Abstract
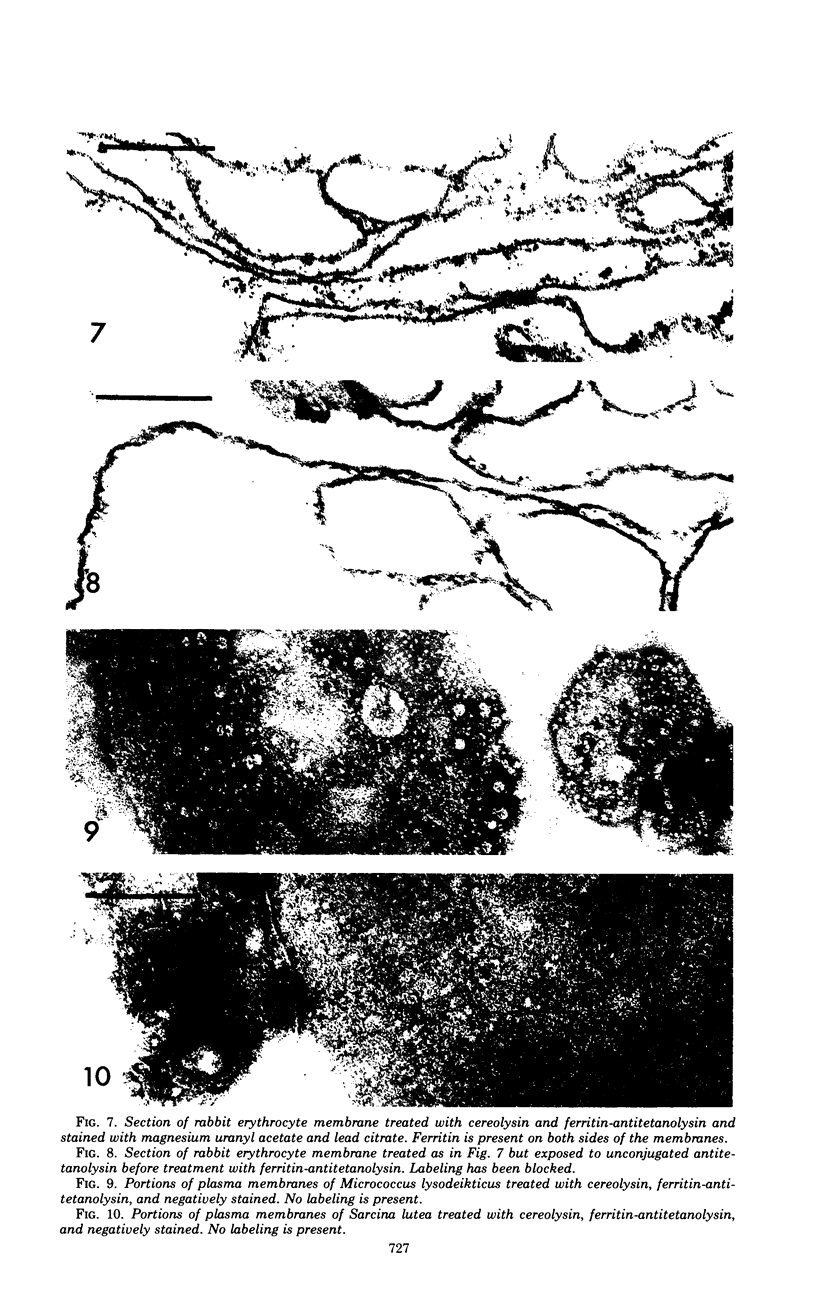
A method is described for the detection of cholesterol in membranes from erythrocytes, mycoplasmas, and bacterial cells by a ferritin-labeling technique. Membranes treated with cereolysin, a bacterial hemolysin which specifically binds to cholesterol, and then treated with ferritin-antitetanolysin, were specifically ferritin-labeled for cholesterol. A similar antigen-antibody system, streptolysin O-ferritin-antistreptolysin, was also used successfully with erythrocyte membranes. There was an uneven distribution of ferritin in erythrocyte membranes suggesting that the distribution of cholesterol may not be entirely random. Mycoplasma gallisepticum was intensely labeled, but Acholeplasma laidlawii with or without cholesterol in the membranes was not labeled, suggesting an unusual location for cholesterol in A. laidlawii membranes. As controls, two of three species of bacterial membranes lacking cholesterol were not ferritin-labeled.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHEIMER A. W., DAVIDSON M. LYSIS OF PLEUROPNEUMONIA-LIKE ORGANISMS BY STAPHYLOCOCCAL AND STREPTOCOCCAL TOXINS. Science. 1965 May 28;148(3674):1229–1231. doi: 10.1126/science.148.3674.1229. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W. Disruption of wall-less bacteria by streptococcal and staphylococcal toxins. J Bacteriol. 1966 May;91(5):1677–1680. doi: 10.1128/jb.91.5.1677-1680.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Grushoff P. Cereolysin: production, purification and partial characterization. J Gen Microbiol. 1967 Jan;46(1):143–150. doi: 10.1099/00221287-46-1-143. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dourmashkin R. R., Rosse W. F. Morphologic changes in the membranes of red blood cells undergoing hemolysis. Am J Med. 1966 Nov;41(5):699–710. doi: 10.1016/0002-9343(66)90031-3. [DOI] [PubMed] [Google Scholar]
- FRASCA J. M., PARKS V. R. A ROUTINE TECHNIQUE FOR DOUBLE-STAINING ULTRATHIN SECTIONS USING URANYL AND LEAD SALTS. J Cell Biol. 1965 Apr;25:157–161. doi: 10.1083/jcb.25.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P., Bernheimer A. W. Interaction of staphylococcal alpha-toxin with artificial and natural membranes. J Bacteriol. 1968 Mar;95(3):1153–1168. doi: 10.1128/jb.95.3.1153-1168.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPEN J. O., ARNOW P. M., SAFFERMAN R. S. Mechanism of protection by sterols against polyene antibiotics. J Bacteriol. 1960 Aug;80:200–206. doi: 10.1128/jb.80.2.200-206.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long R. A., Hruska F., Gesser H. D., Hsia J. C., Williams R. Membrane condensing effect of cholesterol and the role of its hydroxyl group. Biochem Biophys Res Commun. 1970 Oct 23;41(2):321–327. doi: 10.1016/0006-291x(70)90506-1. [DOI] [PubMed] [Google Scholar]
- Oberley T. D., Duncan J. L. Characteristics of streptolysin O action. Infect Immun. 1971 Dec;4(6):683–687. doi: 10.1128/iai.4.6.683-687.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. C., Kamat V. B., Chapman D. The interaction of cholesterol with the sterol free lipids of plasma membranes. Chem Phys Lipids. 1970 Aug;4(3):409–417. doi: 10.1016/0009-3084(70)90039-3. [DOI] [PubMed] [Google Scholar]
- Razin S., Cleverdon R. C. Carotenoids and cholesterol in membranes of Mycoplasma laidlawii. J Gen Microbiol. 1965 Dec;41(3):409–415. doi: 10.1099/00221287-41-3-409. [DOI] [PubMed] [Google Scholar]
- Remsen C. C., Watson S. W., Bernheimer A. W. Evidence for an ordered arrangement in erythrocyte membranes. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1297–1304. doi: 10.1016/0006-291x(70)90007-0. [DOI] [PubMed] [Google Scholar]
- Rottem S., Stein O., Razin S. Reassembly of Mycoplasma membranes disaggregated by detergents. Arch Biochem Biophys. 1968 Apr;125(1):46–56. doi: 10.1016/0003-9861(68)90637-1. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., MILLER F., BARRNETT R. J. ALDEHYDE FIXATION FOR MORPHOLOGICAL AND ENZYME HISTOCHEMICAL STUDIES WITH THE ELECTRON MICROSCOPE. J Histochem Cytochem. 1964 Feb;12:57–71. doi: 10.1177/12.2.57. [DOI] [PubMed] [Google Scholar]
- SRI RAM J., TAWDE S. S., PIERCE G. B., Jr, MIDGLEY A. R., Jr Preparation of antibody-ferritin conjugates for immunoelectron microscopy. J Cell Biol. 1963 Jun;17:673–675. doi: 10.1083/jcb.17.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGT A., KOPP R. LOSS OF SPECIFIC AGGLUTINATING ACTIVITY OF PURIFIED FERRITIN-CONJUGATED ANTIBODIES. Nature. 1964 Jun 27;202:1350–1351. doi: 10.1038/2021350a0. [DOI] [PubMed] [Google Scholar]
- WEBER M. M., KINSKY S. C. EFFECT OF CHOLESTEROL ON THE SENSITIVITY OF MYCOPLASMA LAIDLAWII TO THE POLYENE ANTIBIOTIC FILIPIN. J Bacteriol. 1965 Feb;89:306–312. doi: 10.1128/jb.89.2.306-312.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C. The isolation of protoplasts from Bacillus megaterium by controlled treatment with lysozyme. J Bacteriol. 1953 Dec;66(6):688–695. doi: 10.1128/jb.66.6.688-695.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]





