Abstract
Background
Although memory biases for negatively valenced stimuli have been reliably associated with depression and have been postulated to play a critical role in the maintenance of this disorder, the neural bases of these biases have received little attention. In this study, we tested a model of heightened memory sensitivity for negative information in depression in which neural mechanisms that normally facilitate memory for affective material are over-recruited during encoding of negative material in depression.
Methods
We used functional magnetic resonance imaging to examine amygdala activity and functional connectivity with the hippocampus and caudate-putamen during successful encoding—as assessed by a recognition memory probe one week following scanning—of negative, neutral, and positive pictures by 14 depressed and 12 nondepressed individuals.
Results
Depressed individuals demonstrated greater memory sensitivity than did nondepressed participants to negative, but not to neutral or positive, stimuli. The right amygdala was more active and showed greater functional connectivity with the hippocampus and caudate-putamen during encoding of subsequently remembered negative, but not neutral or positive, stimuli in depressed than in control participants. The degree of memory-related right amygdala responsivity in the depressed participants was significantly correlated with depressive severity.
Conclusions
These findings support the formulation that, in remembering negative information better than nondepressed persons, depressed individuals over-recruit a neural network involved more generally in enhancing memory for affective stimuli, and that the degree to which they over-recruit this system is related to the severity of clinical symptomatology.
Keywords: Depression, Amygdala, Hippocampus, Caudate, Putamen, Memory
Introduction
Cognitive theories of depression (e.g., 1) posit that negative cognitions, derived from dysfunctional self schemas, play a central role in the etiology and course of this disorder. These dysfunctional schemas are hypothesized to bias information processing in depression, with depressed individuals selectively attending to and remembering affectively negative material. Indeed, there is strong evidence that depressed individuals are characterized by negative biases in memory, demonstrating better memory for negative material than do nondepressed individuals, (e.g., 2, 3, 4). Importantly, several theorists have proposed that selective memory for negative information in depression contributes to the duration and severity of depressive episodes, (e.g., 5, 6).
Despite these consistent findings, we know little about the neural underpinnings of enhanced memory for negatively valenced stimuli in depressed relative to nondepressed individuals. Both lesion and functional neuroimaging studies confirm that the amygdala plays an important role in bolstering memory for emotional material. Cahill and his colleagues (7, 8), for example, reported that the generally better recall of affectively valenced than of neutral information is sharply attenuated in patients with lesions confined to the amygdala. Furthermore, using fMRI, Canli found amygdala responsivity to predict subsequent memory performance for affective stimuli both across individuals (9) and across trials (10).
Several investigators have posited that the amygdala facilitates memory for emotional stimuli through modulation of the hippocampus, a structure crucial for episodic memory encoding, (e.g., 11). Packard, Cahill, and McGaugh (12), for example, showed that amygdala stimulation following training facilitated hippocampal-mediated learning in rats and was not blocked by anesthetizing the amygdala prior to a retention test, indicating that the resulting pro-mnemonic effects were not due to lasting changes within the amygdala itself. These findings of amygdala facilitation of hippocampal-dependent learning are echoed in neuroimaging studies of humans by investigators reporting a significant correlation between activation of the amygdala and hippocampus during successful encoding of affective stimuli (13, 14).
The amygdala has also been found to facilitate learning that is dependent on the putamen and caudate, (e.g., 12, 15), a structure complex centrally involved in skill learning, (e.g., 16). Packard and Teather (15), for example, found that amygdala stimulation following training in rats facilitates caudate-putamen mediated learning and, further, that these memory bolstering effects are blocked by anesthetizing the caudate-putamen following training, but not by pre-test amygdala anesthetization. Moreover, given that the amygdala and caudate-putamen comprise nodes of the affective division of the cortico-striatal-pallidal-thalamic (CSPT) loop (17), a circuit involved in the maintenance of information in working memory, (e.g., 18), investigators have posited that the amygdala-caudate-putamen system subserves emotionally-mediated working memory.
The formulation that over-active amygdala-caudate-putamen and/or amygdala-hippocampus systems underlie enhanced memory for negative information in depression is also consistent with findings that depressed individuals have been characterized by greater responsivity to negative stimuli in the amygdala (19–22), hippocampus (19) and caudate-putamen (19) than are nondepressed persons. The relevance of amygdala reactivity to memory in depression has been shown by Roberson-Nay et al. (23) who found that, unlike their nondepressed peers, depressed adolescents showed greater amygdala reactivity when viewing faces that they subsequently remembered versus faces that they subsequently forgot.
The present study was designed to test a model of enhanced memory for negative stimuli in depression in which the neural mechanisms that are involved in bolstering encoding of emotionally valenced material in general are recruited more during encoding of negative material by depressed individuals. More specifically, we test a model in which amygdala activity and consequent modulation of the hippocampus and/or the caudate-putamen is increased during successful encoding of negative stimuli in depression. Based on the literatures reviewed above, we hypothesize that depressed individuals will exhibit better memory for negative material than will nondepressed individuals, as well as greater amygdala activation during successful encoding of negative material. Finally, we predict that amygdala activation during successful encoding of negative stimuli will be more strongly correlated with activation in the hippocampus and caudate-putamen in depressed than in nondepressed participants.
Methods and Materials
Participants
Fourteen individuals diagnosed with Major Depressive Disorder (MDD; 8 females) and 12 nondepressed controls (6 females) with no history of psychiatric disorder participated in this study. Participants were recruited from local psychiatric outpatient clinics as well as through website postings. Inclusion criteria optimized diagnostic homogeneity of our depressed and nondisordered samples and required that all participants: (1) were between the ages of 18 and 50; (2) had no reported history of brain injury, lifetime history of primary psychotic ideation, social phobia, panic disorder, mania, or post-traumatic stress disorder; (3) did not meet diagnostic criteria for current generalized anxiety disorder; (4) had no reported substance abuse within the past six months; and (5) had no physical limitations that prohibited them from undergoing an fMRI examination. Nine of the depressed participants and none of the nondepressed participants were taking antidepressant medication at the time of the study; medicated depressed individuals were required to have maintained a steady antidepressant dosage for one month prior to being scanned.
All depressed participants met criteria for a DSM-IV diagnosis of MDD on the basis of the Structured Clinical Interview for DSM (SCID; 24); none of the control participants met criteria for any current or past Axis I disorder. In addition, all participants completed the Beck Depression Inventory-II (BDI-II; 25). Depressed individuals with comorbid panic disorder or social phobia were excluded from participation in the study. Informed consent was obtained from all participants, and each participant was paid $25 per hour. All aspects of this study complied with the ethical standards for treatment of human participants from the American Psychiatric Association.
Picture Encoding Task
Participants viewed stimuli in the scanner through a projector-directed mirror. The stimuli were selected from the International Affective Picture System (IAPS; 26). A schematic of the in-scanner picture encoding task, adapted from a procedure used by Canli et al. (10), is presented in Figure 1. Each trial lasted 14 seconds and was composed of: 1) picture presentation for the first 2000 ms; 2) picture intensity rating (1 – not intense, 2 – somewhat intense, 3 – quite intense, 4 – extremely intense); and 3) affective valence rating of the picture (1 – negative, 2 – neutral, 3 – positive). A response indicator light in the console room of the scanner was monitored to ensure that participants maintained attention to the task; in addition, in-scanner behavioral data were checked following scanning to ensure there were no missed trials. For the remainder of the trial, participants viewed a fixation cross. Responses were made with a four-button fMRI response box developed at The Lucas Center at Stanford University. Stimulus presentation, timing, and recording of behavioral data during scanning as well as subsequent memory assessment were controlled by a Dell PC running E-prime v1.2 (Psychology Software Tools Inc.; www.pstnet.com/eprime).
Figure 1.
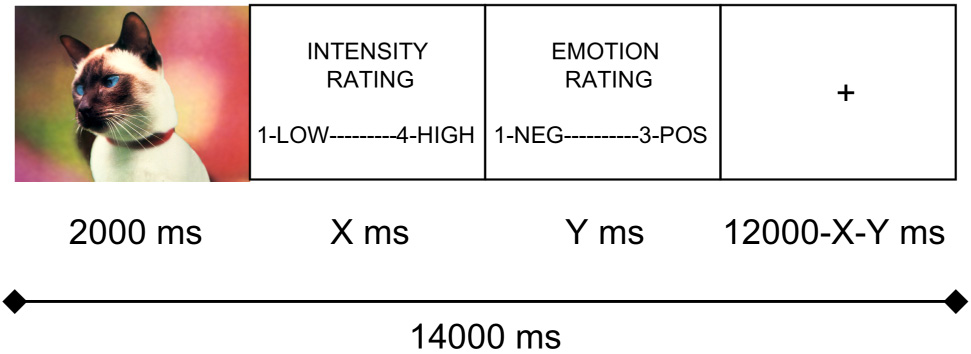
Schematic of individual fMRI memory encoding trials.
Each participant viewed 70 negative (mean normed valence: 2.60; range: 1.3 – 3.9), 70 neutral (mean normed valence: 5.05; range: 4.3 – 5.8), and 70 positive (mean normed valence: 7.30; range: 6.7– 8.3) pictures, for a total of 210 14-second trials completed over five 588-second scanning runs. Stimuli were presented in random order to each participant. Two sets of IAPS stimuli were used for this study. One set was used for the in-scanner encoding portion of the study, and the other set, of equal size and matched for normed intensity and valence, served as foil stimuli for subsequent incidental recognition memory testing; the stimulus set designated as “target” or “foil” varied randomly across participants.
Incidental Recognition Memory Task
One week after the scan, participants returned to the lab to complete the incidental recognition memory portion of the study. The 210 IAPS pictures they had seen the previous week during scanning and 210 foil IAPS pictures were used as stimuli. On each trial, participants first saw a fixation cross presented for 1000 ms that alerted them to the coming memory probe. An IAPS picture probe was then presented along with a key indicating how they should respond. In order to optimize variability in our memory measure to reflect the real variation that is present in recognition of previously seen stimuli as well as to afford us the opportunity to account for this variability in our behavioral and neural analyses, we used a three-point recognition memory probe. Participants were to press “1” if they assessed the picture as previously unseen, “2” if the picture seemed merely familiar, and “3” if participants remembered having seen the picture.
FMRI Data Acquisition
Blood-oxygen level-dependent (BOLD) data were acquired with a 1.5 T General Electric Signa MR scanner. Following scout scanning, two iterations of high order shimming were performed over the whole brain. Next, BOLD data were acquired with a single channel, whole-head imaging coil from 24 axial slices using a spiral pulse sequence (27) [repetition time (TR) = 83 ms/slice, echo time (TE) 40 ms, flip angle = 70°, field of view (FOV) = 24 cm, acquisition time = 2000 ms per frame, number of frames = 299 per run]. Axial slices had 3.75 mm2 in-plane and 4 mm through-plane resolution (with 1 mm between-slice distance). A high resolution structural scan (115 slices, 1 mm2 in-plane and 1.5 mm through-plane resolution, TE = min, flip angle = 15°, FOV = 22cm) was performed following BOLD scanning runs. Head movement was minimized by using a bite-bar formed with each participant’s dental impression.
Analyses: Recognition Memory Data
For each participant, memory sensitivity was calculated for each of the three valence categories. Individual trials from recognition memory testing were categorized as “Hits” if participants had seen the probe picture during scanning and indicated this during testing of recognition memory by assigning it a rating of “3.” Trials were categorized as “False Alarms” if participants had not seen the probe picture during the scan but assigned it a rating of “3,” indicating that they thought they had seen the picture. Hit and False Alarm rates were calculated for each subject for each valence category by dividing the number of hits and false alarms, respectively, by the total number of “3” (i.e.., ‘picture seen’) responses for a particular valence category. These rates were then used to compute sensitivity indices (d’). Given the reliable finding that depressed individuals do not remember information, in general, as well as their nondepressed counterparts (e.g., 28), we controlled for variance introduced by this general memory effect in our estimates of valence-specific memory sensitivity by dividing each participant’s valence-specific (negative and positive) d’ by their d’ for neutral information. A two-way (group repeated over valence) analysis of variance (ANOVA) was conducted on these resultant memory sensitivity indices.
Analyses: BOLD Data
Preprocessing
BOLD images were slice-time corrected using the axial slice with the greatest degree of intersection with the core nuclei of the amygdalae as the reference slice. Images were then motion corrected using a Fourier interpolation algorithm from the AFNI imaging analysis suite (National Institute of Health; http://afni.nimh.nih.gov/). Data for which sudden movement did not exceed 1mm were not corrected further. Scans for which sudden movement fell between 1mm and 3mm were corrected with a despiking algorithm from AFNI that replaced data from individual high motion acquisitions with outlier insensitive estimates. Data were then spatially smoothed with a Gaussian kernel (full width at half maximum = 4 mm) and high-pass filtered with a frequency criterion of one cycle per minute, and then converted to units of percent signal change. Finally, the BOLD data were warped to a common template space (29) to allow comparison between diagnostic groups.
Comparing memory-related amygdala reactivity across valence and diagnosis
Indices of amygdala activity for remembered relative to forgotten stimuli were obtained for positive, negative, and neutral stimuli for each participant. Response amplitude differences for subsequently remembered versus forgotten stimuli were calculated as follows: 1) For each valence, delta functions were computed according to the rule that a picture-viewing event that generated a rating of “3” (picture was seen) during the recognition memory task received a value of 1, and a picture-viewing event that generated a rating of “1” (picture was not seen) during recognition memory testing was given a value of −1; 2) Resulting delta functions for each participant for each valence were convolved with a gamma function to render memory-relevant covariates for fitting with amygdala BOLD timecourses; 3) A least-squares data-fitting procedure (AFNI’s 3dDeconvolve) was conducted on the memory covariates individually, first accounting for nuisance covariates.
To compare the resulting indices of amygdala responsivity to subsequently remembered versus forgotten stimuli as a function of group and valence, two-way (group repeated over valence) ANOVAs were conducted on a voxel-wise basis within the amygdalar region of interest (ROI). The statistical threshold was set at p = .05, corrected, for this analysis and analyses subsequently described. Statistical significance of these comparisons was calculated with the AFNI program AlphaSim, which estimates null hypothesis distributions via multiple Monte Carlo simulations. Probability values for any pairwise contrasts in which the direction of effect was predicted by our hypotheses were calculated as one-tailed; otherwise, p values were calculated as two-tailed.
Calculating psychophysical interaction between amygdala seed regions and the hippocampus and caudate-putamen
We used a procedure similar to that described by Heekeren et al. (30) to calculate the degree of psychophysical interaction between amygdala seed regions and the hippocampus and caudate-putamen. This approach differs from resting-state connectivity analyses in that it permits the calculation of context-dependent correlations in BOLD signal between structures in order to detect task- or performance-dependent co-activity. We implemented this procedure as follows: First, for each participant, an amygdala timecourse was extracted and nuisance covariates were removed. Next, for each valence condition, the resulting “clean” amygdala timecourse was multiplied, on a timepoint-by-timepoint basis, by a gamma function-convolved delta function contrasting successful and unsuccessful encoding events. The fit of the resulting task-by-amygdala timecourse with voxel timecourses within hippocampal and caudate-putamen ROIs was then calculated. A two-way (group repeated over valence) ANOVA was conducted on the resulting fit coefficients at each hippocampus and caudate-putamen voxel.
Results
Participant Characteristics
Table 1 presents the demographic and clinical characteristics of the depressed and nondepressed participants. The two groups of participants did not differ with respect to age, t(24) = 1.22, education t(24) = .17 or gender composition, X2(1,24) = .48, all ps > .05. As expected, the depressed participants had higher scores on the BDI-II than did the nondepressed participants, t(24) = 8.26, p < .05. Table 2 presents additional characteristics of our depressed sample, including antidepressant medication (if any) taken, medication dosage, length of medication period, number of depression-related hospitalizations, duration of current depressive episode, time since first onset of depressive illness, and BDI score.
Table 1.
Participant demographic and clinical data; mean +/− standard error
| Control | Depressed | |
|---|---|---|
| Age | 31.4 +/− 10.2 | 36.5 +/− 10.3 |
| Education | 15.43 +/− 2.6 | 15.27 +/− 1.7 |
| % Female | 50% | 57% |
| BDI-II | .91 +/− 1.4 | 27.6 +/− 10.6 |
Table 2.
Depressed participants: Pharmacological and clinical data
| Participant | Medication and Daily Dosage | Duration of Medication | MDD-related Hospitalizations | Duration of Current Episode (months) | Years Since First Episode | BDI |
|---|---|---|---|---|---|---|
| MDD1 | Venlafaxine (300 mg) | 4 months | 0 | 21 | 29 | 15 |
| MDD2 | none | - | NR | NR | NR | 14 |
| MDD3 | Venlafaxine (450 mg) | 2 years | 1 | 1 | 2 | 24 |
| MDD4 | none | - | 0 | 6 | 25 | 13 |
| MDD5 | Escitalopram; Bupropion (dosage NR) | 1.5 years; 4 years | 0 | 6 | 18 | 45 |
| MDD6 | Venlafaxine (150 mg) | 3 years | 0 | 5 | 20 | 34 |
| MDD7 | Duloxetine (40 mg), Bupropion (300 mg) | 1 year; 1 month | 0 | 36 | NR | 25 |
| MDD8 | none | - | 0 | 8 | 25 | 15 |
| MDD9 | Sertraline (100 mg) | 3 months | 0 | 2 | 2 | 41 |
| MDD10 | none | - | 0 | 10 | 7 | 33 |
| MDD11 | none | - | 0 | 4 | 15 | 39 |
| MDD12 | Venlafaxine (225 mg); Bupropion (300 mg) | 5 months; 5 months | 0 | 14 | 1 | 27 |
| MDD13 | Venlafaxine (150 mg) | 2 years | 0 | 28 | 11 | 33 |
| MDD14 | Venlafaxine (75 mg); Bupropion (100 mg) | 1 year; 1 month | 0 | 16 | 5 | 28 |
Note. NR, not reported.
Intensity Ratings
A two-way (group repeated over valence) ANOVA conducted on stimulus intensity ratings recorded during scanning yielded only a significant main effect of valence, F(2,21) = 80.5, p < .05. Paired samples t-tests contrasting intensity ratings as a function of valence indicate that participants rated negative stimuli as more intense than both neutral, t(25) = 14.23, and positive stimuli, t(25) = 6.41, and positive stimuli as more intense than neutral stimuli t(25) = 5.39, all ps < .05 (see Figure 2 for graphs of these results).
Figure 2.
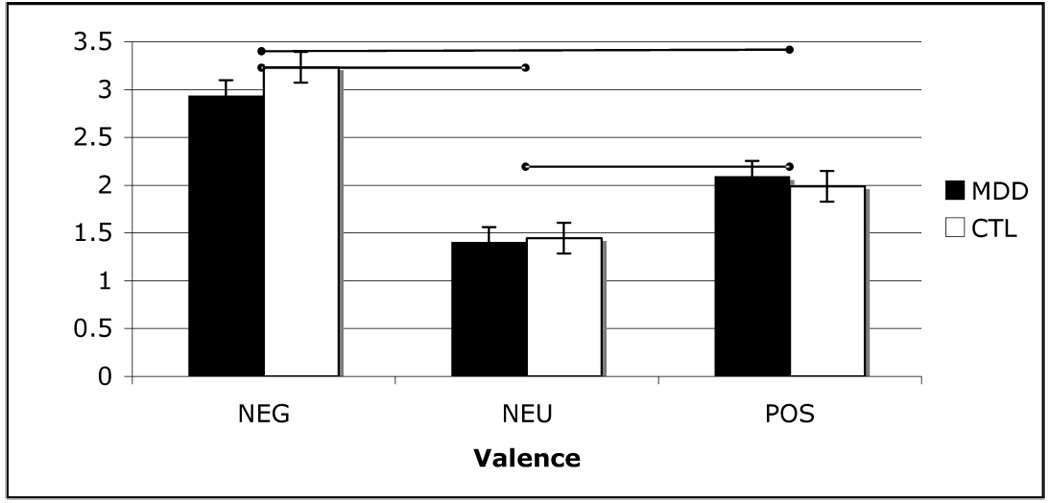
Mean intensity ratings with standard error bars for positive (POS), neutral (NEU) and negative (NEG) stimuli in depressed (MDD) and control (CTL) groups. Mean values connected by bars are significantly different.
Recognition Memory Performance
A two-way (group repeated over valence) ANOVA was conducted on memory sensitivity estimates. No main effects for group, F(1,23) = 2.73, valence, F(1,23) = .47,or their interaction, F(1,23) = 2.73, were obtained, all ps > .05., We then examined differences in specific means to test our a priori hypotheses concerning group performance as a function of valence. These analyses indicated that whereas depressed participants exhibited greater memory sensitivity than did nondepressed controls for negative stimuli, t(24) = 1.88, p < .05, the two groups did not differ in memory performance for positive stimuli, t(24) = 1.17, p > .05. Within-groups contrasts yielded no difference in memory for negative relative to positive stimuli in the depressed group, t(13) = .50. In contrast, the nondepressed participants remembered positive stimuli better than they did negative stimuli, t(11) = 3.01, p < .05. These results are presented graphically in Figure 3.
Figure 3.
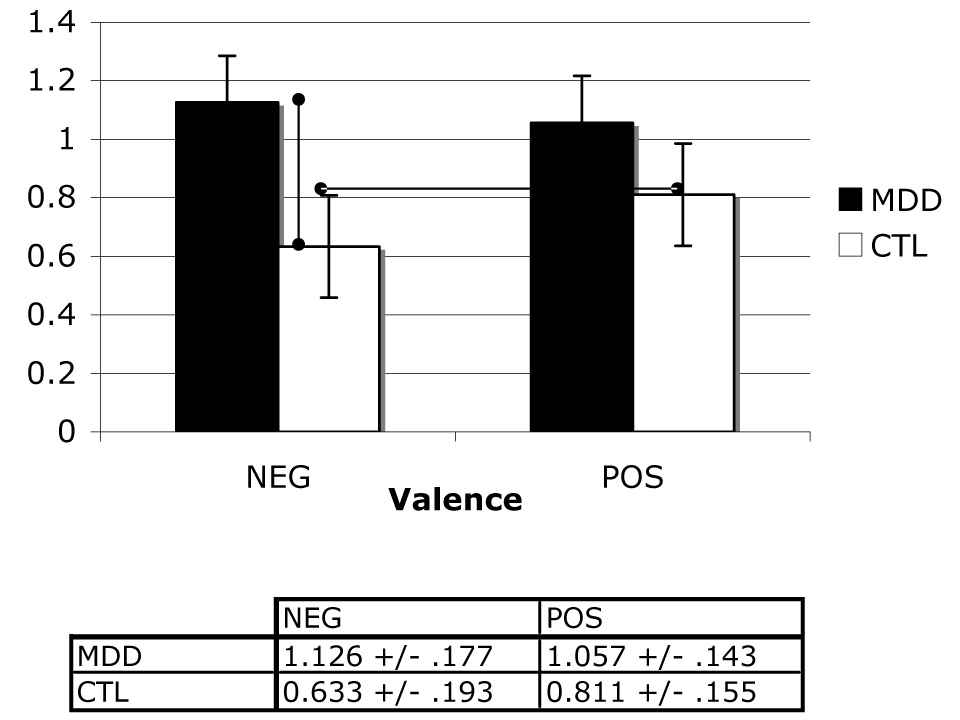
Mean normalized memory sensitivity scores across levels of group and valence factors.
Amygdala ROI Results
Two-way ANOVAs conducted on contrast estimates from the comparison of successful to unsuccessful encoding trials in left amygdala voxels yielded nonsignificant results (peak left amygdala voxel: group, F(1,21) = 1.25; valence, F(2,21) = .23; group by valence interaction, F(2,21) = .65, all ps > .05). The same analysis conducted on voxels within the right amygdala yielded a nonsignificant effect for group, F(1,21) = 1.86, p > .05, and a significant main effect for valence, F(2,21) = 4.92, p < .05, which was qualified by a significant interaction of group and valence F(2,21) = 3.35, p < .05 (all statistics reported from peak right amygdala voxel). Follow-up tests indicated that depressed participants exhibited greater right amygdala responsivity during successful relative to unsuccessful encoding than did nondepressed participants for negative material, t(24) = 2.49, p < .05, but not for neutral or positive material, t(24) = .86 and .09, respectively, both ps > .05. Importantly, this group difference in memory-related responsivity to negative material in the right amygdala was driven by greater amygdala reactivity in depressed than in nondepressed participants to subsequently remembered stimuli, t(24) = 2.32, p < .05, and not by decreased responsivity in depressed participants to subsequently forgotten stimuli, t(24) = .09, p > .05. In addition, within the depressed group, right amygdala responsivity during successful relative to unsuccessful encoding was greater for negative than for both neutral, t(13) = 3.82, p < .05, and positive, t(13) = 2.529, p < .05, stimuli, which did not differ significantly from each other, t(13) = .311, p > .05. In contrast, within the nondepressed group, memory-related right amygdala responsivity did not differ as a function of stimulus valence, all t(11) < 1.60, all ps > .05. These results are presented graphically in Figure 4. Finally, although the subsample sizes are relatively small, it is important to note that Kruskall-Wallis tests — a nonparametric test appropriate for use with small samples that is more sensitive to between-group differences than are ANOVA statistics (31) — yielded no significant differences between medicated and unmedicated MDD participants in memory-related right amygdala responsivity for negative, neutral, or positive stimuli, all ps > .05.
Figure 4.
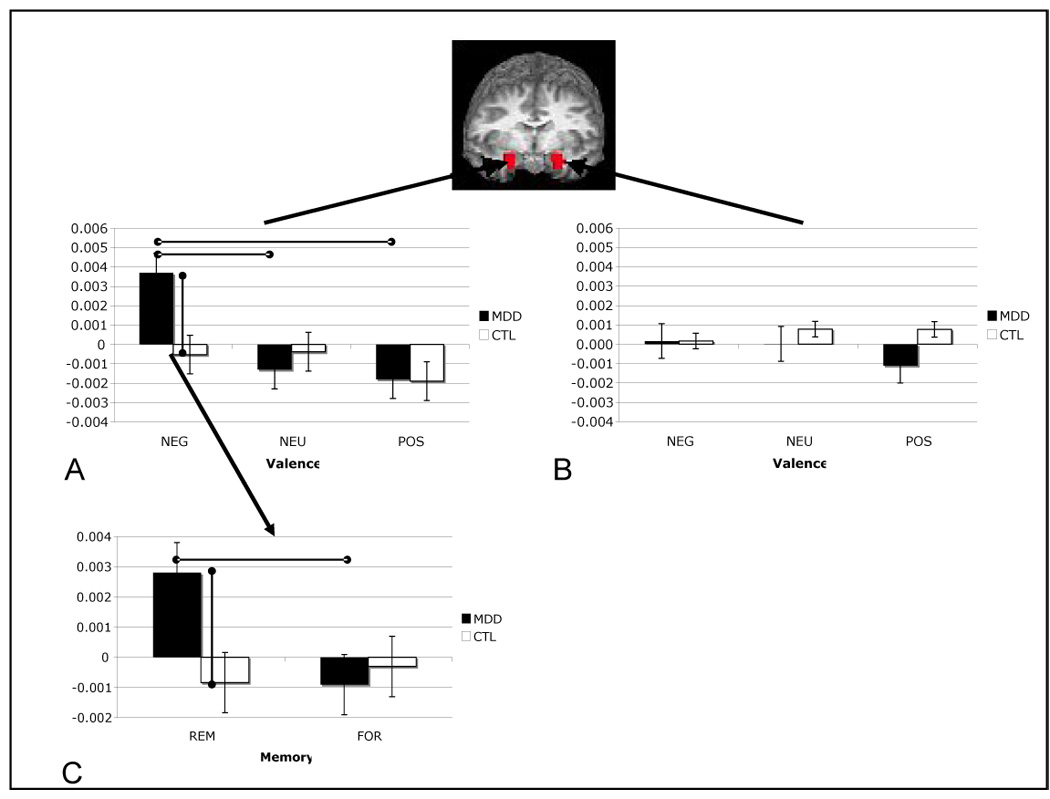
Mean contrast coefficient values from remembered (REM) versus forgotten (FOR) contrast across valence and group variables in peak left amygdala (A; −19, −4, −12) and right amygdala (B; 17, −5, −12) voxels, and REM versus fixation and FOR versus fixation for negative stimuli in each group (C).
Psychophysical Interaction Results
Psychophysical interaction of amygdala with hippocampus
Two-way (group repeated over valence) ANOVAs were conducted on indices of psychophysical interaction with the amygdala at each hippocampal voxel. No effects of group or valence or the interaction of these factors were sufficiently large to satisfy the statistical correction imposed by examining all voxels within this ROI. To decrease the magnitude of the correction factor to our significance threshold, we examined a smaller set of anterior hippocampal voxels found to correlate with the amygdala during effective encoding of affective stimuli (13). While omnibus tests of group and valence effects, and their interaction were not statistically significant, F(1,21) = .91, F(2,21) = .93, F(2,21) = 1.17, respectively, at peak voxel, exploratory between-group contrasts revealed that the degree of psychophysical interaction of the amygdala with the hippocampus was greater in the depressed than in the nondepressed participants during successful encoding of negative, t(24) = 1.84, p < .05, but not of neutral or positive stimuli, t(24) = .44 and 1.05, respectively, both ps > .05. No significant within-group effects of valence were obtained, all ps > .05. These results are presented in Figure 5.
Figure 5.
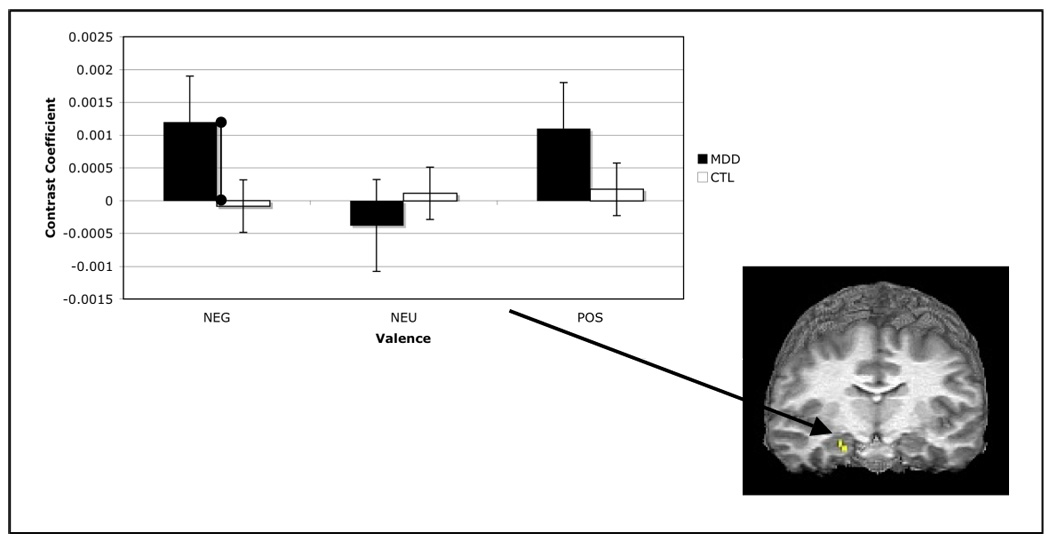
Mean contrast coefficients from analysis of psychophysical interaction between right amygdala and right hippocampus for each level of group and valence. Values shown are from peak hippocampal voxel (22, −11, −12).
Psychophysical interaction of amygdala with caudate-putamen
Analyses of the correlation of memory-related activity in the right amygdala with activation in voxels comprising ipsilateral caudate and putamen showed significant main effects within the right putamen for both group, F(1,24) = 11.54, and valence, F(2,24) = 3.83, both ps < .05, the interaction of group and valence, however, was not significant F(2,24) = 2.67, p < .05. Follow-up tests showed a greater memory-related correlation between the right amygdala and right putamen for depressed than for nondepressed participants for negative, t(24) = 3.55, p < .05, but not for neutral or positive stimuli, ts(24) = 1.22 and .59, respectively, both ps > .05. Further comparisons indicated that, within the depressed group, the amygdala-putamen correlation was greater for negative than for positive stimuli, t(13) = 3.55, p < .05, but not for negative relative to neutral stimuli, t(13) = 1.56, p > .05, or for neutral relative to positive stimuli t(13) = 1.69, p > .05, although the latter two comparisons did approach statistical significance. Within the nondepressed group, the memory-related amygdala-putamen correlation was lower for negative and positive stimuli than for neutral stimuli, ts(11) = 1.99 and 2.77, respectively, both ps < .05; correlations for positive and negative stimuli did not differ from each other, t(11) = 2.78, p < .05. These results are presented in Figure 6.
Figure 6.
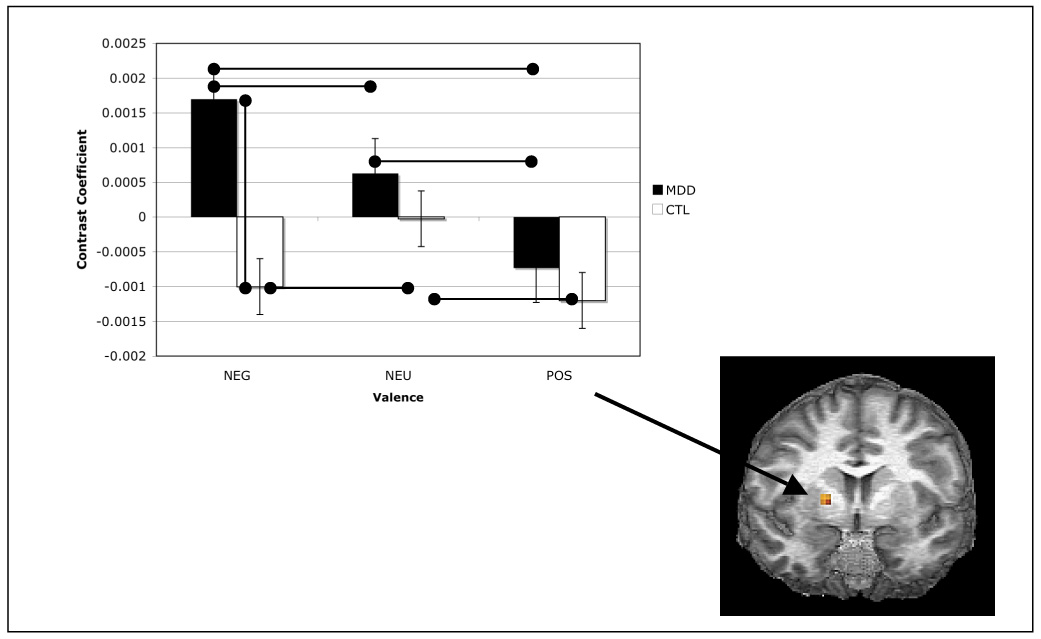
Mean contrast coefficients from analysis of psychophysical interaction between right amygdala and right caudate-putamen for each level of group and valence. Values shown are from peak caudate-putamen voxel (17, 5, 6).
Correlation of Depressive Severity with Amygdala Responsivity
Finally, the severity of depression within the MDD group, as assessed by BDI-II scores, was significantly correlated with memory-related right amygdala activation in response to negative stimuli, r(13) = .63, p < .05, see Figure 7, but not in response to neutral, r(13) = .12, or to positive stimuli, r(13) = −.09, both ps > .05.
Figure 7.
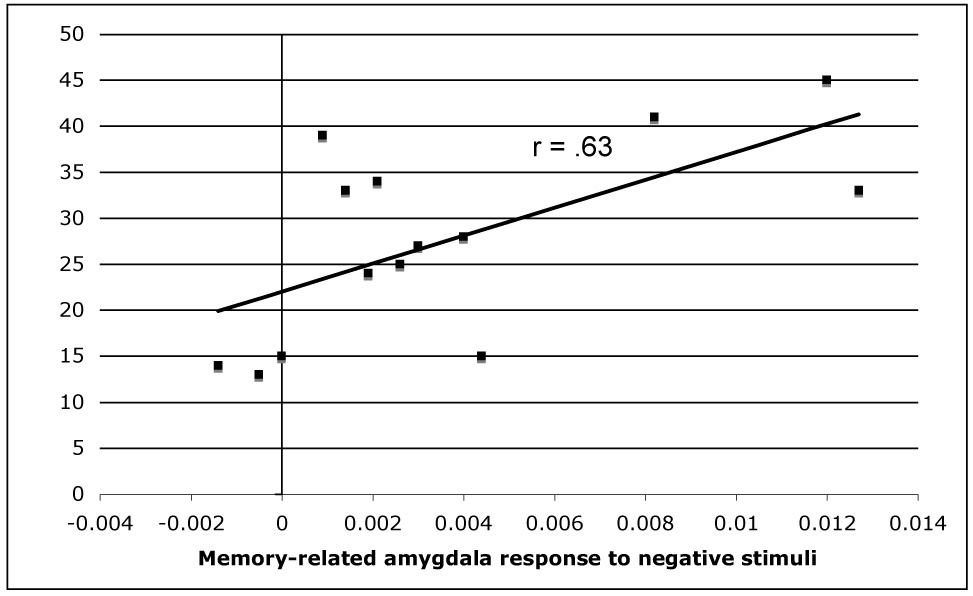
Scatter plot showing positive correlation between BDI-II score in depressed participants and memory-related right amygdala responsivity to negative stimuli.
Discussion
The present study was designed to test a neural model of enhanced memory for negative stimuli in depression. We report behavioral data that replicate previous findings showing better memory for negative information in diagnosed depressed than in nondepressed individuals. We also demonstrate that, compared to their nondepressed counterparts, depressed individuals are characterized by increased activity in the right amygdala during successful encoding of negative, but not of neutral or positive, stimuli. Finally, we find that during successful encoding only of negative stimuli was activity in the right amygdala correlated with activity in both ipsilateral caudate-putamen and hippocampus more strongly in depressed than in nondepressed participants. Taken together, these findings provide support for a neural model of enhanced memory for negative material in depression in which, as they encode negative information, depressed persons over-activate a neural system that subserves encoding of affective material more generally.
This fMRI study is the first to examine the neural substrates of the negative memory bias that has been found in behavioral studies with depressed adults. The present data advance our understanding of depression by elucidating the neural substrates of a consistently reported negative memory bias in this disorder, a process postulated to contribute to the severity of depressive episodes, (e.g., 5, 6). Indeed, this formulation is supported by the finding that severity of depression was significantly correlated with amygdala activity during encoding of negative stimuli that were remembered a week later.
An important aspect of the present findings concerns the specificity of amygdala responsivity and connectivity in depression. Activation differences between depressed and control participants were found for the encoding of subsequently remembered negative, but not positive, stimuli despite the fact that positive stimuli also were rated as more intense than neutral stimuli. Thus, the amygdala responsivity exhibited by depressed participants in response to successfully encoded negative material was not simply reflecting an intensity effect. Moreover, these results do not appear to be related to medication status. Comparisons of amygdala responsivity in medicated and unmedicated MDD participants yielded no significant effects. While the relatively small subsamples in these comparisons dictate that we use caution in interpreting these results, they are nonetheless consistent with the formulation that medicated and unmedicated depressed participants do not differ in memory-related amygdala responsivity.
It is noteworthy that whereas Canli et al. reported greater amygdala activity during effective encoding of affective stimuli in unselected participant samples (e.g., 9, 10) the nondepressed participants in the present study did not exhibit this pattern of activation. This discrepancy may be due to the fact that the nondepressed participants in the present study were selected to have no current or past Axis I disorder and, consequently, were likely characterized by lower levels of psychopathology or distress than were the samples studied by Canli and his colleagues. This is an important consideration in selecting criteria for control groups in psychopathology research, and investigators might examine this formulation more explicitly and systematically in future research.
Investigators working to elucidate the neural substrates of the negative memory bias in depression could expand the neural model presented here by examining the neural underpinnings of both encoding and retrieval processes. It will also be important to design studies that will permit inferences about causality and directionality of influence to be incorporated into neural models of depressotypic processes. For example, the advent of real-time neurofeedback techniques, in which participants can learn to modulate activity in structures such as the amygdala (32) in making corresponding changes to thought and behavior, holds promise that the role of the amygdala in the increased memory sensitivity for negative information in depression may be more clearly elucidated.
Acknowledgements
We thank Hannah Kang and Lauren Atlas for their help with analysis of the data presented in this manuscript. We also thank Anthony Wagner for insights concerning the execution of this study and analysis of the data. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Preparation of this manuscript was supported by Grant MH59259 from the National Institute of Mental Health awarded to Ian H. Gotlib.
A preliminary version of the research detailed in this manuscript was presented at the Annual Meeting for Biological Psychiatry, San Diego, CA, April 17–19, 2007.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Both authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Beck AT. Cognitive therapy and the emotional disorders. New York: International Universities Press; 1976. [Google Scholar]
- 2.Bradley BP, Mogg K, Williams R. Implicit and Explicit Memory for Emotion-Congruent Information in Clinical Depression and Anxiety. Behaviour Research and Therapy. 1995;33(7):755–770. doi: 10.1016/0005-7967(95)00029-w. [DOI] [PubMed] [Google Scholar]
- 3.Ridout N, Astell AJ, Reid IC, Glen T, O'Carroll RE. Memory bias for emotional facial expressions in major depression. Cognition & Emotion. 2003;17(1):101–122. doi: 10.1080/02699930302272. [DOI] [PubMed] [Google Scholar]
- 4.Watkins PC, Mathews A, Williamson DA, Fuller RD. Mood-Congruent Memory in Depression - Emotional Priming or Elaboration. Journal of Abnormal Psychology. 1992;101(3):581–586. doi: 10.1037//0021-843x.101.3.581. [DOI] [PubMed] [Google Scholar]
- 5.Ingram RE. Toward an Information-Processing Analysis of Depression. Cognitive Therapy and Research. 1984;8(5):443–477. [Google Scholar]
- 6.Teasdale JD. Negative Thinking in Depression - Cause, Effect, or Reciprocal Relationship. Advances in Behaviour Research and Therapy. 1983;5(1):3–25. [Google Scholar]
- 7.Adolphs R, Cahill L, Schul R, Babinsky R. Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learning & Memory. 1997;4(3):291–300. doi: 10.1101/lm.4.3.291. [DOI] [PubMed] [Google Scholar]
- 8.Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The Amygdala and Emotional Memory. Nature. 1995;377(6547):295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- 9.Canli T, Zhao Z, Desmond JE, Glover G, Gabrieli JDE. fMRI identifies a network of structures correlated with retention of positive and negative emotional memory. Psychobiology. 1999;27(4):441–452. [Google Scholar]
- 10.Canli T, Zhao Z, Brewer J, Gabrieli JDE, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience. 2000;20(19) doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinvorth S, Levine B, Corkin S. Medial temporal lobe structures are needed to re-experience remote autobiographical memories: evidence from HM and WR. Neuropsychologia. 2005;43(4):479–496. doi: 10.1016/j.neuropsychologia.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Packard MG, Cahill L, McGaugh JL. Amygdala Modulation of Hippocampal-Dependent and Caudate Nucleus-Dependent Memory Processes. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(18):8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42(5):855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- 14.Kensinger EA, Corkin S. Two routes to emotional memory: Distinct neural processes for valence and arousal. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Packard MG, Teather LA. Amygdala modulation of multiple memory systems: Hippocampus and caudate-putamen. Neurobiology of Learning and Memory. 1998;69(2):163–203. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- 16.Atallah HE, Lopez-Paniagua D, Rudy JW, O'Reilly RC. Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nature Neuroscience. 2007;10(1):126–131. doi: 10.1038/nn1817. [DOI] [PubMed] [Google Scholar]
- 17.Alexander GE, Delong MR, Strick PL. Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 18.Levy R, Friedman HR, Davachi L, GoldmanRakic PS. Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. Journal of Neuroscience. 1997;17(10):3870–3882. doi: 10.1523/JNEUROSCI.17-10-03870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu CHY, Williams SCR, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment - A prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61(9):877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 20.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biological Psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 21.Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: Assessment of sustained event-related fMRI amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 22.Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SCR, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry. 2005;57(3):201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, Charney DS, Leibenluft E, Blair J, Ernst M, Pine DS. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An fMRI study. Biological Psychiatry. 2006;60(9):966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-III-R Personality-Disorders (SCID-I) Journal of Personality Disorders. 1995;9(2):83–91. [Google Scholar]
- 25.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. 1979 [Google Scholar]
- 26.Lang PJ, Greenwald MK. Gainesville: Center for Research in Psychophysiology., University of Florida; International affective picture system standardization procedure and results for affective judgments: technical reports 1A–1C. 1993
- 27.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 28.Burt DB, Zembar MJ, Niederehe G. Depression and Memory Impairment - a Metaanalysis of the Association, Its Pattern, and Specificity. Psychological Bulletin. 1995;117(2):285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- 29.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany: Thieme; 1988. [Google Scholar]
- 30.Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG. A general mechanism for perceptual decision-making in the human brain. Nature. 2004;431(7010):859–862. doi: 10.1038/nature02966. [DOI] [PubMed] [Google Scholar]
- 31.Zimmerman DW. Statistical significance levels of nonparametric tests biased by heterogeneous variances of treatment groups. Journal of General Psychology. 2000;127(4):354–364. doi: 10.1080/00221300009598589. [DOI] [PubMed] [Google Scholar]
- 32.Posse S, Fitzgerald D, Gao KX, Habel U, Rosenberg D, Moore GJ, Schneider F. Real-time fMRI of temporolimbic regions detects amygdala activation during single-trial self-induced sadness. Neuroimage. 2003;18(3):760–768. doi: 10.1016/s1053-8119(03)00004-1. [DOI] [PubMed] [Google Scholar]


