Abstract
Purpose
The main goal of this study was to provide the “proof-of-principle” that low-dose paclitaxel is able to change the tumor microenvironment and improve the outcome of intratumoral dendritic cell vaccine in a murine lung cancer model.
Experimental Design
We evaluated the antitumor potential and changes in the intratumoral milieu of a combination of low-dose chemotherapy and dendritic cell vaccine in the Lewis lung carcinoma model in vivo.
Results
The low-dose paclitaxel, which induced apoptosis in ~10% of tumor cells, was not toxic to bone marrow cells and dendritic cells and stimulated dendritic cell maturation and function in vitro. Although tumor cells inhibited dendritic cell differentiation in vitro, this immuno-suppressive effect was abrogated by the pretreatment of tumor cells with low-dose paclitaxel. Based on these data, we next tested whether pretreatment of tumor-bearing mice with low-dose paclitaxel in vivo would improve the antitumor potential of dendritic cell vaccine administered intratumorally. Significant inhibition of tumor growth in mice treated with low-dose paclitaxel plus intratumoral dendritic cell vaccine, associated with increased tumor infiltration by CD4+ and CD8+ T cells and elevated tumor-specific IFN-γ production by draining lymph node cells, was revealed. Using a novel intratumoral microdialysis technique and Luminex technology for collecting and characterizing soluble factors released within the tumor bed for several days in live freely moving animals, we showed that low-dose paclitaxel altered the cytokine network at the tumor site.
Conclusions
Our data indicate that low-dose chemotherapy before intratumoral delivery of dendritic cells might be associated with beneficial alterations of the intratumoral microenvironment and thus support antitumor immunity.
Lung cancer is the leading cause of cancer-related morbidity and mortality, resulting in more than 160,000 deaths per year in the United States and ~1.1 million worldwide. About 80% to 85% of lung cancer cases are non–small-cell lung cancer, and 65% of them have an advanced-stage disease at diagnosis (1, 2). Surgery, chemotherapy, and radiation prolong survival of patients with lung cancer, but the effect is typically temporary and decreased with advanced disease (2–5).
The underlying rationale for high-dose chemotherapy has been to use the most effective therapeutic agents at as high a dose possible to kill the most cancer cells (6). However, 45% of cancer patients eventually succumb to their disease (7). Although almost all patients respond initially to standard chemotherapy, and some patients with limited-stage disease are cured with the combination of chemotherapy and thoracic irradiation, the majority of patients will experience lethal relapse from chemotherapy-resistant micrometastatic disease, and this has resulted in poor long-term survival (8, 9).
These and other problems of traditional maximum-tolerated dose regimens of chemotherapy warranted the development of different approaches, such as metronomic chemotherapy, which involves administration of cytotoxic drugs at doses that are low enough to avoid myelosuppression and other dose-limiting side effects (10, 11). Furthermore, low-dose chemotherapy given on a more frequent basis might have the antiangiogenic effect. For instance, the low toxicity profile and efficacy of low-dose paclitaxel justified its usage in the group of poor-prognosis elderly patients with lung cancer and as a second-line treatment for advanced lung cancer (12, 13). However, in most low-dose studies conducted up to now, even when tumors have disappeared completely, relapses eventually occur and patients die (11).
The limited successes of conventional treatments for patients with lung cancer raised the possibility that a combination therapy may overcome tumor resistance to different treatments. For instance, rationale for combining chemotherapy with immunotherapeutic strategies has been widely discussed and a few reports provide evidence that combining cytotoxic agents with immunostimulation may result in low tumorigenicity and higher immunogenicity when compared with either therapy alone (14–18). However, those studies used pharmacologic, and thus potentially toxic and immunosuppressive, doses of chemotherapeutic drugs. Several recent studies comparing the efficacy of chemotherapy at high and low doses in combination with antiangiogenic drug documented the longest survival in animals that received low-dose chemotherapy and antiangiogenic treatment. This was associated with no side effects and a marked decrease in tumor size (19–21). However, there are no reports that address the therapeutic and immunomodulating potential of low-dose chemotherapeutic agents in a setting of combined chemotherapy with cancer vaccines.
The main goal of this study was to evaluate the therapeutic and primary immunologic effects of low-dose chemotherapy and its combination with intratumoral dendritic cell vaccine for the treatment of lung carcinoma. We tested whether low-dose chemotherapy may change cytokine network at the tumor site and support the antitumor potential of dendritic cell vaccine. We found that although tumor cells inhibited dendritic cell maturation and function in vitro, their immunosuppressive effect was significantly decreased by low-dose paclitaxel. Single administration of low-dose paclitaxel before intralesional dendritic cell vaccine in vivo resulted in significant inhibition of lung cancer growth and was associated with accumulation of CD4+ and CD8+ T cells in the tumor and of IFN-γ–producing tumor-specific T cells in the draining lymph nodes. These results were not achieved when either of the treatments was applied alone. Furthermore, a single injection of paclitaxel resulted in increased expression of monocyte chemoattractant protein 1 (MCP-1) at the tumor site, whereas the application of low-dose paclitaxel followed by dendritic cell vaccine induced an increase in intratumoral MCP-1 and IFN-inducible protein 10 (IP-10) chemokines and a decrease in intratumoral inter-leukin (IL)-1α release in vivo. Altogether, these data suggest that pretreatment of a tumor-bearing host with low-dose paclitaxel might alter the immunologic intratumoral microenvironment, including cytokine network and immunosuppressive activity of tumor cells, and thus be beneficial for the therapeutic potential of the subsequent dendritic cell vaccine.
Materials and Methods
Animals
Male C57BL/6 mice, 6 to 8 weeks old, were obtained from Taconic and housed in transparent plastic cages under pathogen-free conditions, controlled temperature and humidity, and a 12-h light-dark cycle, with sterile food and water ad libitum.
Cell cultures, reagents, and cytokines
Murine 3LL and B16 tumor cells (American Type Culture Collection) and bone marrow cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 100 units/100 μg/mL penicillin/streptomycin, 1 mmol/L sodium pyruvate, 2 mmol/L L-glutamine, and 0.1 mmol/L nonessential amino acids at 37°C in 5% CO2. Tissue culture reagents were from Sigma Chemical or Life Technologies.
The generation of murine bone marrow–derived dendritic cells was done as described (22). Briefly, mouse hematopoietic progenitors were isolated from the bone marrow and depleted of erythrocytes, B and T lymphocytes, and adherent cells. Then, cells were resuspended (0.5 × 106/mL) in culture medium containing granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-4 and cultured for 6 to 7 days. Murine recombinant GM-CSF and IL-4 were from PeproTech and used at 1,000 units/mL to direct dendritic cell differentiation from bone marrow hematopoietic precursor cells.
The cytotoxic agents paclitaxel, cisplatin, and taxotere were from Mayne Pharma. 3LL cells (0.2 × 106/mL) were pretreated with chemotherapeutic agents for 24 and 48 h at a range of concentrations: 0, 10, 50, 100, 200, and 400 nmol/L for paclitaxel; 0, 500, 1,000, 2,000, 4,000, and 8,000 nmol/L for cisplatin; and 0, 1, 10, 25, 50, and 100 nmol/L for taxotere.
Annexin V binding assay
To evaluate apoptosis in 3LL cells, the Annexin V binding assay was done. Tumor cells were double stained with FITC-conjugated Annexin V (PharMingen) and propidium iodide (10 μg/mL; Sigma). Cells undergoing early apoptosis were determined as the percentage of Annexin V–positive propidium iodide–negative cells by FACScan with Cell Quest 1.0 software package (Becton Dickinson).
Flow cytometry
To determine dendritic cell phenotype, cells were washed in fluorescence-activated cell sorting medium (HBSS containing 0.1% bovine serum albumin and 0.1% NaN3) and stained with appropriately diluted antibodies directly conjugated with FITC or phycoerythrin. The following antibodies were used: FITC-labeled antimouse MHC class II, CD86, CD80, CD40, and phycoerythrin-labeled CD11c (PharMingen).
Chemotaxis
Spontaneous and chemokine-induced dendritic cell migration was evaluated using 48-well Transwell system (5 μm pore size, Corning Costar). Recombinant murine dendritic cell chemokines, macrophage inflammatory protein (MIP)-1α (20 ng/mL) or MIP-βh (1 μg/mL; PeproTech), were diluted in RPMI 1640 containing 1% fetal bovine serum (assay medium) and placed (600 AL) in the lower chamber of transwell plates. Assay medium was used to measure spontaneous dendritic cell migration. Dendritic cells (0.5 × 106/mL) were added to transwell inserts in 100 AL and incubated for 4 h at 37°C, 5% CO2. After incubation, cells that trans-migrated through the membrane were collected and acquired on FACScan for 1 min. Dendritic cell migration was calculated as the percentage of transmigrated cells from the control values (spontaneous dendritic cell migration).
Experimental design in vitro
Bone marrow–derived dendritic cell precursors (0.5 × 106/mL) were cocultured with medium or 3LL cells (0.5 × 106/mL) that were untreated or treated with indicated concentrations of chemotherapeutic agents. Dendritic cells and tumor cells were separated by the membrane of 0.4-μm pore size. Tumor cells were added to dendritic cell cultures on day 1 for 48 h. Dendritic cells were harvested on day 7 for the phenotype and function analysis.
Experimental design in vivo
3LL cells (0.5 × 106 in 100 AL of saline) were inoculated s.c. into the back of C57BL/6 mice. Tumors were allowed to develop for 9 days and mice were then randomly divided into four groups: (a) controls, treated with saline; (b) treated with a single i.p. injection of 2 mg/kg paclitaxel; (c) treated with intratumoral injections of dendritic cells (2 × 106 per mouse); and (d) i.p. injection of 2 mg/kg paclitaxel followed by intratumoral dendritic cell injections. In all experiments, the dose of paclitaxel (2 mg/kg) was 1:10 of proven optimal therapeutic dose (23–25) and was not toxic, as assessed in a series of pilot studies. The tumor size was measured thrice a week with calipers and recorded as the tumor area (in square millimeter). All studies consisted of five mice per group. Experiments were independently repeated thrice.
Immunohistochemical analysis
Tumor tissue samples from lung carcinoma–bearing mice were harvested into the optimum cutting temperature compound (Tissue-Tek) and stored at −80°C. Cryostat sections (5 μm) were fixed in cold acetone for 15 min. Slides were washed with PBS and incubated for 1 h at room temperature with the appropriate dilutions of anti-CD4 or anti-CD8 antibodies (PharMingen). Biotinylated mouse anti-rat IgG (Jackson Immuno Research Laboratories) was used as secondary antibody and was applied for 45 min. After developing with the peroxidase chromogen kit (3-amino-9-ethylcarbazol; Biomega) for 8 min, counterstaining was done with hematoxylin.
IFN-γ assay
Lymph node cells (1 × 106/mL) isolated from treated and control mice were added to 24-well plates and stimulated with irradiated (300 Gy) 3LL lung carcinoma or B16 melanoma cells (0.2 × 106/mL). Irradiated B16 cells were used as an irrelevant tumor cell line to verify that IFN-γ–producing T cells in 3LL-bearing and treated mice are specific for 3LL tumor cells. Supernatants were collected 48 h later, centrifuged (2,000 × g, 15 min), and stored at −20°C. Supernatants from nonstimulated lymph node cells were used to measure spontaneous cytokine production. Levels of IFN-γ were assessed by ELISA (R&D Systems).
Analysis of cytokines in intratumoral interstitial fluid
We have recently developed a new technique that allows determining the levels of a number of soluble biomarkers released dynamically within the tumor microenvironment in live freely moving animals (26). The method uses intratumoral insertion of a specially designed two-channel microdialysis probe connected to the micropump and specially designed freely moving collection devise. A 10-mm-long CMA/20 microdialysis probe (CMA Microdialysis) is implanted and fixed inside of a tumor mass or control s.c. tissues, and extracellular interstitial fluid is collected via the micropump-regulated circulation (16 μL/h) of the buffer [4% (w/v) dextran-70 in PBS]. The use of dextran-70 in the circulating buffer helps to counterbalance the high osmolarity of tissue interstitial fluid to prevent probe volume loss and does not interfere with biomarker detection. The outer cannula of the probe is made from nanoporous polyethersulfone and has a nominal molecular weight cutoff of 100,000 Da (26, 27). Multiple samples (~50 μL) were collected from live freely moving animals housed in specially designed chambers/cells and the levels of cytokines, chemokines, and growth factors were detected simultaneously in each sample by the Luminex-based Multiplexed assay. Twenty-plex assays for IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40/p70, IL-13, IL-17, IL-1α, IL-1β. IFN-γ, GM-CSF, IP-10, MIP-1α, MCP-1, Kupffer cells, fibroblast growth factor-β, monokine induced by IFN-γ, tumor necrosis factor-α, and vascular endothelial growth factor were purchased from Biosource International. The Lab MAP assay was done according to the manufacturer’s protocol.
Statistical analysis
For a single comparison of two groups, the Student t test was used after evaluation for normality. If data distribution was not normal, a Mann-Whitney rank sum test was done. For the comparison of multiple groups, one-way or two-way ANOVA was applied. For all statistical analyses, P = 0.05 was considered to be significant. Data are presented as mean ± SE.
Results
Analysis of apoptosis in 3LL cells induced by the cytotoxic agents in vitro
The first goal was to evaluate the sensitivity of 3LL cells to various concentrations of chemotherapeutic agents in vitro. Tumor cells were treated with a range of concentrations of paclitaxel (0–400 nmol/L), cisplatin (0–8,000 nmol/L), or Taxotere (0–100 nmol/L) and harvested for assessing the levels of apoptosis 24 and 48 h later. To express the results related to the apoptosis rates in 3LL cells treated with the chemotherapeutic agents, we subtracted the percentage of spontaneous apoptotic in nontreated tumor cell cultures from chemotherapy-induced apoptosis values. Concentrations of cytotoxic agents that caused <10% to 15% cell death above the background level within 48 h were chosen for the subsequent experiments. For instance, Annexin V binding assay revealed that apoptotic rate determined as the percentage of Annexin V–positive propidium iodide–negative cells treated with paclitaxel at the concentration 50 nmol/L was 9.8 ± 1.3% and 12.2 ± 1.6% at 24 and 48 h, respectively (Fig. 1). Importantly, this dose of paclitaxel was not toxic for murine bone marrow cells: the percentage of cells that underwent early and late stages of apoptosis after 48 h was 11.8% (7.7% + 4.1%), which is comparable with 11.0% (7.2% + 3.8%) for control nontreated bone marrow cells. Higher doses of paclitaxel were used as positive controls and proved its dose-dependent toxic effect on bone marrow cells.
Fig. 1.
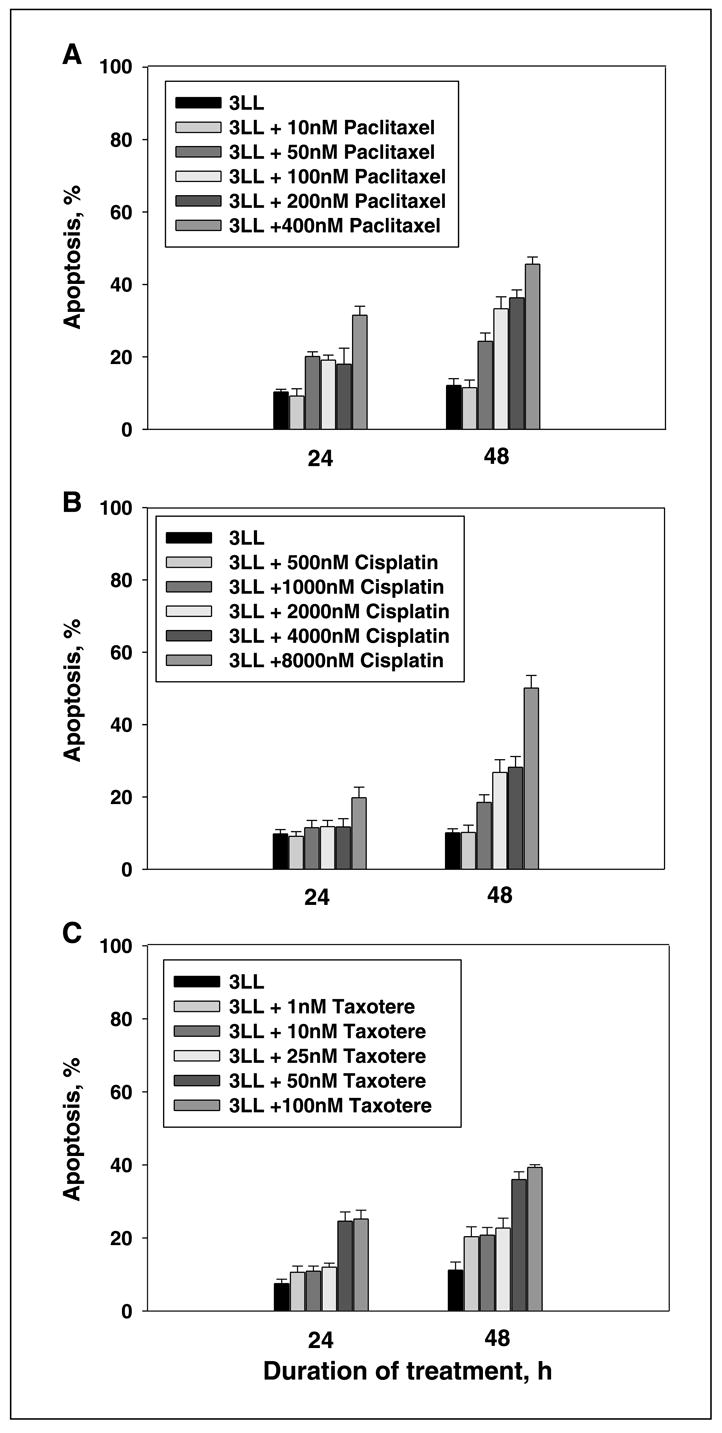
Chemotherapeutic agents induced apoptosis of 3LL cells. 3LL tumor cells were treated with various concentrations of paclitaxel (A), cisplatin (B), and Taxotere (C) in vitro. Apoptosis of tumor cells harvested after 24- and 48-h coincubation with chemotherapeutic agents was assessed byAnnexinV/propidium iodide staining and FACScan analysis. Columns, mean percentage of apoptotic (AnnexinV – positive and propidium iodide – negative) tumor cells from three independent experiments; bars, SE.
Our results also showed that after 48 h of the treatment, taxotere at the range of concentrations between 1 and 25 nmol/L caused 10% to 15% apoptosis of tumor cells (Fig. 1). At the same time, taxotere even at 1 nmol/L induced apoptosis of bone marrow cells up to 30% when compared with control cultures. Because bone marrow cells were sensitive to 1 nmol/L taxotere, and 3LL cells showed high resistance to cisplatin (Fig. 1), only paclitaxel was chosen for further experiments.
Inhibition of dendritic cell maturation and function by 3LL cells in vitro was reversed by pretreatment with low-dose paclitaxel
Murine bone marrow–derived cultures were analyzed at day 7 for the phenotypic characteristics of generated dendritic cells. FACScan analysis revealed that control dendritic cell cultures were repeatedly ~15% CD11c+CD40+, ~20% CD11c+CD86+, and ~10% CD11c+Iab+. The addition of 3LL cells to dendritic cell cultures resulted in significant inhibition of dendritic cell maturation as assessed by the expression of CD40, CD86, and MHC class II molecules on dendritic cells (Fig. 2). However, the immunosuppressive effect of 3LL cells was significantly decreased with pretreatment of tumor cells with 50 nmol/L paclitaxel for 48 h. For instance, in the presence of 3LL cells, the percentage of CD11C+CD40+ dendritic cells decreased from 16.5% (control) to 9.2% (P < 0.05), whereas pretreatment of tumor cells with low-dose paclitaxel recovered the level of CD11C+CD40+ dendritic cells to 29% (Fig. 2). The same pattern was observed for the expression of CD86 and Iab on dendritic cells, but the effect was less dramatic when compared with CD40 expression. Importantly, only live tumor cells were used in these studies, ensuring an appropriate comparison. Furthermore, paclitaxel alone at 50 nmol/L had an immunostimulatory effect on dendritic cell maturation and up-regulated CD40 expression on dendritic cells up to 40% (P < 0.05; Fig. 2). In the presence of paclitaxel, the expression of CD86 and Iab molecules on dendritic cells increased from 18.5% to 44.5% and from 10.9% to 27.3%, respectively (P < 0.05).
Fig. 2.

Paclitaxel blocked the ability of tumor cells to inhibit maturation of dendritic cells. Murine bone marrow dendritic cell (DC) precursors were cultured with medium, 50 nmol/L paclitaxel, or 3LL cells pretreated or non-pretreated with paclitaxel. Tumor cells were added to dendritic cell cultures at day1for 48 h. Dendritic cells were collected 7 d later and analyzed by flow cytometry. Typical data from a representative experiment (n = 3). X-axis and Y-axis, relative fluorescence intensity.
Next, to confirm the FACScan data, we showed that the chemotactic potential and motility of dendritic cells cocultured with 3LL cells were also markedly decreased, as shown by the analysis of dendritic cell migration toward chemokines MIP-1α and MIP-3β. As shown in Fig. 3, up to 75% and 55% reductions of MIP-1α– and MIP-3β–induced dendritic cell migration were observed in dendritic cells treated with tumor cells when compared with the migration of dendritic cells from the control cultures (P < 0.05). However, tumor-induced inhibition of dendritic cell motility was prevented if tumor cells were pretreated with low-dose paclitaxel (P < 0.05). Again, only live tumor cells were used in these experiments to ensure appropriate comparison.
Fig. 3.
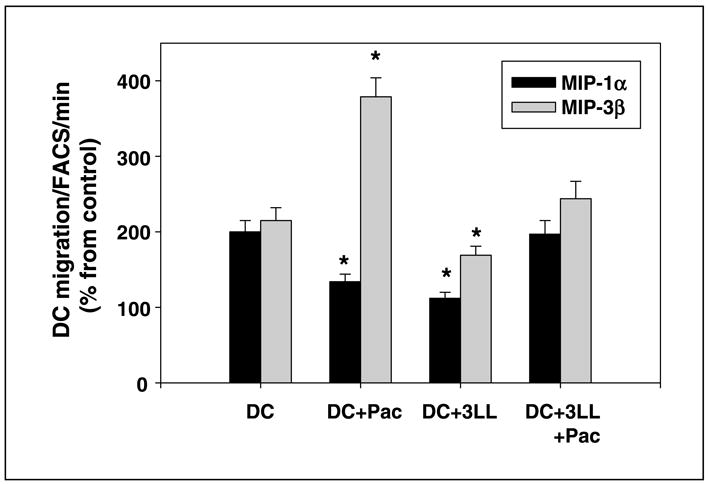
3LL tumor cells inhibited chemokine-induced migration of dendritic cells, whereas pretreatment of tumor cells with low-dose paclitaxel prevented this inhibition. The migratory ability of control cells and dendritic cells treated with tumor cells, paclitaxel-pretreated tumor cells, and paclitaxel was determined. Murine dendritic cells were generated from bone marrow precursors and their chemotaxis was assessed in the 5-μmTranswell system in a 4-h migration assay. The numbers of transmigrated dendritic cells were determined by a 60-s FACScan analysis of triplicate samples. Columns, mean of triplicate measurements from three independent experiments; bars, SE. The values are presented as the percentage from spontaneous migration. P < 0.05, versus ‘‘control’’ values.
Thus, in addition to the immunostimulatory effect of low-dose paclitaxel on dendritic cell differentiation and function, we showed that whereas 3LL cells inhibited dendritic cell maturation and motility, paclitaxel could reverse these effects. These in vitro results served as basic support for therapeutic intratumoral administration of dendritic cells in 3LL-bearing mice after pretreatment with low-dose paclitaxel in our in vivo studies.
Combination of chemotherapy and dendritic cell vaccine caused significant inhibition of lung cancer growth in vivo and augmented T-cell infiltration of the tumor
Next, we investigated the therapeutic effect of a combination of low-dose chemotherapy and dendritic cell vaccine in vivo using s.c. Lewis lung carcinoma model. Animals were divided into four groups: (a) nontreated; (b) treated with i.p. injection of paclitaxel on day 9 (2 mg/kg, 1:10 of an optimal dose); (c) treated with intratumoral injections of immature dendritic cells on days 11 and 13; and (d) i.p. injection of paclitaxel followed by intratumoral dendritic cell injections (Fig. 4A). To avoid the limitations with the isolation of unknown tumor antigens in 3LL model, we injected dendritic cells intralesionally. Intratumoral delivery of dendritic cell vaccine in the absence of defined tumor antigens has a number of advantages compared with the conventional dendritic cell administration (28, 29). As shown in Fig. 4A, there was a gradual increase in tumor size in tumor-bearing mice treated with saline. After day 13, reduced tumor growth was observed in groups 2, 3, and 4 when compared with the nontreated controls (Fig. 4A; P < 0.05). However, the combination of chemotherapy with dendritic cell vaccine resulted in the strongest decrease in 3LL tumor growth when compared with either therapy alone or control groups. For example, on day 21 after 3LL cell injection, the mean tumor sizes in groups 1, 2, 3, and 4 were 458.2 ± 44.8, 303.6 ± 9.3, 342.8 ± 25.0, and 191.8 ± 33.8 mm2 (P < 0.05, group 4 versus other three groups), respectively. Next, we assessed how the inhibition of tumor growth was correlated with lymphocyte attraction and homing at the tumor site. Tumors from all experimental groups were removed on day 21 and examined for CD4 and CD8 expression. There was a marked increase in CD4 and CD8 T-cell infiltration observed in the group of animals that received combination therapy, allowing us to speculate that inhibition of tumor growth in these mice might be directly or indirectly related to the development of antitumor immunity (Fig. 4B).
Fig. 4.
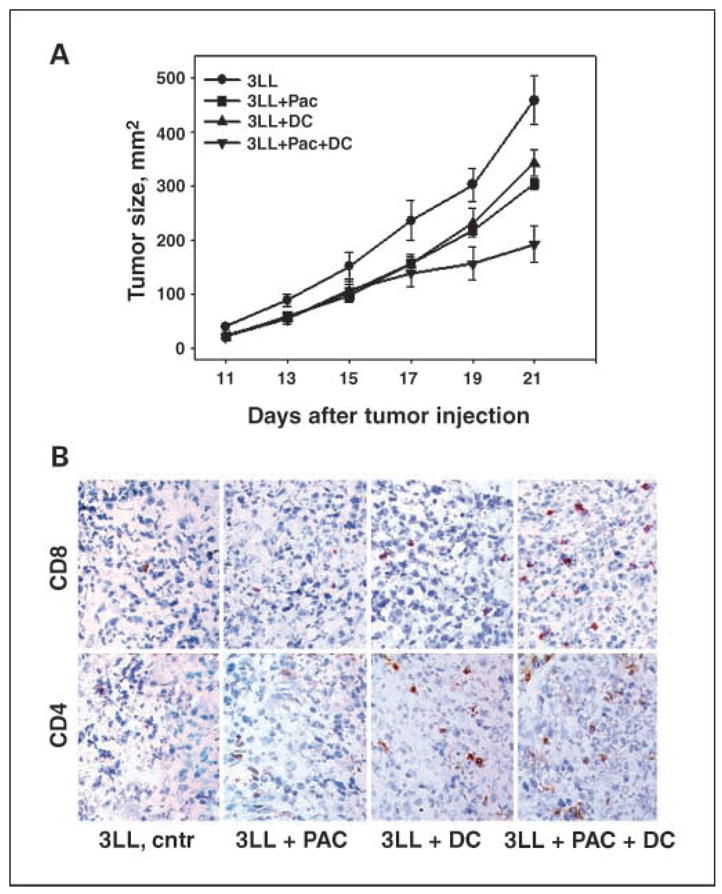
A, antitumor potential of combination chemo-immunotherapy was stronger than either therapy alone in the murine lung cancer model. C57BL/6 mice were inoculated s.c. with 0.5 × 106 3LL cells, and the treatment protocol was initiated 9 d later, when an s.c. mass became visible (~3 × 3 mm2). Experimental groups consisted of five mice per group. Experiments were independently repeated thrice. Points, mean tumor size (mm2) from three independent experiments; bars, SE. *, P < 0.05, two-wayANOVA for 3LL + Pac + dendritic cell group versus other treatments. B, combination therapy augmentedT-cell infiltration of the tumor. Twenty-one days after tumor cell injection, control and treated mice were sacrificed and the tumors were sectioned and stained for CD4 and CD8 expression. Representative sections are shown from untreated control and paclitaxel-, dendritic cell vaccine –, and combination therapy – treated mice. Magnification, ×40.
Combination chemo-immunotherapy of lung cancer resulted in increased IFN-γ production by draining lymph node cells
To evaluate potential generation of tumor-specific cytotoxic lymphocytes, IFN-γ production was assessed in cultures of regional lymph node cells isolated from saline-, paclitaxel-, dendritic cell–, and paclitaxel/dendritic cell–treated mice. IFN-γ was measured in the supernatants of lymph node cells, which were (a) nonstimulated, (b) stimulated with irradiated 3LL lung cancer, and (c) stimulated with irradiated irrelevant B16 melanoma cells. Figure 5 shows that lymph node cells isolated from mice treated with low-dose paclitaxel plus dendritic cell vaccine released significantly higher levels of IFN-γ on stimulation with irradiated 3LL cells than lymphocytes from other groups (P < 0.05): they produced 288.4 ± 3.4 pg/mL IFN-γ, whereas IFN-γ production by cells isolated from control, paclitaxel-, and dendritic cell–treated groups was 0.5 ± 0.01, 3.2 ± 0.01, and 156 ± 14.5 pg/mL, respectively. IFN-γ was secreted in minute amounts in response to the irrelevant syngeneic murine melanoma cells and was not secreted spontaneously, indicating that the observed IFN-γ response was immunologically specific to 3LL tumor cells (Fig. 5).
Fig. 5.

Effect of low-dose paclitaxel and dendritic cell vaccine on IFN-γ secretion by draining lymph node cells. Draining lymph node cells were isolated on day 21 after tumor injection from each experimental group. The cells (1 × 106/mL) were unstimulated or stimulated with irradiated 3LL or B16 cells (0.2 × 106) and incubated in 24-well tissue culture plates for 48 h. The supernatants were collected and evaluated by ELISA for the production of IFN-γ. Columns, mean of triplicate samples (n = 3); bars, SE. P < 0.05, versus medium.
Thus, the clinical response achieved with the combination of low-dose paclitaxel and dendritic cell vaccine was associated with increased tumor-specific IFN-γ production by draining lymph node lymphocytes. Together with increased tumor infiltration by CD4+ and CD8+ T cells, these data indicate that intratumoral delivery of dendritic cells after low-dose chemotherapy might be associated with up-regulation of antitumor immunity and beneficial alteration of intratumoral microenvironment.
Systemic chemotherapy and local dendritic cell vaccine modulated cytokine network at the tumor site
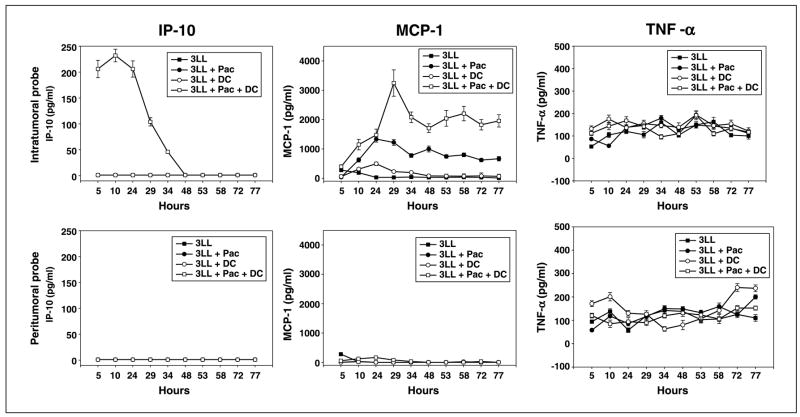
In the next set of experiments using implantation of microdialysis probes and MAPX Luminex technology for protein detection, we assessed alterations of intratumoral cytokine network in mice treated with chemotherapeutic, immunotherapeutic, or chemoimmunotherapeutic means. Microdialysis probes were implanted intratumorally or in close nonmalignant tissue at day 21 after tumor injection. Figure 6 shows the results of a representative experiment (n = 2) when intratumoral cytokines were assessed during a 3-day period in the established 3LL tumors after the low-dose paclitaxel, dendritic cell vaccine, or combination therapy. Secretion of cytokines in tumor-free cutaneous tissue served as a control. Nontreated tumors served as an additional control. Systemic administration of low-dose paclitaxel was accompanied by up to 20- to 30-fold up-regulation of MCP-1 level in the tumor mass, whereas the level of IL-1α was decreased almost twice. The combination therapy was associated with an additional increase in intratumoral MCP-1 and IP-10 and a decrease in intratumoral IL-1α. As can be seen in Fig. 6, after combination therapy, the increase in intratumoral MCP-1 and IP-10 release continued up to 77 and 48 h, respectively. For instance, 72 h after implantation of micro-dialysis probe, the levels of intratumoral MCP-1 were 29.9 ± 2.2, 620.5 ± 55.0, 80.3 ± 6.8, and 1,823.4 ± 178.2 pg/mL in untreated tumor-bearing mice and lung cancer–bearing mice treated with low-dose paclitaxel, intratumoral dendritic cell vaccine, or combination therapy, respectively. No effects of therapy on cytokine production in control nonmalignant areas were determined. The therapy did not change tumor necrosis factor-α, fibroblast growth factor-β, IL-4, IL-6, IL-10, and MIP-1α production. The release of proinflammatory mediators MCP-1 and IP-10 and the decrease in intratumoral IL-1α in the group of animals that received low-dose paclitaxel and dendritic cell vaccine were correlated with significant inhibition of tumor growth. Thus, these data suggest that chemo-immunotherapy might cause specific alterations in the intratumoral microenvironment, which can be beneficial for up-regulating antitumor responses and inhibition of tumor growth.
Fig. 6.
Alterations of intratumoral cytokine network in 3LL-bearing mice treated with chemo-immunotherapy in vivo. C57BL/6 mice received 0.5 × 106 lung carcinoma cells s.c. and, 9 d later, the treatment protocol was initiated. CMA/20 microdialysis probes were implanted on day 21αnd aliquots (~50 AL/3–4 h) of intratumoral and control interstitial fluid were collected for 3 d in live freely moving mice. Cytokines, chemokines, and growth factors were assessed in each sample by a multiplexed analysis. 3LL, Lewis lung carcinoma; Pac, paclitaxel: DC, dendritic cell vaccine. Results of three representative cytokines from 20 tested. Two independent experiments provided similar data.
Discussion
Recent studies have shown the benefits of combining chemotherapy with immunotherapy for the treatment of cancer. Although it is common to consider conventional chemotherapy as an immunosuppressive modality with a negative cumulative effect on the function of nonmalignant tissues and the immune system (25, 30, 31), there are no data characterizing the potential immunomodulating properties of low-dose chemotherapy. We initiated incorporation of low-dose chemotherapy into the vaccine therapy protocols, wherein the immunotherapeutic component should target both primary and metastatic tumors and hopefully induce specific antitumor immunologic memory. We thus intended to “prepare” tumor-bearing hosts for a better response to dendritic cell vaccine by using low-dose chemotherapy. We speculated that tumor cell modification by low-dose chemotherapeutic agents might change the local tumor microenvironment in a way that increases the source of tumor antigens for immune cells, up-regulates their functional activity, and thus accelerates and sustains antitumor immunity and overall therapeutic effect. Our data support this hypothesis. We showed that the combination of low-dose paclitaxel followed by intratumoral injection of dendritic cell vaccine was much more efficient for the treatment of murine lung carcinoma than either dendritic cell vaccine or paclitaxel alone in terms of the inhibition of tumor growth, CD4+ and CD8+ T-cell infiltration of the tumor, and induction of a tumor-specific immune response in regional lymph nodes. In addition, we showed that the chosen concentration of paclitaxel was not toxic to bone marrow cells and even stimulated dendritic cell maturation and function. Furthermore, tumor-induced inhibition of dendritic cell maturation and motility was reversed by the pretreatment of tumor cells with low-dose paclitaxel. The fact that paclitaxel stimulates dendritic cells and protects them from tumor-mediated inhibition supports the design of intratumoral administration of dendritic cells after pretreatment of tumor-bearing mice with low-dose chemotherapy.
Recently, Ramanathapuram et al., using a α-tocopheryl succinate (a nontoxic vitamin E analogue) in combination with dendritic cell vaccine to treat murine tumors, showed that the combination therapy was efficient in inhibiting tumor growth when compared with α-tocopheryl succinate alone. The clinical effect of combination therapy correlated with increased production of IFN-γ by spleenic and lymph node lymphocytes (32). In addition, α-tocopheryl succinate was not toxic to normal cells but selectively killed tumor cells (33–35). These and other data are in agreement with our findings suggesting that supplementing the chemotherapy with dendritic cell vaccine might be beneficial for cancer treatment (4, 14, 17).
It is now well accepted that tumors exist as a tissue collection of many interdependent cell types, which results in the production of a variety of cytokines and other factors that directly or indirectly orchestrate a number of important cellular processes that eventually lead to tumor eradication or, in contrast, support tumor survival (26). Tumor cells, together with stromal and tumor-infiltrating cells, produce a variety of pro-tumorigenic cytokines with paracrine and autocrine functions. These factors might up-regulate proliferation of tumor cells, stimulate neovascularization, or suppress tumor cell recognition by the immune system. Among them are IL-1, IL-6, IL-8, IL-10, IL-13, tumor necrosis factor-α, transforming growth factor-β, vascular endothelial growth factor, and others (36–38). For example, IL-10 and IL-13 may serve as tumor growth factors and suppressors of immune responses. Chemo-kine (C-X-C motif) ligand 8 plays a role in mediating many human carcinoma–derived angiogenesis and tumorigenesis (39). However, cytokines may play different roles in tumor development and their expression might cause pleiotropic effects, ranging from accelerating spreading and invasion of tumor cells to inducing antitumor immune responses. This depends on the stage of tumor growth, specific tumor types, or cytokine concentrations. For instance, whereas tumor necrosis factor-α provides pro-oncogenic activities in many solid tumors (40), glioma-bearing mice deficient in tumor necrosis factor develop larger tumors and have reduced survival compared with their wild-type controls (41).
The role of many intratumoral cytokines in stimulating tumor growth and spreading, as well as in the induction of antitumor immune response in vivo, is currently unclear. In spite of a high translational potential of regulating intratumoral cytokine network and signaling, there are no studies exploring the mechanisms of alteration of cytokine expression at the tumor site during tumor progression or antitumor therapy. Evaluation of cytokines in the tumor is based on several methodologic approaches. Historically, immunohistochemical strategies were the most widely used in spite of very limited quantitative applicability. Isolation of tumor cells from fresh tumor implants by enzymatic digestion provides a possibility for a quantitative determination of cytokine production by ELISA or flow cytometry. Laser capture microdissection might extract small amounts of tumor cells from paraffin-embedded slides, which can be used in gene expression or proteomic assays (42, 43). However, none of these techniques has a capability to study intratumoral factors in dynamic tumor response to therapy. In the present study, for the first time, we combined intratumoral microdialysis and multiplexed protein arraying to profile the cytokine network in vivo in a complex process of tumor progression exemplified by chemo-immunotherapy-induced inhibition of tumor growth. The results show that the application of low-dose paclitaxel with intratumoral dendritic cell vaccine was associated with an increase in intratumoral MCP-1 and IP-10 chemokines and a decrease in IL-1α, which correlated with inhibition of tumor growth, increased CD4 and CD8 T-cell infiltration, and increased tumor-specific IFN-γ production by draining lymph node cells. The antitumor potential associated with MCP-1 and IP-10 chemokines and the pro-tumoral properties of IL-1α were described earlier. For example, low-level MCP-1 secretion with modest macrophage infiltration resulted in tumor formation, whereas a high secretion was associated with massive macrophage infiltration into the tumor mass, leading to its destruction within a few days (44). IP-10 is a known inhibitor of tumor angiogenesis and a powerful chemoattractant for monocytes and T lymphocytes (45, 46). IL-1α, which is abundant at tumor sites (47), might induce proliferation, adhesion, and migration of tumor cells (48, 49). Thus, it is now apparent that the tumor microenvironment, serving as an interaction arena between different cell types, serves also as an interaction ground between different intratumoral factors.
Our data suggest that low-dose chemotherapeutic agents and dendritic cell vaccine might function in rationally selected combinations to achieve better tumor control associated with decreased toxicity and, most importantly, more effective therapeutic potential. We are currently extending these findings using different tumor cell lines with different classes of chemotherapeutic agents.
Acknowledgments
NIH grant 2RO1CA084270 (M.R. Shurin).
References
- 1.O’Mahony D, Kummar S, Gutierrez ME. Non-small-cell lung cancer vaccine therapy: a concise review. J Clin Oncol. 2005;23:9022–8. doi: 10.1200/JCO.2005.02.3101. [DOI] [PubMed] [Google Scholar]
- 2.Stinchcombe TE, Lee CB, Socinski MA. Current approaches to advanced-stage non-small-cell lung cancer: first-line therapy in patients with a good functional status. Clin Lung Cancer. 2006;7(Suppl 4):S111–7. doi: 10.3816/clc.2006.s.002. [DOI] [PubMed] [Google Scholar]
- 3.Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;350:379–92. doi: 10.1056/NEJMra035536. [DOI] [PubMed] [Google Scholar]
- 4.Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12:878–87. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 5.Ruttinger D, Winter H, van den Engel NK, et al. Immunotherapy of lung cancer: an update. Onkologie. 2006;29:33–8. doi: 10.1159/000090341. [DOI] [PubMed] [Google Scholar]
- 6.Fong KM, Yang IA, Zimmerman PV, Bowman RV. Cochrane systematic reviews of treatments for lung cancer. Respir Med. 2005;99:1071–8. doi: 10.1016/j.rmed.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Pirozynski M. 100 years of lung cancer. Respir Med. 2006;100:2073–84. doi: 10.1016/j.rmed.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 9.Schiller JH. Current standards of care in small-cell and non-small-cell lung cancer. Oncology. 2001;61(Suppl1):3–13. doi: 10.1159/000055386. [DOI] [PubMed] [Google Scholar]
- 10.Shaked Y, Emmenegger U, Francia G, et al. Low-dose metronomic combined with intermittent bolus-dose cyclophosphamide is an effective long-term chemotherapy treatment strategy. Cancer Res. 2005;65:7045–51. doi: 10.1158/0008-5472.CAN-05-0765. [DOI] [PubMed] [Google Scholar]
- 11.Shaked Y, Emmenegger U, Man S, et al. Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005;106:3058–61. doi: 10.1182/blood-2005-04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correale P, Cerretani D, Remondo C, et al. A novel metronomicchemotherapyregimenofweeklyplatinum and daily oral etoposide inhigh-risk non-small cell lung cancerpatients. Oncol Rep. 2006;16:133–40. doi: 10.3892/or.16.1.133. [DOI] [PubMed] [Google Scholar]
- 13.Ramalingam S, Belani CP. Taxanes for advanced non-small cell lung cancer. Expert Opin Pharmacother. 2002;3:1693–709. doi: 10.1517/14656566.3.12.1693. [DOI] [PubMed] [Google Scholar]
- 14.Lake RA, Robinson BW. Immunotherapy and chemotherapy– a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 15.Schultz ES, Schuler G. [Malignant melanoma. Diagnosis and therapy] Hno. 2005;53:928–39. doi: 10.1007/s00106-005-1326-y. [DOI] [PubMed] [Google Scholar]
- 16.van der Most RG, Currie A, Robinson BW, Lake RA. Cranking the immunologic engine with chemotherapy: using context to drive tumor antigen cross-presentation towards useful antitumor immunity. Cancer Res. 2006;66:601–4. doi: 10.1158/0008-5472.CAN-05-2967. [DOI] [PubMed] [Google Scholar]
- 17.Nowak AK, Lake RA, Robinson BW. Combined chemo-immunotherapy of solid tumours: improving vaccines? Adv Drug Deliv Rev. 2006;58:975–90. doi: 10.1016/j.addr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Koppold B, Sauer G, Buening H, et al. Chemotherapeutic agents enhance AAV2-mediated gene transfer into breast cancer cells promoting CD40 ligand-based immunotherapy. J Cancer Res Clin Oncol. 2006;132:787–94. doi: 10.1007/s00432-006-0127-3. [DOI] [PubMed] [Google Scholar]
- 19.Bello L, Carrabba G, Giussani C, et al. Low-dose chemotherapy combined with an antiangiogenic drug reduces human glioma growth in vivo. Cancer Res. 2001;61:7501–6. [PubMed] [Google Scholar]
- 20.Tan GH, Tian L, Wei YQ, et al. Combination of low-dose cisplatin and recombinant xenogeneic endoglin as a vaccine induces synergistic antitumor activities. Int J Cancer. 2004;112:701–6. doi: 10.1002/ijc.20449. [DOI] [PubMed] [Google Scholar]
- 21.Hou JM, Liu JY, Yang L, et al. Combination of low-dose gemcitabine and recombinant quail vascular endothelial growth factor receptor-2 as a vaccine induces synergistic antitumor activities. Oncology. 2005;69:81–7. doi: 10.1159/000087303. [DOI] [PubMed] [Google Scholar]
- 22.Shurin MR, Pandharipande PP, Zorina TD, et al. FLT3 ligand induces the generation of functionally active dendritic cells in mice. Cell Immunol. 1997;179:174–84. doi: 10.1006/cimm.1997.1152. [DOI] [PubMed] [Google Scholar]
- 23.Saad SY, Najjar TA, Alashari M. Cardiotoxicity of doxorubicin/paclitaxel combination in rats: effect of sequence and timing of administration. J Biochem Mol Toxicol. 2004;18:78–86. doi: 10.1002/jbt.20012. [DOI] [PubMed] [Google Scholar]
- 24.Yamori T, Sato S, Chikazawa H, Kadota T. Anti-tumor efficacy of paclitaxel against humanlung cancer xenografts. Jpn J Cancer Res. 1997;88:1205–10. doi: 10.1111/j.1349-7006.1997.tb00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu B, Kusmartsev S, Cheng F, et al. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clin Cancer Res. 2003;9:285–94. [PubMed] [Google Scholar]
- 26.Shurin MR, Shurin GV, Lokshin A, et al. Intratumoral cytokines/chemokines/growth factors and tumorinfiltrating dendritic cells: friends or enemies? Cancer Metastasis Rev. 2006;25:333–56. doi: 10.1007/s10555-006-9010-6. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbloom AJ, Ferris RL, Sipe DM, et al. In vitro and in vivo protein sampling by combined microdialysis and ultrafiltration. J Immunol Methods. 2006;309:55–68. doi: 10.1016/j.jim.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Triozzi PL, Khurram R, Aldrich WA, Walker MJ, Kim JA, Jaynes S. Intratumoral injection of dendritic cells derived in vitro in patients with metastatic cancer. Cancer. 2000;89:2646–54. doi: 10.1002/1097-0142(20001215)89:12<2646::aid-cncr18>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 29.Crittenden MR, Thanarajasingam U, Vile RG, Gough MJ. Intratumoral immunotherapy: using the tumour against itself. Immunology. 2005;114:11–22. doi: 10.1111/j.1365-2567.2004.02001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–6. [PubMed] [Google Scholar]
- 31.Shin JY, Lee SK, Kang CD, et al. Antitumor effect of intratumoral administration of dendritic cell combination with vincristine chemotherapy in a murine fibro-sarcoma model. Histol Histopathol. 2003;18:435–47. doi: 10.14670/HH-18.435. [DOI] [PubMed] [Google Scholar]
- 32.Ramanathapuram LV, Hahn T, Dial SM, Akporiaye ET. Chemo-immunotherapy of breast cancer using vesiculated α-tocopheryl succinate in combination with dendritic cell vaccination. Nutr Cancer. 2005;53:177–93. doi: 10.1207/s15327914nc5302_7. [DOI] [PubMed] [Google Scholar]
- 33.Neuzil J, Weber T, Gellert N, Weber C. Selective cancer cell killing by α-tocopheryl succinate. Br J Cancer. 2001;84:87–9. doi: 10.1054/bjoc.2000.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prasad KN, Kumar B, Yan XD, Hanson AJ, Cole WC. α-Tocopheryl succinate, the most effective form of vitamin E for adjuvant cancer treatment: a review. J Am Coll Nutr. 2003;22:108–17. doi: 10.1080/07315724.2003.10719283. [DOI] [PubMed] [Google Scholar]
- 35.Israel K, Yu W, Sanders BG, Kline K. Vitamin E succinate induces apoptosis in human prostate cancer cells: role for Fas in vitamin E succinate-triggered apoptosis. Nutr Cancer. 2000;36:90–100. doi: 10.1207/S15327914NC3601_13. [DOI] [PubMed] [Google Scholar]
- 36.Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119:2026–9. doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]
- 37.Ohshima K, Akaiwa M, Umeshita R, Suzumiya J, Izuhara K, Kikuchi M. Interleukin-13 and interleukin-13 receptor in Hodgkin’s disease: possible autocrine mechanism and involvement in fibrosis. Histopathology. 2001;38:368–75. doi: 10.1046/j.1365-2559.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 38.Ellis MJ, Jenkins S, Hanfelt J, et al. Insulin-like growth factors in human breast cancer. Breast Cancer Res Treat. 1998;52:175–84. doi: 10.1023/a:1006127621512. [DOI] [PubMed] [Google Scholar]
- 39.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neurooncol. 2005;7:122–33. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-α as a tumour promoter. Eur J Cancer. 2006;42:745–50. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Villeneuve J, Tremblay P, Vallieres L. Tumor necrosis factor reduces brain tumor growth by enhancing macrophage recruitment and microcyst formation. Cancer Res. 2005;65:3928–36. doi: 10.1158/0008-5472.CAN-04-3612. [DOI] [PubMed] [Google Scholar]
- 42.Hemmerlein B, Markus A, Wehner M, Kugler A, Zschunke F, Radzum HJ. Expression of acute and late-stage inflammatory antigens, c-fms, CSF-1, and human monocytic serine esterase1, in tumor-associated macrophages of renal cell carcinomas. Cancer Immunol Immunother. 2000;49:485–92. doi: 10.1007/s002620000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujii A, Oshima K, Hamasaki M, et al. Differential expression of cytokines, chemokines and their receptors in follicular lymphoma and reactive follicular hyperplasia: assessment by complementary DNA microarray. Oncol Rep. 2005;13:819–24. [PubMed] [Google Scholar]
- 44.Nesbit M, Schaider H, Miller TH, Herlyn M. Low-level monocyte chemoattractant protein-1stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J Immunol. 2001;166:6483–90. doi: 10.4049/jimmunol.166.11.6483. [DOI] [PubMed] [Google Scholar]
- 45.Nakanishi T, Imaizumi K, Hasegawa Y, et al. Expression of macrophage-derived chemokine (Mdendritic cell)/CCL22 in human lung cancer. Cancer Immunol Immunother. 2006;55:1320–9. doi: 10.1007/s00262-006-0133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Angiolillo AL, Sgadari C, Taub DD, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. JExp Med. 1995;182:155–62. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elaraj DM, Weinreich DM, Varghese S, et al. The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clin Cancer Res. 2006;12:1088–96. doi: 10.1158/1078-0432.CCR-05-1603. [DOI] [PubMed] [Google Scholar]
- 48.Sawai H, Funahashi H, Yamamoto M, et al. Interleukin-1α enhances integrin a(6)h(1) expression and metastatic capability of human pancreatic cancer. Oncology. 2003;65:167–73. doi: 10.1159/000072343. [DOI] [PubMed] [Google Scholar]
- 49.Wolf JS, Chen Z, Dong G, et al. IL (interleukin)-1α promotes nuclear factor-κB and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clin Cancer Res. 2001;7:1812–20. [PubMed] [Google Scholar]