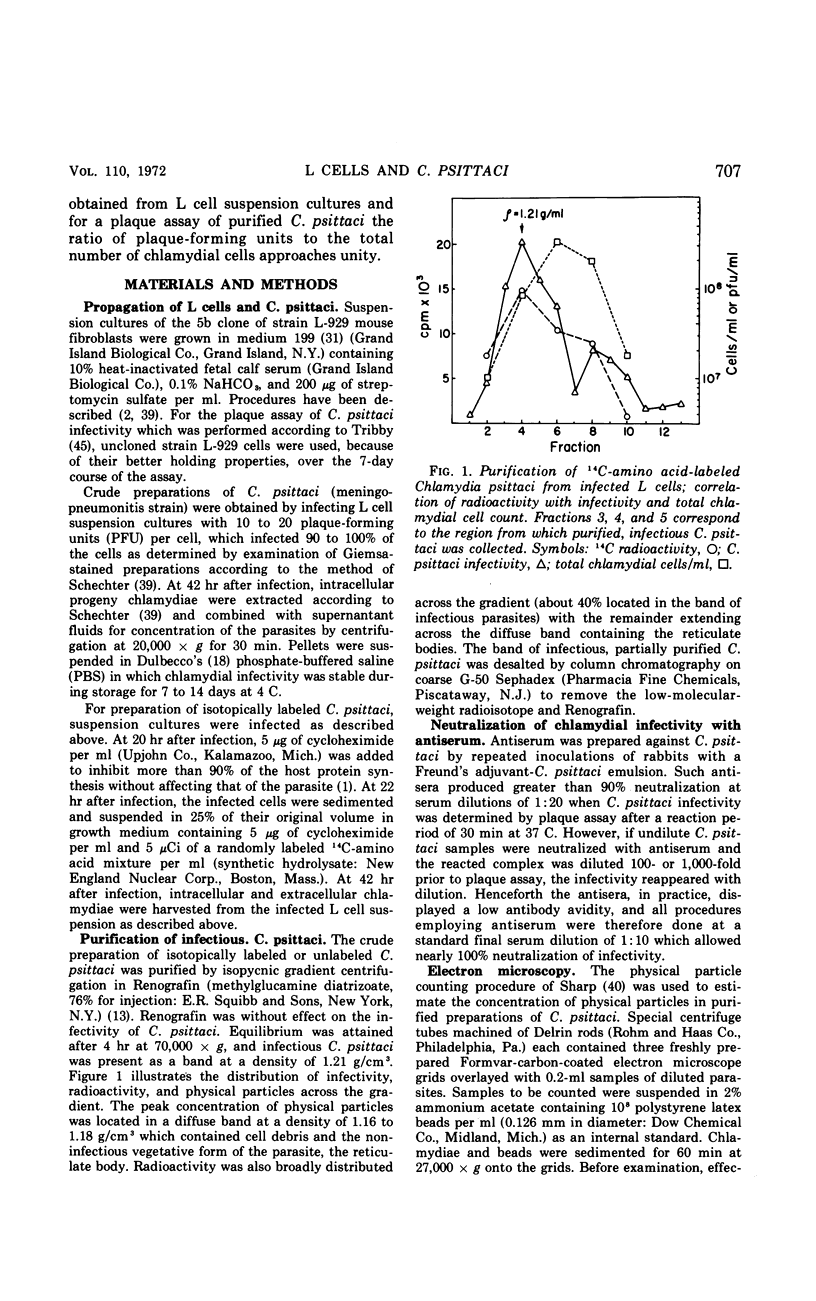
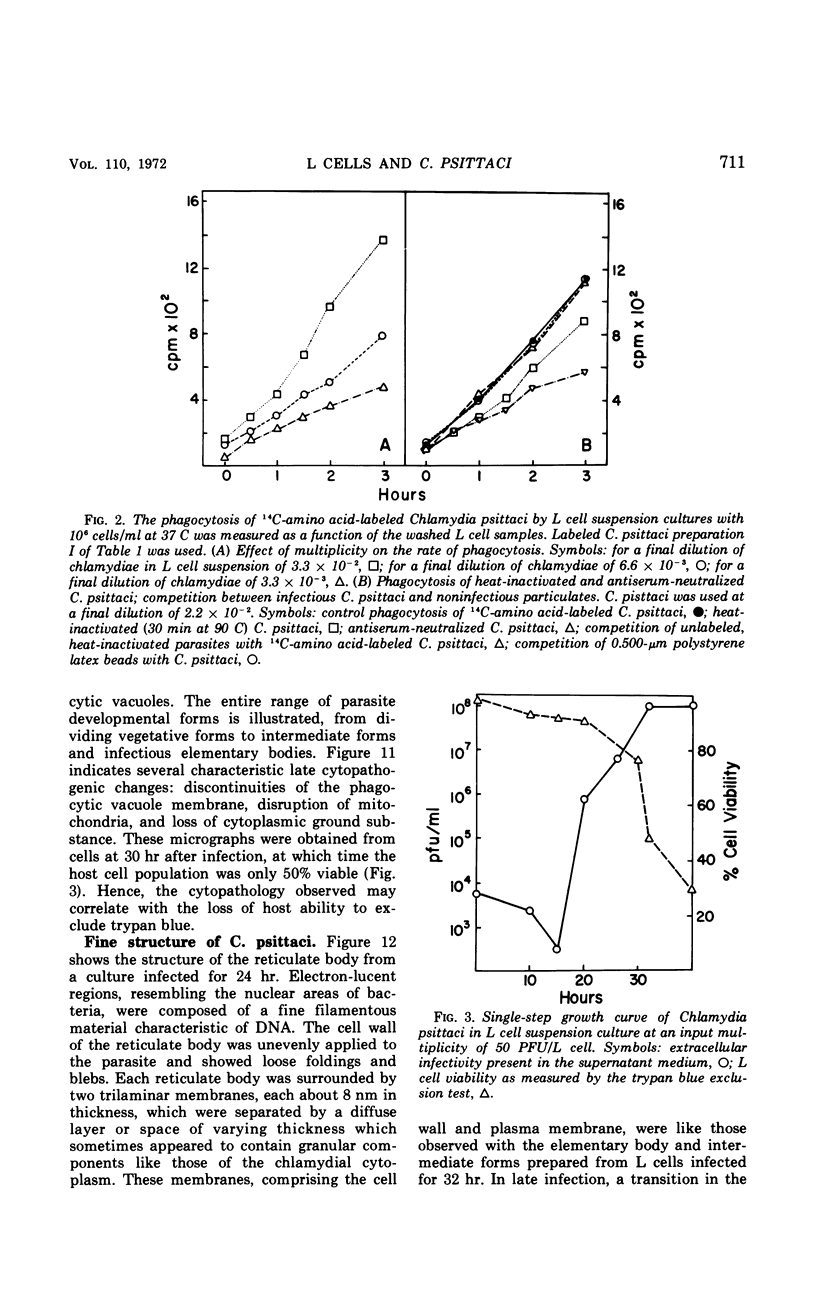
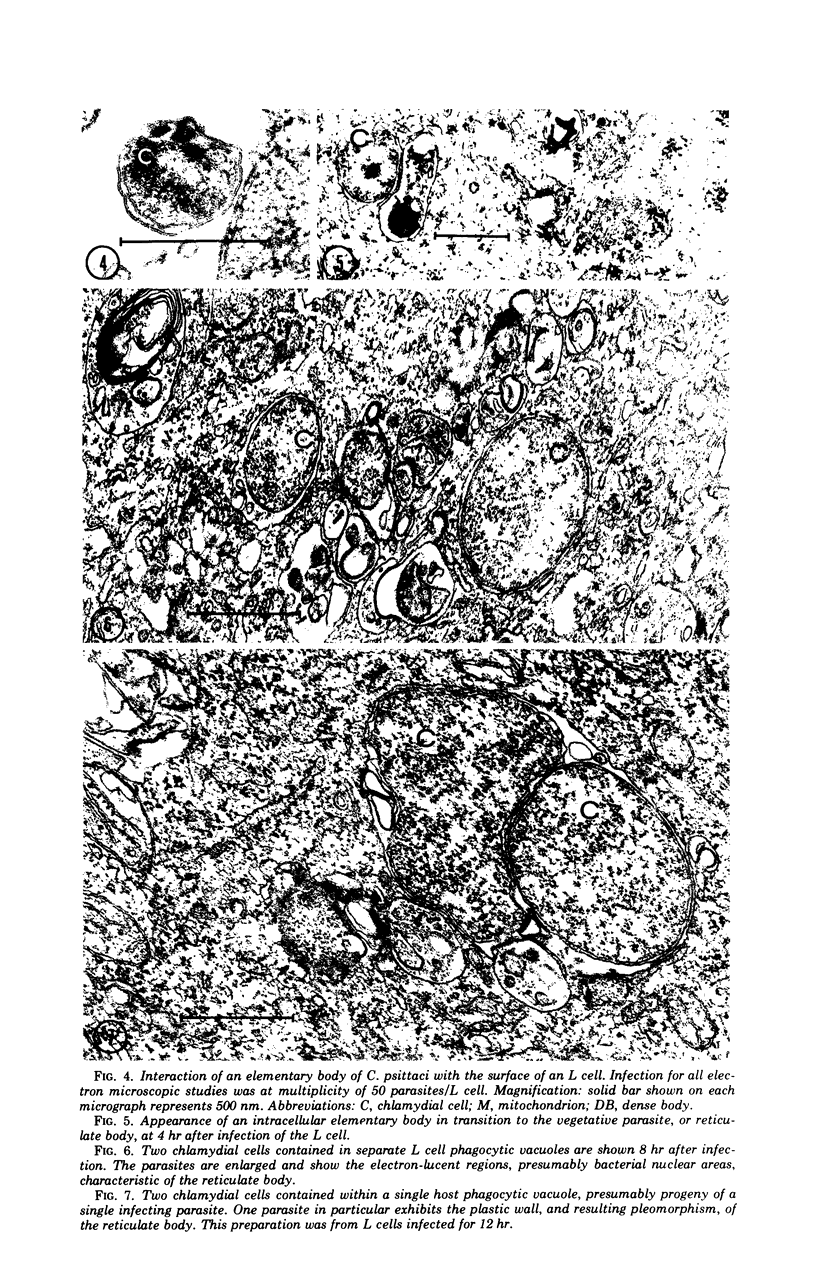
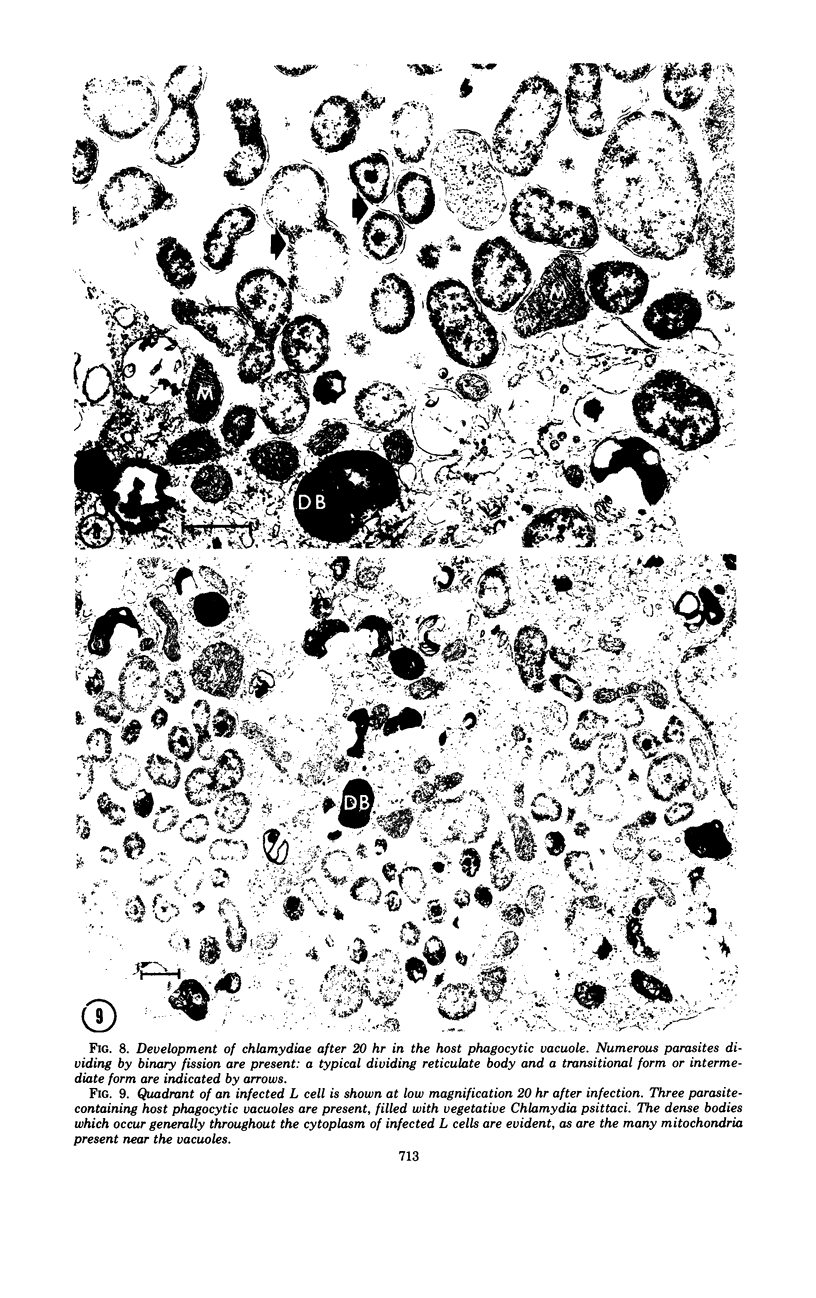
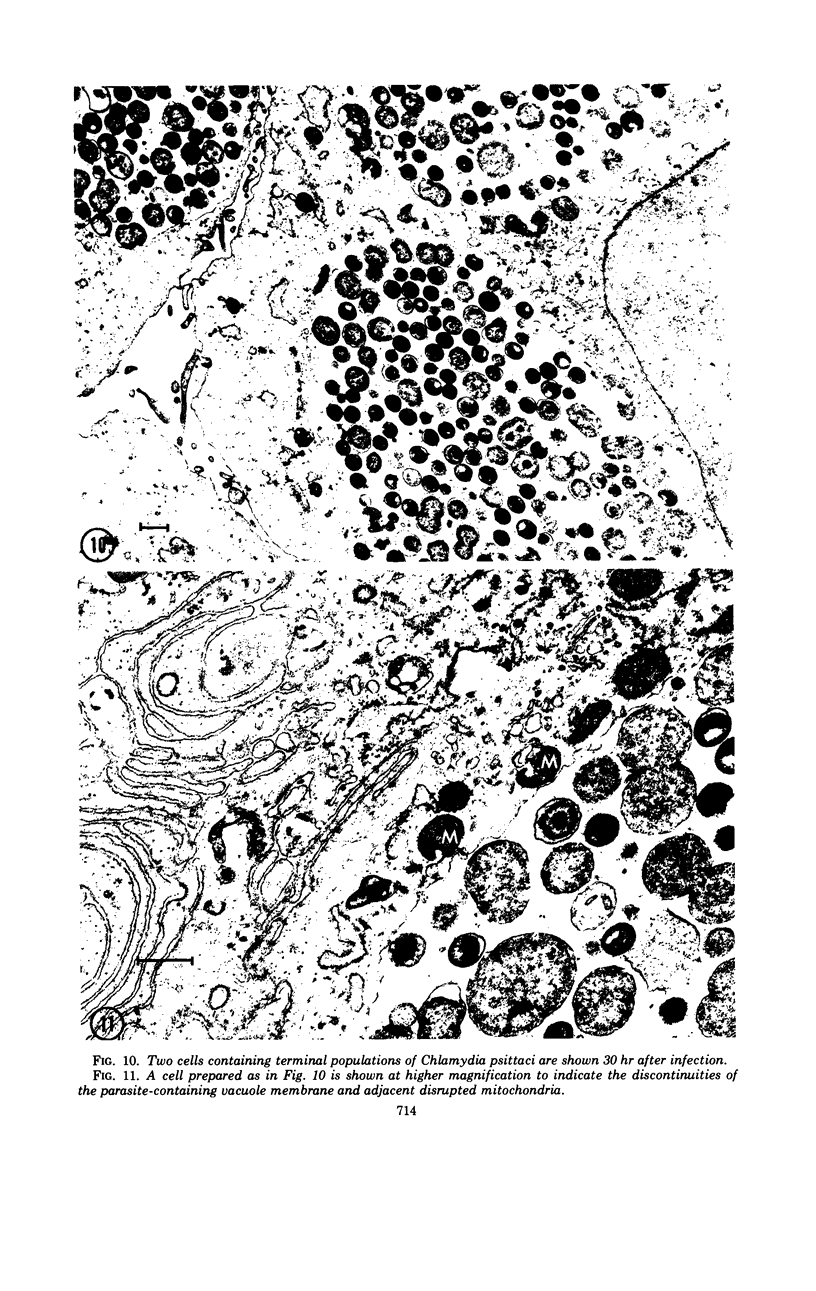
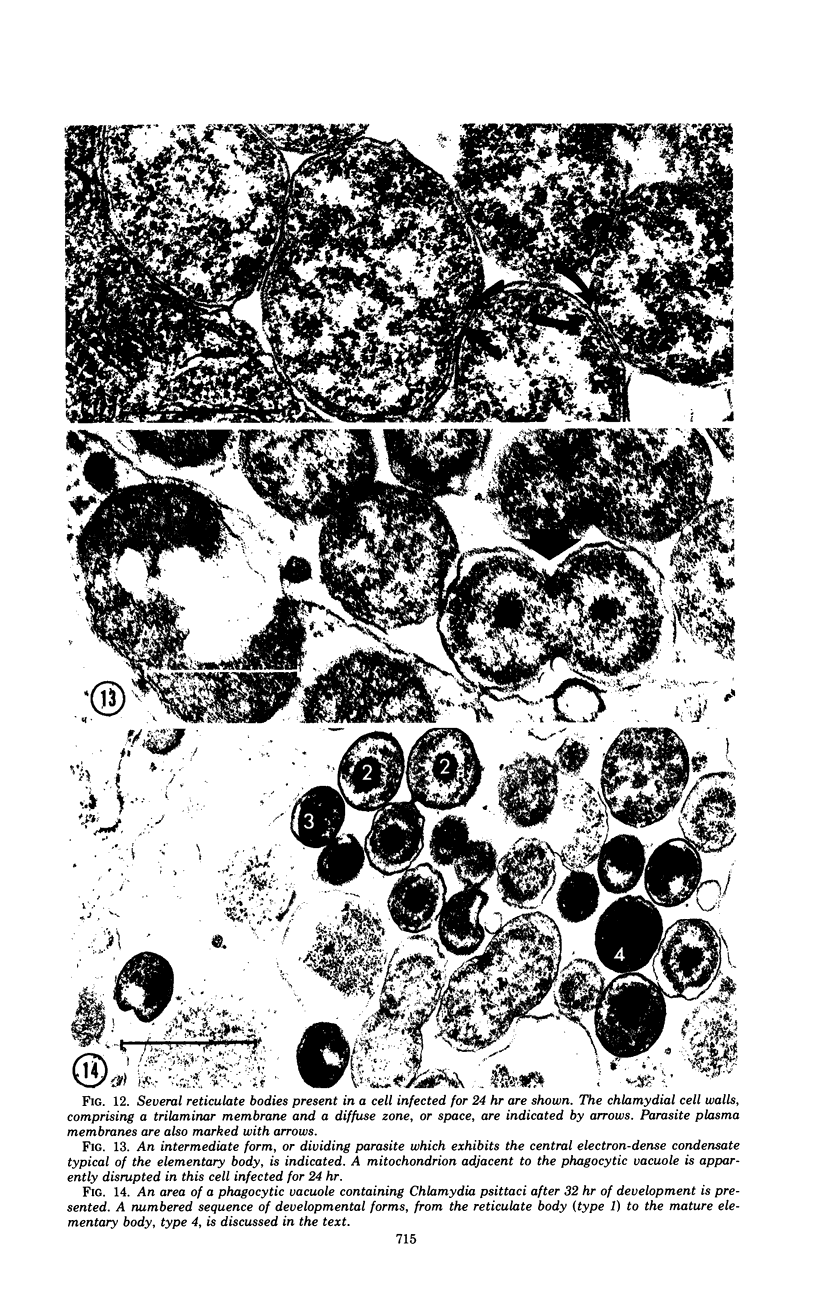
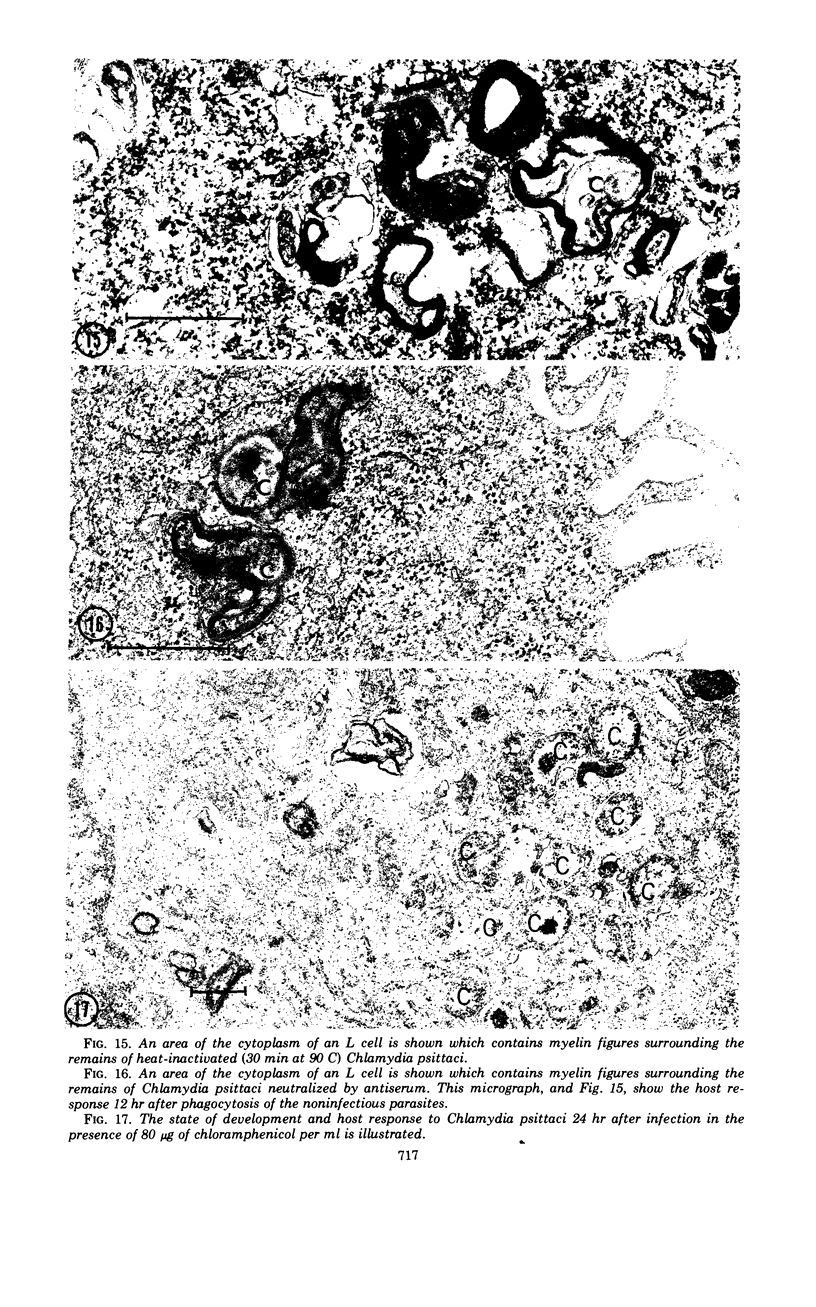
The entry and development of Chlamydia psittaci in the L cell was studied by using purified, infectious parasites at high multiplicity. Entry of the parasite was accomplished by an act of phagocytosis by the host which was independent of an adsorption stage but was temperature-dependent. Kinetic studies of phagocytosis performed with 14C-amino acid-labeled, purified parasites indicated that the rate of phagocytosis was directly proportional to the multiplicity of inoculation. Electron microscopy of cells infected at high multiplicity with purified infectious C. psittaci showed that phagocytosed chlamydiae were segregated in a host phagocytic vacuole throughout their developmental cycle which consisted of the transition of infecting elementary bodies to reticulate bodies dividing by binary fission, followed by the reemergence of a population of elementary bodies. The process of the transition was examined and a proposed sequence of intermediate bodies is presented. In isopycnic gradients of fractionated, infected L cells, chlamydial phagocytic vacuoles were apparent as a dense band distinct from lysosome and mitochondrion peaks, as indicated by acid phosphatase and cytochrome oxidase activities. Chlamydiae inactivated by heat or neutralized by antiserum were phagocytosed and appeared in lysosomes within 12 hr after infection according to electron microscopy; however, chlamydiae which were continuously inhibited in their development by chloramphenicol were retained intact in the cell for 24 hr without lysosomal response. The possibility of a lysosomal inhibitor on the native parasite is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., VALENTINE R. C. Virus particle adsorption, III. Adsorption of viruses by cell monolayers and effects of some variables on adsorption. Biochim Biophys Acta. 1960 Jun 3;40:400–410. doi: 10.1016/0006-3002(60)91380-9. [DOI] [PubMed] [Google Scholar]
- ALLISON A. C., VALENTINE R. C. Virus particle adsorption. II. Adsorption of vaccinia and fowl plague viruses to cells in suspension. Biochim Biophys Acta. 1960 Jun 3;40:393–399. doi: 10.1016/0006-3002(60)91379-2. [DOI] [PubMed] [Google Scholar]
- Alexander J. J. Effect of infection with the meningopneumonitis agent on deoxyribonucleic acid and protein synthesis by its L-cell host. J Bacteriol. 1969 Feb;97(2):653–657. doi: 10.1128/jb.97.2.653-657.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. J. Separation of protein synthesis in meningopneumonitisgent from that in L cells by differential susceptibility to cycloheximide. J Bacteriol. 1968 Feb;95(2):327–332. doi: 10.1128/jb.95.2.327-332.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. R., Hopps H. E., Barile M. F., Bernheim B. C. Comparison of the ultrastructure of several rickettsiae, ornithosis virus, and Mycoplasma in tissue culture. J Bacteriol. 1965 Nov;90(5):1387–1404. doi: 10.1128/jb.90.5.1387-1404.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT H. S., LUFT J. H. zeta-Collidine as a basis for buffering fixatives. J Biophys Biochem Cytol. 1959 Aug;6(1):113–114. doi: 10.1083/jcb.6.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENSCH K., GORDON G., MILLER L. THE FATE OF DNA-CONTAINING PARTICLES PHAGOCYTIZED BY MAMMALIAN CELLS. J Cell Biol. 1964 Apr;21:105–114. doi: 10.1083/jcb.21.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTHET J., DE DUVE C. Tissue fractionation studies. I. The existence of a mitochondria-linked, enzymically inactive form of acid phosphatase in rat-liver tissue. Biochem J. 1951 Dec;50(2):174–181. doi: 10.1042/bj0500174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers W. E., Finkenstaedt J. T., de Duve C. Lysosomes in lymphoid tissue. I. The measurement of hydrolytic activities in whole homogenates. J Cell Biol. 1967 Feb;32(2):325–337. doi: 10.1083/jcb.32.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BOZEMAN F. M., CAMPBELL J. M., HUMPHRIES J. W., SAWYER T. K. Study on growth of Rickettsia. V. Penetration of Rickettsia tsutsugamushi into mammalian cells in vitro. J Exp Med. 1959 Mar 1;109(3):271–292. doi: 10.1084/jem.109.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
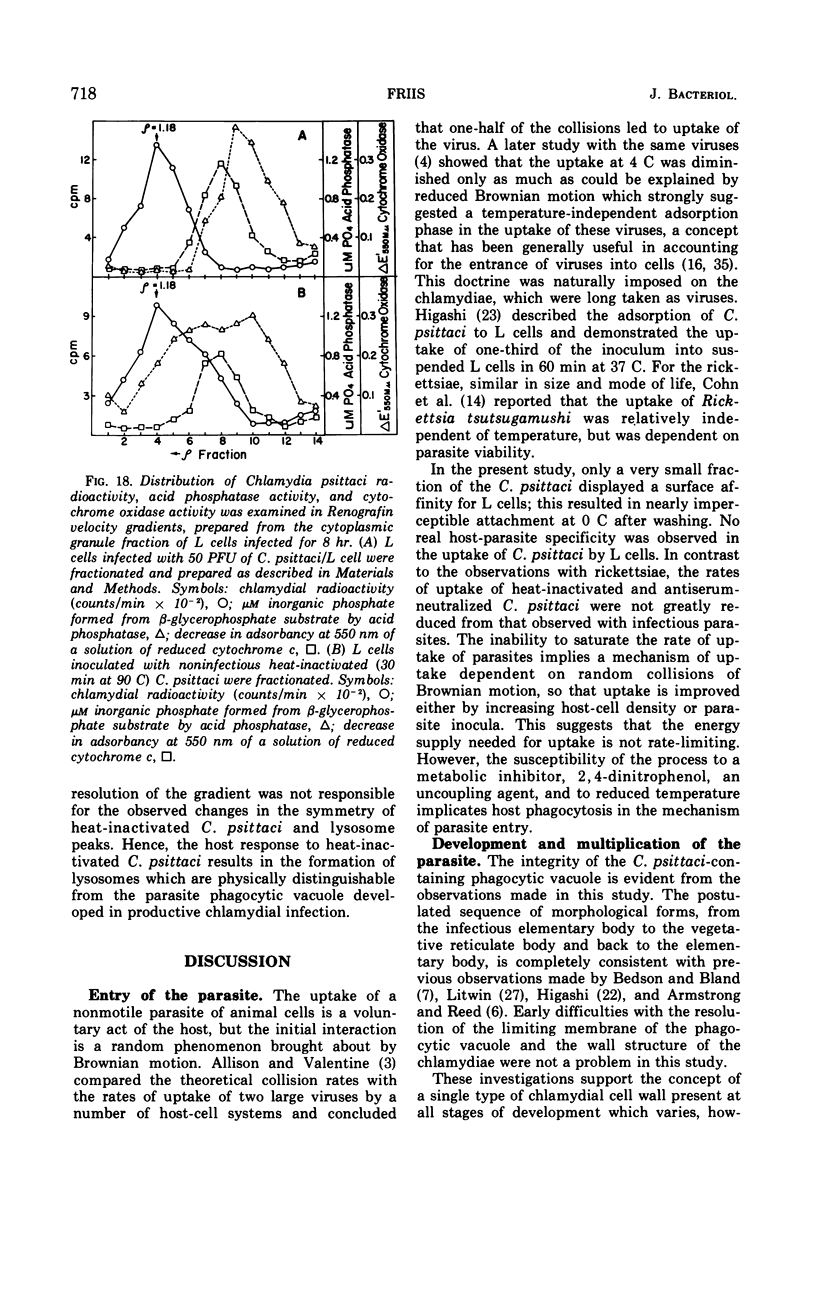
- Cahn F. H., Fox M. S. Fractionation of transformable bacteria from ocompetent cultures of Bacillus subtilis on renografin gradients. J Bacteriol. 1968 Mar;95(3):867–875. doi: 10.1128/jb.95.3.867-875.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUNCE A. L. THE ISOLATION OF NUCLEI FROM TUMOR CELLS. Exp Cell Res. 1963;24:SUPPL9–SUPPL9:143. doi: 10.1016/0014-4827(63)90253-2. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S. Pentration of animal viruses into cells. Prog Med Virol. 1965;7:1–43. [PubMed] [Google Scholar]
- ERLANDSON R. A., ALLEN E. G. THE ULTRASTRUCTURE OF MENINGOPNEUMONITIS. Virology. 1964 Mar;22:410–418. doi: 10.1016/0042-6822(64)90031-5. [DOI] [PubMed] [Google Scholar]
- HIGASHI N. ELECTRON MICROSCOPIC STUDIES ON THE MODE OF REPRODUCTION OF TRACHOMA VIRUS AND PSITTACOSIS VIRUS IN CELL CULTURES. Exp Mol Pathol. 1965 Feb;76:24–39. doi: 10.1016/0014-4800(65)90021-3. [DOI] [PubMed] [Google Scholar]
- HIGASHI N., TAMURA A., IWANAGA M. Developmental cycle and reproductive mechanism of the meningopneumonitis virus in strain L cells. Ann N Y Acad Sci. 1962 Mar 5;98:100–121. doi: 10.1111/j.1749-6632.1962.tb30536.x. [DOI] [PubMed] [Google Scholar]
- ITO S., VINSON J. W. FINE STRUCTURE OF RICKETTSIA QUINTANA CULTIVATED IN VITRO AND IN THE LOUSE. J Bacteriol. 1965 Feb;89:481–495. doi: 10.1128/jb.89.2.481-495.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITWIN J., OFFICER J. E., BROWN A., MOULDER J. W. A comparative study of the growth cycles of different members of the psittacosis group in different host cells. J Infect Dis. 1961 Nov-Dec;109:251–279. doi: 10.1093/infdis/109.3.251. [DOI] [PubMed] [Google Scholar]
- LITWIN J. The growth cycle of the psittacosis group of micro-organisms. J Infect Dis. 1959 Sep-Oct;105:129–160. doi: 10.1093/infdis/105.2.129. [DOI] [PubMed] [Google Scholar]
- Lépinay A., Orfila J., Anteunis A., Boutry J. M., Orme-Roselli L., Robineaux R. Etude en microscopie électronique du développement et de la morphologie de Chlamydia psittaci dans les macrophages de souris. Ann Inst Pasteur (Paris) 1970 Aug;119(2):222–231. [PubMed] [Google Scholar]
- MITSUI Y., FUJIMOTO M., KAJIMA M. DEVELOPMENT AND MORPHOLOGY OF TRACHOMA AGENT IN THE YOLK SAC CELL AS REVEALED BY ELECTRON MICROSCOPY. Virology. 1964 May;23:30–45. doi: 10.1016/s0042-6822(64)80005-2. [DOI] [PubMed] [Google Scholar]
- MORGAN J. F., MORTON H. J., PARKER R. C. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950 Jan;73(1):1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- McLIMANS W. F., DAVIS E. V., GLOVER F. L., RAKE G. W. The submerged culture of mammalian cells; the spinner culture. J Immunol. 1957 Nov;79(5):428–433. [PubMed] [Google Scholar]
- Moulder J. W. Glucose Metabolism of L Cells Before and After Infection with Chlamydia psittaci. J Bacteriol. 1970 Dec;104(3):1189–1196. doi: 10.1128/jb.104.3.1189-1196.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. The relation of the psittacosis group (Chlamydiae) to bacteria and viruses. Annu Rev Microbiol. 1966;20:107–130. doi: 10.1146/annurev.mi.20.100166.000543. [DOI] [PubMed] [Google Scholar]
- PHILIPSON L. THE EARLY INTERACTION OF ANIMAL VIRUSES AND CELLS. Prog Med Virol. 1963;5:43–78. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON J. D. The ultrastructure of cell membranes and their derivatives. Biochem Soc Symp. 1959;16:3–43. [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter E. M. Synthesis of nucleic acid and protein in L cells infected with the agent of meningopneumonitis. J Bacteriol. 1966 May;91(5):2069–2080. doi: 10.1128/jb.91.5.2069-2080.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Manire G. P. Effect of penicillin on the multiplication of meningopneumonitis organisms (Chlamydia psittaci). J Bacteriol. 1968 Oct;96(4):875–880. doi: 10.1128/jb.96.4.875-880.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Matsumoto A., Manire G. P., Higashi N. Electron microscopic observations on the structure of the envelopes of mature elementary bodies and developmental reticulate forms of Chlamydia psittaci. J Bacteriol. 1971 Jan;105(1):355–360. doi: 10.1128/jb.105.1.355-360.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I. Cell Wall Synthesis by Chlamydia psittaci Growing in L Cells. J Bacteriol. 1970 Dec;104(3):1176–1188. doi: 10.1128/jb.104.3.1176-1188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]