Abstract
Rad is the prototypic member of a new class of Ras-related GTPases. Purification of the GTPase-activating protein (GAP) for Rad revealed nm23, a putative tumor metastasis suppressor and a development gene in Drosophila. Antibodies against nm23 depleted Rad-GAP activity from human skeletal muscle cytosol, and bacterially expressed nm23 reconstituted the activity. The GAP activity of nm23 was specific for Rad, was absent with the S105N putative dominant negative mutant of Rad, and was reduced with mutations of nm23. In the presence of ATP, GDP⋅Rad was also reconverted to GTP⋅Rad by the nucleoside diphosphate (NDP) kinase activity of nm23. Simultaneously, Rad regulated nm23 by enhancing its NDP kinase activity and decreasing its autophosphorylation. Melanoma cells transfected with wild-type Rad, but not the S105N-Rad, showed enhanced DNA synthesis in response to serum; this effect was lost with coexpression of nm23. Thus, the interaction of nm23 and Rad provides a potential novel mechanism for bidirectional, bimolecular regulation in which nm23 stimulates both GTP hydrolysis and GTP loading of Rad whereas Rad regulates activity of nm23. This interaction may play important roles in the effects of Rad on glucose metabolism and the effects of nm23 on tumor metastasis and developmental regulation.
Ras-related GTP-binding proteins are a superfamily consisting of many members that play important roles in cell proliferation and differentiation (1), intracellular vesicular trafficking (2), cytoskeletal rearrangement (3), cell cycle regulation (4), and glucose transport into cells (5, 6). These GTPases cycle between GTP-bound (active) and GDP-bound states (inactive) in a controlled manner, stimulated by interaction with GTPase-activating proteins (GAPs) present in the cytoplasm of the cells (7, 8). Conversely, the reloading of the GTPases is made possible by the action of guanine nucleotide exchange factors (GEFs), which facilitates the exchange of free GTP for G protein-bound GDP (7), or is slowed by the presence of GDP dissociation inhibitors (9).
Rad is the prototype of a new subfamily of Ras-related GTPases, which are of molecular mass 33–35 kDa and lack typical prenylation motifs at the C terminus. It was initially identified by subtraction cloning and is overexpressed in skeletal muscle of a subset of humans with type 2 diabetes (10). Overexpression of Rad in 3T3-L1 adipocytes and C2C12 myocytes causes a marked reduction in glucose uptake in response to insulin stimulation, suggesting that it contributes to the insulin resistance of diabetes (6). Rad also interacts with calmodulin and calmodulin kinase II (11) and β-tropomyosin (12). These interactions are enhanced by an increase in the calcium influx and favor the GDP-bound form of Rad. The other two members of Rad family are Gem/Kir and Rem. Gem/Kir was identified in activated T lymphocytes (13) and v-abl transformed pre-B cells (14). Rem was cloned as a PCR product by using primers derived from conserved regions of Rad and Gem/Kir, is expressed in heart, lung, and other tissues, and is regulated in expression by inflammation in mice (45). Little is known about the regulators of the Rad family members. We have previously shown that Rad is regulated by a cytosolic GAP protein, which is distinct from all of the known GAPs that were tested (15).
NM23 is a putative tumor metastasis suppressor that was also originally identified by subtraction cloning in murine melanomas of differing metastatic potential (16). In human tumors, decreased expression of nm23 correlates with poorer prognosis in melanoma, neuroblastoma, and breast, hepatocellular, colorectal, and gastric carcinomas (17). Conversely, transfection of nm23 into some tumor cell lines is associated with reduced metastatic potential (18) and cell motility (19). The homologue of nm23 in Drosophila is the Awd (abnormal wing discs) protein (20). Mutations in awd result in developmental abnormalities of imaginal disc structures (21). Mutation in Awd of Pro 97 to Ser produces no phenotype by itself, but can synergize with a mutations of a putative GAP protein, which affects eye color (prune or pn) to produce larval death and hence is referred to as the killer of prune mutation (Awdkpn). NM23 is also identical to the enzyme nucleoside diphosphate (NDP) kinase in mammalian cells that is a major supplier of nucleoside triphosphates (NTPs) such as GTP, using ATP as a phosphate donor (22).
In the present study, we show that nm23 and Rad participate in a novel interaction in which nm23 acts as a bifunctional regulator of Rad, promoting both GTP hydrolysis and GTP reloading whereas Rad appears to regulate nm23 NDP kinase activity and its ability to undergo autophosphorylation. We show that this interaction may play an important role in the effects of these proteins on tumor cell growth and may also play roles in development and glucose metabolism.
Experimental Procedures
Recombinant Proteins.
Ral, Rho, Gem (gifts of L. Feig, Tufts University), Ran (gift of I. Macara, University of Vermont), Rad, and nm23-H1 were cloned into pGEX-2T vectors; Ras (gift of L. Feig) was cloned into pGEX-3T vectors. Expression of the glutathione S-transferase (GST) fusion proteins was induced with 1 mM IPTG for 1–3 hr. The fusion proteins were affinity-purified on glutathione-Sepharose beads and were quantitated by SDS/PAGE using BSA as a standard.
GAP Assay.
The GTP hydrolysis was assessed by preloading 1 μg of GST-Rad-Sepharose (20 pmol) with 3 μCi (1 Ci = 37 GBq) of [α-32P]GTP in a buffer consisting of 50 mM Tris⋅HCl (pH 7.4), 1 mM MgCl2, 1 mM DTT, and 1 mg/ml bovine albumin at 25°C for 5 min followed by three washings with the same buffer at 4°C. The [α-32P]GTP-Rad was incubated with the GAP preparation in a final volume of 100 μl at 25°C for 5 min, the beads were washed two more times with the same buffer at 4°C, and the bound nucleotides were eluted in 20 μl of buffer containing 1% SDS and 50 mM EDTA at 65°C for 5 min. The radioactivity in the supernatant was normalized, and the labeled nucleotides were resolved on polyethyleneimine-cellulose thin layer chromatography plates with a mobile phase of 0.75 M KH2PO4 (pH 3.4) (15). The dried plates were exposed to film, and the radioactivity was visualized on autoradiograms. One unit of the GAP activity was defined as the amount able to catalyze the conversion of 50% of radioactive GTP to GDP with 20 pmol of GST-Rad at 25°C in 5 min.
In some experiments, the GAP assay was carried out in the solution free from glutathione-Sepharose beads and any washings by incubating 0.5–1 μg of GST-Rad and 10 ng to 1 μg of GST-nm23 either alone or together with 3–5 μCi of [α-32P]GTP in 100 μl total volume. The reaction was allowed to proceed at 25°C for 5 min and was quenched with 1 ml of cold GAP buffer, and 1 μl of the mixture was directly spotted on the TLC to resolve radioactive GDP and GTP.
To determine whether GTP hydrolysis for Rad-GAP occurred in situ, i.e., in the absence of dissociation and reassociation, GST-Rad (1 μg/assay) was incubated in the hydrolysis buffer minus DTT, in the dark, with 10 μCi of 8-azido-[γ-32P]GTP at 25°C for 5 min. Samples were then transferred to a 48-multiwell cell culture plate prechilled on ice and were incubated for another 5 min. UV irradiation was performed by using an UV lamp (254 nm) 6 cm from the samples for the time indicated, then was quenched by diluting the samples in 10-fold excess of hydrolysis buffer containing 10 mM DTT. The radiolabeled GST-Rad was rebound to GSH-Sepharose beads by incubation at 4°C for 2 hr, the free nucleotides were washed away, and hydrolysis was initiated by incubating the washed GST-Rad with 1 μg nm23 in 100 μl at 25°C for 5 min. Samples were washed again, and proteins were resolved by 12% SDS/PAGE and autoradiography.
Purification and Sequencing of Rad-GAP.
Sprague–Dawley rats (250–300 g) were killed by CO2 inhalation, and the livers were immediately perfused with ice-cold PBS and frozen at −80°C. All subsequent purification steps were carried out at 4°C. In a typical trial, ≈350 g of liver were homogenized by using a Potter-Elvehjem homogenizer in 1 liter of buffer containing 50 mM Tris⋅HCl (pH 7.4), 1 mM EGTA, 1 mM DTT, 1 mM MgCl2, 1 mM PMSF, and 2 mM benzamidine (TED buffer). Samples were centrifuged at 30,000 × g for 30 min, and the supernatant was clarified by centrifugation twice at 200,000 × g for 60 min. Solid ammonium sulfate was added to the final supernatant with gentle stirring over 60 min to 55% saturation. Samples were centrifuged again at 30,000 × g for 20 min, and the supernatant was directly applied onto a phenyl-Sepharose column (Amersham Pharmacia, 2.6 × 35 cm) that had been preequilibrated with 1.8 liters of TED containing 2 M (NH4)2SO4. After washing with 1.8 liters of the same buffer, Rad-GAP activity was eluted with TED in two phases: initially with a decreasing linear gradient of (NH4)2SO4 from 2 M to 0 M and then a linear gradient of Triton X-100 from 0 to 0.2%. The active fractions were pooled and dialyzed against a buffer containing 20 mM KH2PO3 (pH 6.0), 1 mM EGTA, and 1 mM DTT (phosphate-EGTA-DTT, PED buffer). The dialyzed samples were applied to a Blue-4 column (Sigma, 1.6 × 40 cm) and were washed with 400 ml of PED. Elution was carried out with PED containing a linear gradient of NaCl from 0 to 1 M. After extensive dialysis against PED, the pooled active fractions were chromatographed on a Yellow-2 column (Sigma, 1.6 × 18 cm). The column was washed with 150 ml of PED, and Rad-GAP activity was eluted with PED containing a linear gradient of β-NAD+ from 0 to 10 mM. The active fractions were concentrated by using a Centriprep (Amicon, cut off 10 kDa) to 0.6 ml in PED. Finally, the sample was applied to a Mono S column (Amersham Pharmacia, 1 ml), and elution was carried out with PED containing step-linear gradient of NaCl from 0 to 1 M. The Mono S fraction size was 0.5 ml. At each purification step, small aliquots of the samples were supplemented with 10% glycerol and stored at −80°C until assay of GAP-specific activity.
Fraction 20 of Mono S eluate that contained the highest specific activity was concentrated with a Centricon (Amicon, cut off 10 kDa) and was resolved by 12% SDS/PAGE followed by Copper stain. The negative stained bands were cut and destained. The sequence analysis was carried out at the Harvard Microchemistry Facility by collisionally activated dissociation on a Finnigan-MAT (San Jose, CA) TSQ-7000 triple quadrupole mass spectrometer.
Immunoprecipitation and Immunoblotting.
Rad was transfected to a mouse skeletal muscle cell line C2C12 by retroviral infection (15). The cells were maintained in DMEM containing 10% FBS to confluence, then were switched to DMEM with 1% calf serum to promote differentiation to myotubes. The cells were lysed in the immunoprecipitation buffer (50 mM Tris⋅HCl, pH 7.4/100 mM NaCl/1 mM PMSF/1 mM benzamidine/5 μg/ml aprotinin/10 μg/ml leupeptin/1 mM MgCl2/1 mM EGTA/1% Triton X-100) at 4°C for 1 hr. After centrifugation at 13,000 × g for 15 min, aliquots of the supernatant were used for immunoprecipitation and direct blotting with a polyclonal rabbit antiserum generated against a bacteria-expressed Rad-GST (15) or a polyclonal rabbit antiserum against a peptide corresponding to C-terminal amino acid residues 175–194 of human nm23 H1 (Santa Cruz Biotechnology, catalog no. SC343). In the immunodepletion experiment, human skeletal muscle was solubilized in the above lysis buffer supplemented with 0.1% SDS. The anti-nm23 antibody was from Santa Cruz Biotechnology (catalog no. SC-465).
Determination of the GTP Formation.
Two approaches were used to examine the ability of nm23 to convert GDP⋅Rad to GTP⋅Rad. To assess the ability of nm23 to catalyze the transfer of the γ-phosphate from [γ-32P]ATP to GDP⋅Rad to form [γ-32P]GTP⋅Rad, Sepharose-coupled GST-Rad (1 μg/assay) was preincubated with 10 mM GDP at 37°C for 30 min. The free GDP was washed out, and the GDP-loaded GST-Rad was incubated with [γ-32P]ATP (10 μCi) in the absence or presence of 0.1 μg of GST-nm23. The nucleotides bound to Rad were determined by TLC as described above.
The second approach was to immobilize GDP on Rad by UV cross-linking to determine whether nm23 could promote the formation of GTP⋅Rad in situ. GST-Rad, GST-Gem, or GST-Ras (at 1 μg/assay) was preincubated with 10 mM GDP at 37°C for 30 min. Samples were chilled at 4°C, and UV cross-linking was performed by using a lamp (254 nm) 6 cm from the samples for 30 min. The Sepharose beads were washed, followed by incubation with 10 μCi [γ-32P]ATP in the presence of 1 μg GST-nm23 at 25°C for 5 min, and the proteins were resolved by SDS/PAGE and autoradiography. To determine the amount of GDP covalently attached to the G proteins before transfer of the γ-phosphate by nm23, we prepared [α-32P]GDP by incubating 100 μCi [α-32P]GTP with 10 μg GST-Rad and 5 μg GST-nm23 (total volume, 100 μl), as described above. After 5 min, the entire reaction mixture was spotted onto the TLC plate and developed as above. The GDP spot was scrapped off the plate, and the radioactive GDP was extracted with 0.5 ml of cold GAP buffer at 4°C overnight. This yielded ≈25 μCi of [α-32P]GDP (25% yield), which co-migrated with the nonradioactive GDP standard. GST-Rad, GST-Gem, and GST-Ras were then loaded with [α-32P]GDP (3 μCi/sample) at 37°C for 30 min followed by extensive washes. The [α-32P]GDP-loaded G proteins were then subjected to the UV irradiation and resolved by SDS/PAGE, as described above.
NM23 Autophosphorylation Assay.
GST-fusion proteins of wild-type nm23 or nm23kpn with a mutation homologous to Awdkpn (0.5 μg) was incubated in the absence or presence of 1 μg of GST-Rad in 20 μl GAP buffer lacking BSA. The reaction was initiated by the addition of 5 μCi of [γ-32P]ATP. After 5 min at 25°C, the reaction was stopped with Laemmli buffer; the samples were incubated for an additional 10 min and were subjected to 12% SDS/PAGE with and without boiling. The nonboiling procedure was used to preserve the phosphorylation of nm23 on the histidine residues. The gels were dried and exposed to the Kodak O-Mat films and were quantitated by scanning densitometry.
Analysis of Rad and NM 23 in Vivo in Melanoma Cells.
K-1735 TK melanoma cells overexpressing the murine nm23–1 or the control vector (gifts of P. Steeg, National Cancer Institute, National Institutes of Health, Bethesda, MD) (18) were transfected with the pBabe-puro vectors containing the full-length human Rad cDNA or S105N-Rad. Stably transfected cells were established by continued passage in puromycin-containing media (2 μg/ml, Sigma). Cells (1 × 105 cells) plated in triplicate samples in 24-well plates were starved in DMEM containing 0.1% BSA for 72 hr. After stimulation with FBS at the indicated concentrations for 16 hr, DNA synthesis was assessed by pulse-labeling the cells for 1 hr with 2 μCi of [3H]thymidine in 500 μl. The reaction was stopped by two washes with PBS, followed by precipitation in cold 10% trichloroacetic acid for 1 hr. The precipitated DNA was solubilized in 200 μl of 0.2 N NaOH-0.2% SDS and was neutralized with 50 μl of 10% trichloroacetic acid, and the incorporated [3H]thymidine was measured in a scintillation counter.
Results
Purification and Sequencing of Rad-GAP.
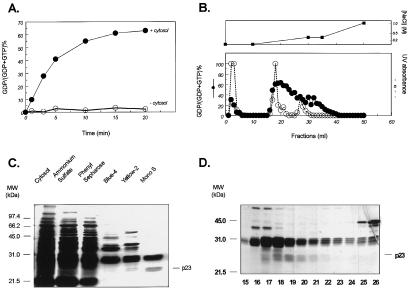
The human Rad GTPase is a 35-kDa molecule that specifically binds GTP but has a low intrinsic GTPase activity and is not activated by Ras-GAP or other known GAP molecules (15). As previously shown (15) and illustrated again in Fig. 1, however, cytosolic extracts of several cell types and tissues, especially liver and lung, contain a Rad-GAP activity. Thus, although GST-Rad alone hydrolyzed only 5% of the bound GTP to GDP by 5 min at 25°C, incubation of 1 μg of GST-Rad in the presence of 10 μg of rat liver cytosol resulted in a marked enhancement of hydrolysis, reaching 40% by 5 min (Fig. 1A).
Figure 1.
Purification of Rad-GAP from rat liver. GST-Rad-Sepharose was loaded with [α-32P]GTP and was incubated with or without GAP preparations as described in Experimental Procedures. The beads were washed, and the bound nucleotides were eluted in 1% SDS and 50 mM EDTA at 65°C for 5 min, were separated by PEI-TLC, and were quantitated on a PhosphorImager (Molecular Dynamics). The data are expressed as the percent conversion of [α-32P]GTP to [α-32P]GDP. (A) [α-32P]GTP-bound GST-Rad was incubated in the absence or presence of 10 μg cytosol for the time between 0 and 20 min. (B) After buffer exchange, the active fractions from Yellow 2 column were subjected to Mono S separation. Elution was carried out with step-and-linear gradient of NaCl between 0 and 1 M. The upper panel shows the salt gradient, and the lower panel shows the Rad GAP activity (closed circle, solid line) and UV absorbance (open circle, dashed line). (C) GAP preparations from each purification step were resolved by 10% SDS/PAGE followed by silver stain. (D) Resolution of the Mono S active fractions by 12% SDS/PAGE and silver stain.
Purification of Rad-GAP was therefore initiated by using rat liver cytosolic extract (470 ml containing 15.5 g of protein). After precipitation with 55% saturated ammonium sulfate, the supernatant was sequentially purified by chromatography on a phenyl-Sepharose column, a Blue 4 column, a Yellow 2 column, and finally FPLC on a Mono S column with a step-linear gradient of NaCl (Table 1; Fig. 1B). All column fractions were assayed for Rad-GAP activity, and the protein patterns of the most active fractions were resolved by SDS/PAGE and silver staining (Fig. 1C). The active fractions from the Mono S column were better resolved on a 12% gel, which showed a cluster of three bands at ≈31 kDa and a cluster of two bands at 20–23 kDa (Fig. 1D), the latter corresponding better with peak GAP activity. All five species were subjected to tryptic hydrolysis, and two or three peptides of each were sequenced. The peptides derived from the two lower bands matched exactly the sequence of residues 7–18, 115–128, and 129–143 of rat nm23 (23) whereas the peptides from the upper bands were identical to rat GST. The latter represents a contaminant that simply co-purified with nm23 because GST has no Rad-GAP activity. The fold of purification to the Mono S step was at least 2,530, with an overall yield of 0.5% (Table 1). Purification at the SDS/PAGE gel step was estimated at >20,000-fold.
Table 1.
Purification of Rad-GAP from rat liver
| Step | Total protein, mg | Activity, units | Specific activity, units/mg | Recovery, % | Purification, -fold |
|---|---|---|---|---|---|
| Cytosol | 15,510 | 439,000 | 28 | 100 | 1.0 |
| Ammonium sulfate | 9,792 | 360,900 | 37 | 82.2 | 1.3 |
| Phenyl-sepharose | 731 | 70,000 | 96 | 16 | 3.4 |
| Blue-4 | 142.8 | 68,000 | 476 | 15.5 | 16.8 |
| Yellow-2 | 0.93 | 2,980 | 3204 | 0.68 | 113.2 |
| Mono S | 0.031 | 2,222 | 71613 | 0.52 | 2,530 |
The table represents one trial using 350 g of rat liver. Specific activity was determined with serially diluted pooled samples at each purification step. One unit is defined as the amount able to catalyze conversion of 50% of radioactive GTP to GDP by 1 μ of GST-Rad at 25°C in 5 min.
NM23 Acts as a Rad-GAP and Is Associated with Rad in Vivo.
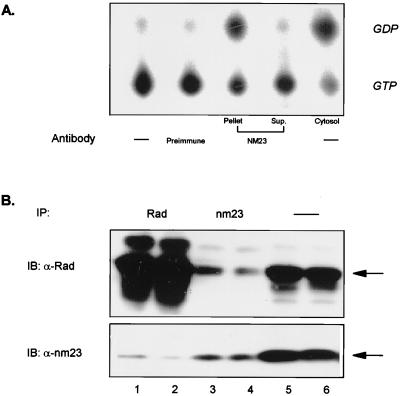
To confirm that nm23 was functioning as a Rad-GAP, human skeletal muscle cytosolic extracts were subjected to two rounds of immunoprecipitation with an antibody against human nm23, and the supernatant and precipitates were tested in the GAP assay. As seen in Fig. 2A, the Rad-GAP activity was almost completely retained in the immunocomplex whereas the nm23-depleted cytosol lost most of the GAP activity toward Rad. A low level of interaction between Rad and nm23 could also be detected in intact cells by co-immunoprecipitation and immunoblotting with anti-Rad and anti-nm23 antibodies (Fig. 2B). The level of co-precipitation correlated with the level of Rad protein expression (data not shown).
Figure 2.
NM23 as the source of Rad-GAP activity and its association with Rad in vivo. (A) Human skeletal muscle extracts were prepared as described in Experimental Procedures and were subjected to two rounds of immunoprecipitation (IP) with an anti-human nm23 antibody. The immunocomplex and the cytosol before and after nm23-depletion were used for the GAP assay as described in Fig. 1. (B) C2C12 myotubules overexpressing Rad were solubilized and subjected to immunoprecipitation for 2 hr with anti-Rad (1:100) and anti-nm23 (1:50) antibodies, then were subjected to SDS/PAGE and immunoblotting with both antibodies. Starting from the left, lanes 1 and 2 represent an IP with anti-Rad antibody; lanes 3 and 4 with anti-nm23 antibody; and lanes 5 and 6 represent straight blotting.
The Action of NM23 as a Rad-GAP Can Be Reconstituted By Purified Proteins in Vitro.
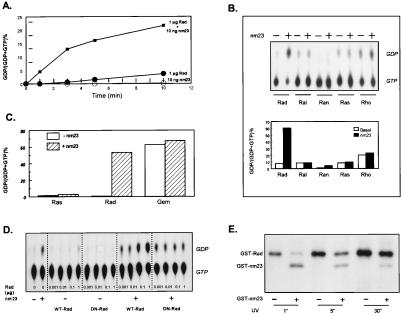
In humans, there are two forms of nm23, H1 and H2, which share 90% sequence homology (23). To directly assess GAP activity, recombinant nm23 H1 was expressed as a GST fusion protein and was incubated in vitro with bacterially expressed GST-Rad in the presence of [α-32P]GTP. When incubated alone, 10 ng of GST-nm23 exhibited no detectable GTP hydrolysis above base line at 25°C over 10 min whereas GST-Rad alone slowly hydrolyzed GTP, owing to its intrinsic GTPase activity. When GST-Rad was co-incubated with GST-nm23, there was a marked increase in the rate of GTP-hydrolysis, which varied between 20- and 500-fold at the early time points (Fig. 3A).
Figure 3.
NM23 is a Rad-specific GAP. (A) GST-Rad (1 μg) and GST-nm23 (10 ng), alone or together, were incubated with [α-32P]GTP at 25°C for 0–10 min. The reaction was quenched with addition of 10-fold cold buffer, and 1 μl was spotted onto a PEI-TLC plate as described above. (B) One microgram of GST-Rad, GST-Ral, GST-Ran, GST-Ras, and GST-Rho were loaded with 3 μCi of [α-32P]GTP, were washed extensively, and then were incubated in the absence or presence of 0.1 μg of GST-nm23 at 25°C for 5 min. The bound nucleotides were eluted and resolved by TLC. The upper panel shows a representative TLC profile; the lower panel depicts the percent conversion of GTP to GDP quantitated by using a PhosphorImager and represents the average of two separate assays. (C) One microgram of GST-Rad and GST-Gem on GSH-Sepharose beads were loaded with 3 μCi of [α-32P]GTP, were washed and incubated with or without 1 μg of GST-nm23 at 14°C or 25°C for 5 min. The bound radioactivity was eluted, and nucleotides were detected as described above. (D) Wild-type GST-Rad and GST-S105NRad were incubated with 3 μCi of [α-32P]GTP in the absence or presence of 50 ng GST-nm23 at 25°C for 5 min. The nucleotides were resolved by TLC. (E) One microgram of GST-Rad was incubated in the DTT-free buffer with 3 μCi of 8-azido-[γ-32P]GTP at 25°C, in the dark, for 5 min. Samples were UV irradiated with UV at 6 cm for the time indicated. The samples were transferred to DTT-containing buffer, and nucleotides were rebound to GSH-Sepharose beads. After 3 washes, the labeled GST-Rad was incubated with or without 1 μg of GST-nm23 and was washed again, and the proteins were resolved by 12% SDS/PAGE.
To determine whether nm23 was specific for Rad, we expressed a panel of Ras-related GTPases as GST fusion proteins, loaded them with [α-32P]GTP, and assessed hydrolysis of the G protein-bound GTP in the presence or absence of GST-nm23. In the absence of nm23, the nucleotide eluted from Rad, Ral, Ran, Ras, and Rho was mainly GTP, although Rho had a slightly higher intrinsic GTPase activity and contained both GTP and GDP. As noted above, in the presence of GST-nm23, the majority of GTP associated with Rad was converted to GDP whereas those eluted from other G proteins remained mainly GTP (Fig. 3B). We also tested the GAP activity of nm23 against another member of the Rad family, Gem. Although Gem displayed exceptionally high intrinsic GTPase activity, this was not further enhanced by the addition of nm23 (Fig. 3C). Thus, nm23 behaves as a Rad-specific GAP.
Because nm23 could also serve to convert GTP to GDP, two additional experiments were performed to confirm the role of Rad as the active GTPase in this pair. In the first, we tested whether GTP-binding to Rad was a prerequisite for GTP hydrolysi by using S105N-Rad, a point mutation analogous to S17N-Ras, that is devoid of GTP-binding but still binds GDP (15). As predicted, in the presence of nm23, this putative dominant negative mutant of Rad failed to hydrolyze GTP to a level beyond that carried out by the NDP kinase activity of nm23 (Fig. 3D)
More importantly, we determined whether the GTPase activity of Rad could be enhanced by nm23 for GTP covalently bound to Rad. As shown in Fig. 3E, when 8-azido-[γ-32P]GTP was covalently bound to Rad, addition of nm23 caused reduction in amount of [γ-32P] labeling on Rad, indicating that GTP was being hydrolyzed in situ. This effect was greatest at the shortest times of cross-linking because increasing the duration of UV exposure resulted in a loss of Rad's GTPase activity due to denaturation of the protein. These results prove that Rad⋅GTP can be converted to Rad⋅GDP without nucleotide dissociation/reassociation by a mechanism requiring an active Rad GTPase and accelerated by nm23.
Mutations of NM23 Impair Its GAP Activity.
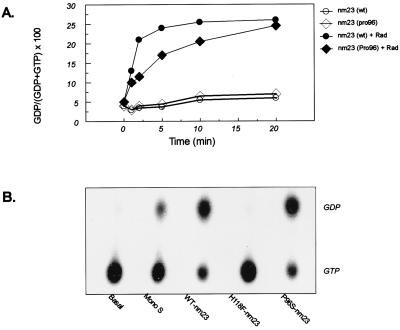
Previous studies of nm23 have defined two functionally important mutants of this protein. One change of Pro96 to Ser96 is analogous to the killer-of-prune mutation in the Drosophila homologue of nm23 (Awdkpn). This mutation does not affect NDP kinase activity but causes larval death if complemented with the reduction or loss of gene products of the prune locus (24). The second is point mutation of His118 to Phe mutant that abolishes the histidine kinase activity of nm23 (22). The GAP activity of nm23 was reduced with mutations at either of these residues. When GTP hydrolysis was analyzed by using increasing concentrations of nm23 or nm23(Pro96) alone, no difference in the low level of GTP hydrolysis was observed. However, in the presence of Rad, nm23(Pro96) failed to accelerate GTP hydrolysis to the same extent as the wild-type protein (Fig. 4A). This was especially apparent at the lower concentrations of nm23. The kinase-negative mutant of nm23 (His118Phe) lost its Rad-GAP activity, even at high concentrations of the protein (Fig. 4B). Thus, the ability of nm23 to act as a Rad-GAP is altered in both the mammalian homologue of the killer-of-prune mutant and a mutant of nm23 defective in phosphotransferase activity.
Figure 4.
Mutations of NM23 impair its GAP activity. (A) Wild-type and nm23(Pro96) (50 ng) were incubated with 3 μCi of [α-32P]GTP in the absence or presence of 1 μg of GST-Rad at 25°C for 5 min. The reaction was stopped by the addition of cold buffer, and the nucleotides were resolved as above. (B) [α-32P]GTP-loaded Rad was incubated in the absence or presence of one of the following: 60 ng/1 μl of Mono S fraction 20, 0.1 μg each of wild-type nm23, H118F-nm23, and P96S-nm23. The Rad-bound nucleotides were eluted and resolved by TLC.
NM23 Is a Functional “GEF” for Rad, Gem, and Ras.
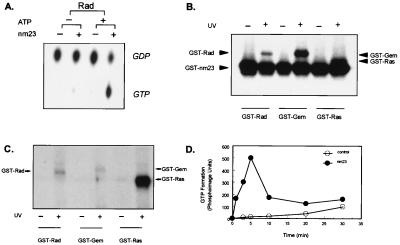
The traditional role of nm23 as an NDP kinase is to convert NDPs to the corresponding NTPs by using ATP as a phosphate donor (23). Despite a number of studies, it has remained controversial as to whether nm23 might be able to activate GTP-binding proteins by facilitating GDP to GTP conversion in situ (25). We reasoned that the direct interaction between Rad and nm23 creates a unique microenvironment in which this refueling process, i.e., conversion of GDP⋅Rad to GTP⋅Rad, might occur. To test this hypothesis, we prepared and isolated [α-32P]GDP and studied the reloading by using two experimental designs. First, GST-Rad was loaded with the [α-32P]GDP, and then [α-32P]GTP formation in the absence or presence of nm23 and nonradioactive ATP (to provide the γ-phosphate) was monitored. After incubation, the GST-Rad bound to glutathione-Sepharose beads was washed, and the associated nucleotides were eluted for analysis. In the absence of either nm23 or ATP, virtually all of the nucleotides bound to Rad remained as [α-32P]GDP. However, when both nm23 and ATP were present, there was a significant conversion of [α-32P]GDP⋅Rad to [α-32P]GTP⋅Rad (Fig. 5A).
Figure 5.
NM23 is a functional GEF for Rad, Gem, and Ras. (A) [α-32P]GDP was prepared as described in Experimental Procedures. GST-Rad (1 μg each) was loaded with this [α-32P]GDP (3 μCi each) at 37°C for 30 min, followed by incubation in the absence or presence of 1 μg of GST-nm23 and 10 mM ATP at 25°C for 5 min. The bound nucleotides were eluted and resolved by TLC. (B) One microgram each of GST-Rad, GST-Gem, and GST-Ras were loaded with 10 mM nonradioactive GDP at 37°C for 30 min, followed by UV irradiation. The protein was rebound to the GSH-Sepharose beads and was washed 3 times. The GDP-bound Rad was incubated in the presence of 10 μCi [γ-32P]ATP and 1 μg of GST-nm23 at 25°C for 5 min, then was resolved by 12% SDS/PAGE. (C) One microgram of GST-Rad, GST-Gem, and GST-Ras were incubated with 3 μCi of [α-32P]GDP prepared as described above at 37°C for 30 min. Samples were UV-irradiated for 30 min and were resolved by 12% SDS/PAGE. (D) GST-Rad-Sepharose was incubated with 10 mM nonradioactive GDP at 37°C for 30 min followed by 3 washes. Aliquots were incubated with 10 μCi [γ-32P]ATP with or without 0.1 μg of GST-nm23 at 25°C for the time indicated. The beads were washed, and the nucleotides were eluted and resolved by TLC, and the amount of [γ-32P]GTP was quantitated by using a PhosphorImager.
The mechanism of this “reloading” could be two-fold: Rad-bound [α-32P]GDP could be released into solution, converted to [α-32P]GTP by nm23, then rebound to Rad. Alternatively, the [α-32P]GDP⋅Rad could be converted to [α-32P]GTP⋅Rad in situ as a result of transphosphorylation by nm23. To address this issue, we covalently attached GDP to Rad by UV cross-linking, then incubated the complex with nm23 and [γ-32P]ATP as the phosphate donor. Fig. 5B shows that nm23 was able to catalyze the transphosphorylation of Rad⋅GDP to Rad⋅GTP in situ. This transphosphorylation occurred to an even greater extent to GDP bound to Gem and Ras (Fig. 5B). The lower extent of GTP-reloading of Rad as compared with the other G-proteins was not the result of difference in binding or UV cross-linking of GDP to Rad, Gem, and Ras (Fig. 5C). Rather, these data, together with the previous results, suggest that nm23 can “reload” Rad and other GTPases but that the net level of GTP achieved for Rad is lower because of the specific GAP-activity of nm23 for Rad, which converts the newly formed GTP⋅Rad back to GDP⋅Rad. This notion was further supported by the time course of GTP-reloading to Rad in the presence of nm23, which revealed a rapid formation of GTP⋅Rad that peaked by 5 min and then declined by 30 min (Fig. 5D). Thus, nm23 plays a dual functional role for Rad. Depending on the initial state of Rad, nm23 may act as a functional Rad-GAP and promote hydrolysis of Rad⋅GTP to Rad⋅GDP or act as a functional Rad-GEF by converting Rad⋅GDP to Rad⋅GTP. These two effects offset one another, finally reaching a balance in the ratio of Rad⋅GTP to Rad⋅GDP.
Rad Is also a Regulator of NM23.
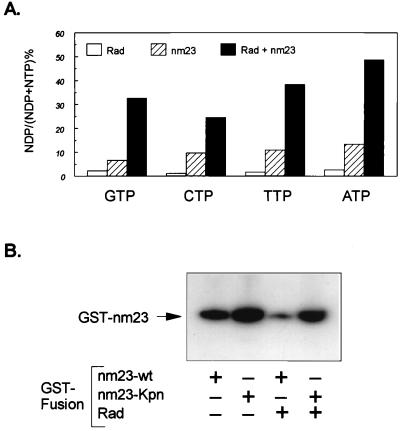
It is known that the NDP kinase activity of nm23 can transfer γ-phosphate from all of the nucleoside triphosphates to form corresponding nucleoside diphosphates (26). Thus, although it is clear that nm23 acts to promote GTP hydrolysis by the Rad GTPase, it is also possible that nm23 itself could act on GTP or other nucleoside triphosphates in solution to form nucleotide diphosphates. To determine whether there was an effect of Rad on the NDP kinase activity of nm23, [α-32P] labeled GTP, CTP, TTP, and ATP were incubated with GST-Rad alone, GST-nm23 alone, or a mixture of the two together. Whereas GST-Rad alone had a very small effect on the conversion of the NTPs to NDPs over 5 min, 50 ng of GST-nm23 did convert significant amounts of the NTPs to NDPs. When both Rad and nm23 were present, they synergized to produce much greater conversion of all four NTPs to their respective NDPs (Fig. 6A). Because Rad does not bind CTP or TTP and has very low affinity for ATP (15), the synergism of Rad and nm23 toward CTP, TTP, and ATP is likely attributable to the enhancement of the catalytic activity of nm23 by Rad. The effect of Rad and nm23 on GTP, however, is more complex, because Rad does bind GTP and is itself an active GTPase. The lack of effect of S105N-Rad to synergize with nm23 in conversion of GTP to GDP suggests that this mutant of Rad not only lacks GTPase activity (because of a lack of GTP binding), but also that the GTP bound form of Rad is required for regulation of the NDP kinase activity of nm23.
Figure 6.
Rad is a regulator of NM23. (A) GST-nm23 (50 ng) was incubated with 3 μCi each of [α-32P]GTP, [α-32P]CTP, [α-32P]TTP, and [α-32P]ATP in the absence or presence of 1 μg GST-Rad. After 5 min at 25°C, the reaction was stopped with 10-fold cold buffer, and the nucleotides were resolved by PEI-TLC [mobile phase = 0.1 M LiCl and 0.06 M (NH4)SO4] and were quantitated by PhosphorImager analysis, and the percent conversion was calculated as the ratio of NDP/(NDP + NTP) × 100. (B) Wild-type and Pro96 GST-nm23 (0.5 μg) was incubated with 5 μCi of [γ-32P]ATP in the absence or presence of 1 μg of GST-Rad at 25°C for 5 min. After addition of 2 × SDS/PAGE sample buffer (pH 8.8), the mixture was incubated at 25°C for 10 min, and proteins were resolved by 10% SDS/PAGE.
The second important catalytic feature of nm23 is its ability to undergo autophosphorylation on histidine and serine residues. These effects occur using mainly ATP as the phosphate donor (18, 26). To determine the effect of Rad on nm23 autophosphorylation, we incubated the two GST-fusion proteins in the presence of [γ-32P]ATP and analyzed the phosphorylation by SDS/PAGE. Care was taken to maintain the phosphohistidine in place by keeping these aliquots at alkaline pH and avoidance of boiling (27). In the absence of boiling, autophosphorylation of both wild-type, and to an even greater extent nm23(Pro96), was easily detected after incubation with [γ-32P] ATP (Fig. 6B). Addition of Rad markedly decreased the autophosphorylation of wild-type nm23. Autophosphorylation of the killer-of-prune mutant was also inhibited, but to a lesser extent (Fig. 6B). This may reflect the decreased interaction between the kpn mutant and Rad, which was also observed in the GAP assay (see Fig. 4A). When similar samples were analyzed after boiling to assess serine phosphorylation, the overall levels of incorporation of 32P were lower, but the pattern was similar (data not shown). These data suggest that the interaction of nm23 with Rad may decrease both its histidine and serine phosphorylation.
Rad and NM23 in the Regulation DNA Synthesis.
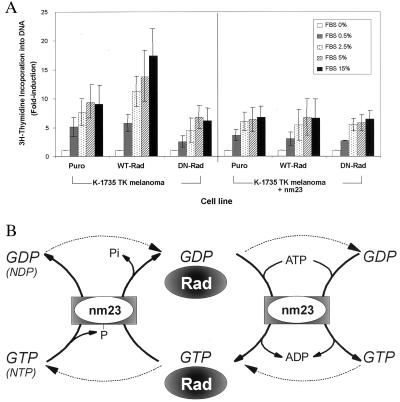
To address the functional role of the Rad-nm23 interaction in vivo, K-1735 TK melanoma cells were established overexpressing either wild-type Rad or S105N-Rad in the presence or absence of coexpression of nm23, and the incorporation of [3H]thymidine into DNA was assessed after serum stimulation (Fig. 7A). All cell lines responded to serum stimulation; however, cells overexpressing wild-type Rad showed a significantly two-fold greater response to serum than the Puro controls. In contrast, cells overexpressing the S105N-mutant of Rad showed slightly lower levels of response to serum stimulation as compared with the control cells. In the cells overexpressing wild-type Rad, coexpression of nm23 blocked the effect of wild-type Rad to increase the levels of DNA synthesis induced by serum.
Figure 7.
The in vivo significance of Rad and NM23 interaction. (A) Effects of Rad and nm23 on serum stimulation of thymidine incorporation. K-1735 TK melanoma cells were stimulated with serum as indicated for 16 hr, were pulsed with 2 μCi of [3H]thymidine in 500 μl of sample per well for 1 hr, and were lysed and assayed for incorporation of radioactivity into trichloroacetic acid-precipitated DNA. Data are presented as fold induction by serum relative to the medium control for each cell line. Values are the mean of three independent experiments, each in triplicate; error bars indicate the standard error of the mean. (B) Model of a coordinate, bidirectional, and bimolecular regulation of Rad and NM23. (Left) (i) Rad-bound GTP is hydrolyzed by Rad GTPase activity stimulated by nm23 to Rad-bound GDP in situ. (ii) γ-phosphate of NTPs are transferred to the histidine residue of nm23 (intermediate), and NDPs are formed. The phosphate can be transferred to NDPs to form new NTPs. (iii) The dotted lines denote that GTP may dissociate from Rad and be converted to GDP by nm23, which is then rebound to Rad. (Right) (i) Rad-bound GDP is transphosphorylated to form Rad-bound GTP in situ. (ii) GDP is phosphorylated by nm23 to form GTP at the expense of ATP in solution. (iii) the dotted lines denote that GDP may dissociate from Rad and be converted to GTP by nm23, which is then rebound to Rad. The balance between GTP⋅Rad and GDP⋅Rad may be maintained through interactions with nm23 and other yet-determined molecules.
Discussion
Rad is the prototypic member of a new family of Ras-related GTPases, which also includes Gem and Kir. Although Rad was originally identified in the muscles of a type 2 diabetic human by subtraction cloning, the exact functions of this GTPase remain unknown. Our previous studies have suggested potential roles for Rad in regulation of glucose uptake (6), calcium-mediated signaling (11), and possible cell motility or contraction (12). NM23 is an ≈17-kDa protein that was also originally identified by differential hybridization and has been suggested to act as a tumor metastasis suppressor because it is more highly expressed in tumors of low metastatic potential. Subsequently, nm23 was shown to exist in two isoforms (H1 and H2) and to be identical to human nucleoside diphosphate (NDP) kinases A and B, the major enzymes involved in furnishing the cell with all NTPs (except for ATP) (28). NM23 also acts as a histidine kinase (22) and has been suggested to play a role in transcriptional regulation (29). The Drosophila homologue of nm23 (Awd) has been shown to be critical to development of wing, leg, and eye-antenna imaginal disc structures, as well as the ovaries, brain, and proventriculus (24), and a point mutation of Awd (the kpn mutation) complements mutations at the prune locus to produce larval death (30, 31).
In the present study, we have demonstrated a unique interaction between Rad and nm23. Thus, by protein purification, immunodepletion, and studies of recombinant proteins in vitro, we have demonstrated that nm23 acts as a Rad-GAP. This enhanced conversion of Rad⋅GTP to Rad⋅GDP occurs even if the GTP is covalently cross-linked to Rad. Thus, although nm23 might be acting to promote hydrolysis of GTP, which has dissociated from Rad and then rebinds as GDP or may act in concert with Rad to promote GTP hydrolysis, it is clear that a significant amount of Rad⋅GTP is converted to Rad⋅GDP without dissociation of the nucleotide. NM23 acts as a specific Rad-GAP. It does not act on other Ras-related GTPases, such as Ral, Ran, Ras, and Rho, or even the closely related GTPase Gem. A unique form of nm23 termed DR-nm23 has been identified in leukemic cells and is ≈65% identical to nm23 H1 and nm23 H2 (32). Whether this protein could serve as a GAP for Gem or Kir remains to be determined. Although we have not yet mapped the domain of Rad involved in the GAP interaction, preliminary studies indicate that the interaction probably does not involve the C terminus (calmodulin-binding domain) of Rad because calmodulin neither enhances nor interferes with the Rad-GAP activity of nm23 (11) nor the N-terminal 88-aa extension of Rad, since truncation of this domain does not modulate the effect of nm23 as a Rad-GAP (data not shown). Full activity of nm23 as a Rad-GAP depends on proline at position 96 (kpn mutant) and histidine at position 118. Whether these affect the affinity of interaction or are required for maintaining the active conformation of the protein remains to be determined.
Because of its ability to generate GTP from GDP, a number of laboratories have proposed that nm23 might be able to activate GTP-binding proteins in situ. This concept was originally proposed for tubulin (33) but has been extended to many other G-proteins, including the trimeric G-Proteins (34–36), members of the Ras family (37), Arf (38), and tubulin (33). However, this concept has been challenged by Randazzo et al., who have argued that kinetically one cannot exclude these effects as being indirect: i.e., involving dissociation and reassociation of the GDP and GTP with conversion occurring in the cytoplasm (25).
Our data demonstrate that nm23 not only functions as a Rad-specific GAP but also can reload GDP to GTP in situ on Rad, thereby acting as a functional GEF for this GTPase. Because this can also occur with GDP covalently attached to Rad, we believe that our data provide clear evidence that nm23 is able to recharge G proteins. In contrast to the GAP-activity of nm23, which is Rad-specific, this “reloading” activity is not G-protein specific. The exact mechanisms by which nm23 “recharges” Rad (and other G-proteins) with GTP in cells, however, may be two-fold. NM23 may phosphorylate the Rad-bound GDP in situ to form a Rad⋅GTP. Alternatively, nm23 may phosphorylate the GDP that has been released from Rad during the incubation, and the newly created GTP may rebind to Rad. In either case, nm23 works functionally like a GEF to promote the conversion from “inactive” GDP⋅Rad to “active” GTP⋅Rad. A summary of this bidirectional modulation of Rad by nm23 is shown schematically in Fig. 7B.
Equally surprising to the regulation of Rad by nm23 is the possibility that Rad may also be an important modulator of nm23 activity. The NDP kinase activity of nm23 provides all nucleoside triphosphates for DNA/RNA synthesis, UTP for polysaccharide synthesis, CTP for lipid synthesis, and GTP for microtubule polymerization, protein elongation, and signal transduction (20). Rad appears to increase this NDP kinase activity of nm23. In addition, Rad reduces the level of histidine phosphorylation of nm23, as well as the heat stable phosphorylation of nm23, which occurs on serine residues, both of which may play a role in the tumor metastasis suppressing activity of this protein (17).
In the present study, we find that the interaction between Rad and nm23 appears to play a significant role in control of tumor cell growth. Thus, in melanoma cells, Rad acts to increase serum-stimulated DNA synthesis whereas nm23 blocks this effect. A similar effect is observed on cell growth in vivo in human breast cancer cells (Y.-H.T., D. Vicent, P. Watson, J.Z., and C.R.K., unpublished work). This is particularly interesting because the expression of nm23 gene has been shown to inversely correlate with metastatic potential in several tumor types (17, 18). The exact mechanism of these effects at present is unclear. We and others have previously shown that nm23 may mediate part of its effect by altering cell motility (19, 39) and the response of cells to transforming growth factor β, serum, insulin-like growth factor-1, and platelet-derived growth factor (18, 19, 40). We have also shown that Rad interacts in vivo with β-tropomyosin, calmodulin, and calmodulin-dependent kinase II and that these interactions are favored by the GDP bound form of Rad. Tropomyosin is clearly indicated in the contraction mechanism and cytoskeletal organization, both of which play central roles in the cell motility. In transgenic animals, overexpression of Rad alters skeletal and cardiac muscle function (Jacob Ilany, Philip Bilan, Sania Kapur, James Caldwell, Mary-Elizabeth Patti, Andre Marette, and C.R.K., unpublished work). Calmodulin and calmodulin-dependent kinase II may participate in calcium mediated signaling involved in cellular movement (41).
An equally intriguing potential role of the Rad-nm23 interaction would be in Drosophila development. As noted above, a missense mutation in the Drosophila homologue of nm23 (Awdkpn) results in no apparent phenotype but becomes lethal when it occurs together with a defect at another locus termed prune (pn) (24) that encodes a protein with some homology to the catalytic domains of mammalian GAPs (24, 42). Although the exact interaction between pn and Awdkpn is unclear, several investigators have proposed a model in which these mutant proteins interact with another protein to produce a product that is toxic (42–44). Based on our data, one could hypothesize that this additional protein is the Drosophila homologue of Rad (dRad), or dRad might be under the control of Awd and the product of prune, with the former providing both GAP and GEF activities whereas the latter serves only as a GAP. If this were the case, a missense mutation in the awd protein, the kpn mutation, might cause an impairment of the GAP activity (as suggested by our data) that does not produce a phenotype as long as the GAP activity of the product of the prune locus keeps the dRad in the GDP-bound state. However, if the Awdkpn mutation occurred together with the reduction in prune expression, the reduced GAP activity for dRad would be uncompensated and lead to a lethal phenotype. Whether mammals also have a prune-like protein that serves as an additional or alternative regulator of Rad needs further consideration.
In summary, our data indicate that nm23 and Rad form a novel complex for bidirectional, bimolecular regulation both in vitro and in vivo. In this complex, nm23 acts functionally as both a Rad-GAP and a Rad-GEF, determining the delicate balance between GTP⋅Rad and GDP-Rad. Simultaneously, Rad appears to regulate nm23 and play a role in the balance between various NTP and NDP levels. In vivo, this interaction modifies the growth rate of tumor cells. The signals that regulate this system and the roles Rad and nm23 play in development and metabolism are clearly worthy of further study.
Acknowledgments
We thank T. L. Azar for excellent secretarial assistance, L. Feig (Tufts University) for the pGEX-constructs of ras, ral, and rho, I. Macara of the University of Vermont for pGEX-ran, K. Kelly for pGEX-gem, and P. Steeg for the mouse melanoma cells. This work was supported in part by National Institutes of Health Grants DK 45935 (C.R.K.), RO1 DK 47919 (C.J.R.), and RO1 CA 37393 (B.R.Z.), an individual National Research Service Award, DK 09193 (J.Z.), Joslin's Institutional Training Grant DK 07260, and Joslin's Diabetes and Endocrinology Research Center Grant P30 DK 36836.
Abbreviations
- GAP
GTPase-activating protein
- GEF
guanine nucleotide exchange factor
- GST
glutathione S-transferase
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 27, 1999.
References
- 1.Lange-Carter C A, Johnson G L. Science. 1994;265:1458–1461. doi: 10.1126/science.8073291. [DOI] [PubMed] [Google Scholar]
- 2.Zerial M, Stenmark H. Curr Opin Cell Biol. 1993;5:613–620. doi: 10.1016/0955-0674(93)90130-i. [DOI] [PubMed] [Google Scholar]
- 3.Hall, A. (1994) Annu. Rev. Cell Biol.31–54. [DOI] [PubMed]
- 4.Ren M, Drivas G, D'Eustachio P, Rush M G. J Cell Biol. 1993;120:313–323. doi: 10.1083/jcb.120.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cormont M, Bortoluzzi M N, Gautier N, Mari M, Van Obberghen E, Le Marchand-Brustel Y. Mol Cell Biol. 1996;16:6879–6886. doi: 10.1128/mcb.16.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moyers J S, Bilan P J, Reynet C, Kahn C R. J Biol Chem. 1996;271:23111–23116. doi: 10.1074/jbc.271.38.23111. [DOI] [PubMed] [Google Scholar]
- 7.Feig L A. Science. 1993;260:767–768. doi: 10.1126/science.8484117. [DOI] [PubMed] [Google Scholar]
- 8.Trahey M, McCormick F. Science. 1987;238:542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- 9.Garrett M D, Kabcenell A K, Zahner J E, Kaibuchi K, Sasaki T, Takai Y, Cheney C M, Novick P J. Growth Regul. 1993;331:233–238. doi: 10.1016/0014-5793(93)80343-s. [DOI] [PubMed] [Google Scholar]
- 10.Reynet C, Kahn C R. Science. 1993;262:1441–1444. doi: 10.1126/science.8248782. [DOI] [PubMed] [Google Scholar]
- 11.Moyers J S, Bilan P J, Zhu J, Kahn C R. J Biol Chem. 1997;272:11832–11839. doi: 10.1074/jbc.272.18.11832. [DOI] [PubMed] [Google Scholar]
- 12.Zhu J, Bilan P J, Moyers J S, Antonetti D A, Kahn C R. J Biol Chem. 1996;271:768–773. doi: 10.1074/jbc.271.2.768. [DOI] [PubMed] [Google Scholar]
- 13.Maguire J, Santoro T, Jensen P, Siebenlist U, Yewdell J, Kelly K. Science. 1994;265:241–244. doi: 10.1126/science.7912851. [DOI] [PubMed] [Google Scholar]
- 14.Cohen L, Mohr R, Chen Y Y, Huang M, Kato R, Dorin D, Tamanoi F, Goga A, Afar D, Rosenberg N, et al. Proc Natl Acad Sci USA. 1994;91:12448–12452. doi: 10.1073/pnas.91.26.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu J, Reynet C, Caldwell J S, Kahn C R. J Biol Chem. 1995;270:4805–4812. doi: 10.1074/jbc.270.9.4805. [DOI] [PubMed] [Google Scholar]
- 16.Steeg P S, Bevilacqua G, Kopper L, Thorgeirsson U P, Talmadge J E, Liotta L A, Sobel M E. J Natl Cancer Inst. 1988;80:200–204. doi: 10.1093/jnci/80.3.200. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald N J, De La Rosa A, Benedict M A, Freije J M P, Krutsch H, Steeg P S. J Biol Chem. 1993;268:25780–25789. [PubMed] [Google Scholar]
- 18.Leone A, Flatow U, King C R, Sandeen M A, Margulies I M K, Liotta L A, Steeg P S. Cell. 1991;65:25–35. doi: 10.1016/0092-8674(91)90404-m. [DOI] [PubMed] [Google Scholar]
- 19.Kantor J D, McCormick B, Steeg P S, Zetter B R. Cancer Res. 1993;53:1971–1973. [PubMed] [Google Scholar]
- 20.Biggs J, Hersperger E, Steeg P S, Liotta L A, Shearn A. Cell. 1990;63:933–940. doi: 10.1016/0092-8674(90)90496-2. [DOI] [PubMed] [Google Scholar]
- 21.Rosengard A M, Krutzsch H C, Shearn A, Biggs J R, Barker E, Margulies I M K, King C R, Liotta L A, Steeg P S. Nature (London) 1989;342:177–180. doi: 10.1038/342177a0. [DOI] [PubMed] [Google Scholar]
- 22.Wagner P D, Vu N D. J Biol Chem. 1995;270:21758–21764. doi: 10.1074/jbc.270.37.21758. [DOI] [PubMed] [Google Scholar]
- 23.Stahl J A, Leone A, Rosengard A M, Porter L, King C R, Steeg P S. Cancer Res. 1991;51:445–449. [PubMed] [Google Scholar]
- 24.Dearolf C R, Tripoulas N, Biggs J, Shearn A. Dev Biol. 1988;129:169–178. doi: 10.1016/0012-1606(88)90171-6. [DOI] [PubMed] [Google Scholar]
- 25.Randazzo P A, Northup J K, Kahn R A. J Biol Chem. 1992;267:18182–18189. [PubMed] [Google Scholar]
- 26.Tepper A D, Dammann H, Bominaar A A, Veron M. J Biol Chem. 1994;269:32175–32180. [PubMed] [Google Scholar]
- 27.Kowluru A, Seavey S E, Rhodes C J, Metz S A. Biochem J. 1996;313:97–107. doi: 10.1042/bj3130097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jong A, Ma J J. Arch Biochem Biophys. 1991;291:241–246. doi: 10.1016/0003-9861(91)90129-7. [DOI] [PubMed] [Google Scholar]
- 29.Postel E H, Berberich S J, Flint S J, Ferrone C A. Science. 1993;261:478–480. doi: 10.1126/science.8392752. [DOI] [PubMed] [Google Scholar]
- 30.Hadorn E, Mitchell H K. Proc Natl Acad Sci USA. 1951;37:650–665. doi: 10.1073/pnas.37.10.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biggs J, Tripoulas N, Hersperger E, Dearolf C, Shearn A. Genes Dev. 1988;2:1333–1343. doi: 10.1101/gad.2.10.1333. [DOI] [PubMed] [Google Scholar]
- 32.Venturelli D, Martinez R, Melotti P, Casella I, Peschle C, Cucco C, Spampinato G, Darzynkiewicz Z, Calabretta B. Proc Natl Acad Sci USA. 1995;92:7435–7439. doi: 10.1073/pnas.92.16.7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penningroth S M, Kirschner M W. J Mol Biol. 1977;115:643–673. doi: 10.1016/0022-2836(77)90108-5. [DOI] [PubMed] [Google Scholar]
- 34.Kimura N, Shimada N. Biochem Biophys Res Commun. 1988;151:248–256. doi: 10.1016/0006-291x(88)90586-4. [DOI] [PubMed] [Google Scholar]
- 35.Lacombe M L, Jakobs K H. Trends Pharmacol Sci. 1992;13:46–48. doi: 10.1016/0165-6147(92)90020-7. [DOI] [PubMed] [Google Scholar]
- 36.Bominaar A A, Molijn A C, Pestel M, Veron M, Van Haastert P J M. EMBO J. 1993;12:2275–2279. doi: 10.1002/j.1460-2075.1993.tb05881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohtsuki K, Ikeuchi T, Yokoyama M. Biochim Biophys Acta. 1986;882:322–330. doi: 10.1016/0304-4165(86)90254-0. [DOI] [PubMed] [Google Scholar]
- 38.Randazzo P A, Northup J K, Kahn R A. Science. 1991;254:850–853. doi: 10.1126/science.1658935. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald N J, Freije J M P, Stracke M L, Manrow R E, Steeg P S. J Biol Chem. 1996;271:25107–25116. doi: 10.1074/jbc.271.41.25107. [DOI] [PubMed] [Google Scholar]
- 40.Hsu S, Huang F, Wang L, Banerjee S, Winawer S, Friedman E. Cell Growth Differ. 1994;5:909–917. [PubMed] [Google Scholar]
- 41.Pauly R R, Bilato C, Sollott S J, Monticone R, Kelly P T, Lakatta E G, Crowe M T. Cirulation. 1995;91:1107–1115. doi: 10.1161/01.cir.91.4.1107. [DOI] [PubMed] [Google Scholar]
- 42.Teng D H F, Engele C M, Venkatesh T R. Nature (London) 1991;353:437–440. doi: 10.1038/353437a0. [DOI] [PubMed] [Google Scholar]
- 43.Sturtevant A H. Genetics. 1956;41:118–123. doi: 10.1093/genetics/41.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hackstein J D. Eur J Cell Biol. 1992;58:429–444. [PubMed] [Google Scholar]
- 45.Finlin B S, Andres D A. J Biol Chem. 1997;272:21982–21988. doi: 10.1074/jbc.272.35.21982. [DOI] [PubMed] [Google Scholar]