Abstract
Rotavirus is the major cause of severe dehydrating diarrhoea in young children worldwide. Considerable research has been carried out on rotavirus disease in India. This review collated data from 46 epidemiological studies to determine rotavirus positivity rates and genotypes of infecting rotavirus strains from various settings in India. Studies on diarrhoea presenting to hospitals, neonatal rotavirus infections, symptomatic and asymptomatic infections in the community and nosocomial enteric infections were included. Rotavirus positivity rates varied greatly between different settings - diarrhoea hospitalizations (20%), neonatal infections (35%), symptomatic and asymptomatic infections in the community (15.1% and 6.3% respectively) and nosocomial enteric infections (22.5%). Among diarrhea hospitalizations, the commonest G types were G1 and G2 while commonest P types were P[8], P[6] and P[4]. Region specific neonatal infections by bovine-human reassortants have been reported, in addition to several recently described unusual strains, which may be evidence of zoonotic infection and/or reassortment. The emergence of several new strains highlights the need for intensive strain surveillance before and after the introduction of a new vaccine.
Keywords: Genotyping, India, rotavirus
Rotaviruses are the major cause of severe gastroenteritis in infants and young children worldwide. Since the first description in humans in 1973, and their subsequent recognition as a major human pathogen, there have been a large number of studies on the structure, pathogenesis and epidemiology of these viruses. Their clinical relevance, structural complexity and unique morphogenesis strategies have prompted extensive research on these viruses in recent years, using molecular biological techniques1.
It is estimated that rotavirus is responsible for 611,000 deaths annually with 80 per cent of these taking place in poorer countries2. Vaccines offer the most promising tool for preventing morbidity and mortality caused by rotavirus. The first licensed rotavirus vaccine was withdrawn due to a temporal association with intussusception3. Efforts to develop new, safer vaccines are now underway with two vaccines having been licensed in Mexico and the United States after extensive safety trials4. Testing of candidate vaccines derived from two rotavirus strains which caused asymptomatic infections in neonates in India are also being carried out. The potential availability of rotavirus vaccines in the near future, including recently licensed vaccines from Merck and Glaxo SmithKline5,6 as well as vaccines currently in development in India highlights the need to better define the epidemiology and disease burden associated with rotavirus. The epidemiological profile of these viruses in India will be of considerable importance to both policy makers and vaccine developers in determining the composition, dosage and schedule for a vaccine to be used in India.
Structure and classification
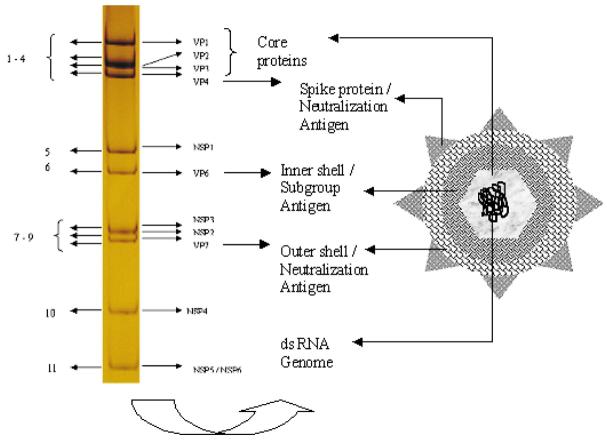
Rotaviruses are double stranded RNA viruses comprising a genus within the family Reoviridae. The mature virus particles are triple layered, approximately 70 nm in diameter, and possess icosahedral symmetry. The rotavirus genome consists of 11 segments of double-stranded RNA, that code for 6 structural viral proteins (VP1, VP2, VP3, VP4, VP6 and VP7) and 6 non-structural proteins (NSP1 - NSP6)7, with gene segment 11 encoding both NSP5 and 6. The genome is encompassed by an inner core consisting of VP2 with small amounts of VP1 and VP3 proteins. The intermediate layer or inner capsid is made of VP6, which determines group and subgrouping specificities. The outer capsid layer is composed of two proteins, VP7 and VP4 that elicit neutralizing antibody responses (Fig. 1).
Fig. 1.
Structure of rotavirus. Coding assignments of 11 RNA segment (left) and schematic diagram of ratavirus (right).
Rotaviruses are classified by a scheme of groups and multiple serotypes/genotypes within each group. The classification of rotavirus into seven different groups (A-G) is based on the antigenic specificity of the VP6 capsid proteins, as well as on the pattern of electrophoretic mobility of the 11 RNA segments of the viral genome. Of the seven groups, only groups A, B and C are known to infect humans. Severe, life-threatening disease in children worldwide is caused predominantly by group A rotaviruses. Within group A, four different subgroups (SG); SGI, SGII, SGI and II, and nonI/nonII, have been distinguished on the basis of VP6 diversity, of which human strains are possibly only from SGI or SGII8.
Further typing schemes to describe rotavirus strains are based on the proteins of the outer capsid that elicit neutralizing antibodies- VP7 (G serotypes) and VP4 (P serotypes). G- and P- serotypes were defined based on their reactivity to specific monoclonal antibodies (MAbs). While the use of MAbs for VP7 was relatively easy, cross reactivity between serotypes precluded use of MAbs for VP4 serotyping. Variability in the genes encoding the two outer capsid proteins VP7 and VP4 form the basis of the current strain typing of group A rotaviruses into G and P genotypes respectively. All known G serotypes correspond with genotypes; more P genotypes than serotypes have been identified. Currently, a rotavirus strain is identified by a G genotype, indicated by a number, followed by its P type. To distinguish strains identified by P genotyping from those identified by P serotyping, the dual serotype/genotype nomenclature is used. P genotypes are expressed as P followed by a number in square brackets whereas P serotypes are designated as P with serotype number, followed by corresponding genotype in square brackets (e.g., the same strain could be represented as G9P[6], when both G and P genotypes are used, or G9P2A[6] when G genotype is followed by P serotype/genotype classification). At least 15 G genotypes and 25 P genotypes have been identified to date9.
Rotavirus detection and strain characterization
Laboratory procedures for diagnosis of rotavirus include electron microscopy (EM), passive latex agglutination assays (LA), electropherotyping using polyacylamide gel electrophoresis (PAGE), enzyme-linked immunosorbent assays (ELISA) and reverse transcription - polymerase chain reaction (RT-PCR)10. In recent years, ELISA has become the method of choice for screening. The sensitivity of routine diagnostics is high since the amount of virus excreted by a child with rotavirus diarrhoea (∼1010 viruses/g of stool) far exceeds the level of detection (∼107 viruses/g of stool)11.
Early studies on strain surveillance identified rotavirus serotypes using neutralization assays. Monoclonal antibodies to specific serotypes were used. New methods have greatly improved data on circulating rotavirus strains and include multiplex RT-PCR based genotyping, hybridization assays and nucleotide sequencing.
The rapid evolution of rotaviruses by a variety of mechanisms provides one of the major challenges in epidemiological studies. Interspecies transmission plays an important role in the diversity of rotaviruses. The ability of rotaviruses to reassort during co-infections results in the exchange of genetic material between human and animal viruses and generates novel viruses. Different mechanisms have been used to describe the evolution of rotaviruses. These include genetic drift, wherein accumulation of point mutations generates genetic lineages leading to the emergence of antibody escape mutants, and genetic shift through gene reassortment during dual infection of a single cell12. Hence methods of virus typing need to be regularly monitored and updated to identify emerging novel strains of epidemiological importance.
Rotavirus epidemiology in India
Considerable research has been carried out on rotavirus disease in India in different settings. Collation of data from these studies is frequently not possible due to the differences in study design, populations examined, and the testing methodologies used. The studies have, however, documented the marked diversity of rotavirus strains circulating in India, as well as the prevalence of unusual strains. Since the introduction and wider use of molecular biology-based typing methods, strains with G and P genotypes other than those considered common have increasingly been reported. G1, G2 and G4 strains are seen in almost all parts of the country, while there are few reports on G3 strains. In the past 5 years, four VP7 (G6, G8, G10 and G12) and one VP4 genotype (P[19]) have been newly identified as causes of diarrhoea in humans13.
A review in 2001 presented data from studies published from India on rotaviral disease14, but provided only the limited genotyping information available at the time. A review published in 2003 described the distribution of rotavirus in various settings in India published till 200215, but does not include a number of very recent publications describing unusual strains, particularly those that are potentially zoonotic in origin. In this review, we included studies published from 1990 to 2005 to collate data on the molecular epidemiology of rotaviruses in India. The studies were categorized as (i) rotavirus diarrhoea presenting to hospitals, (ii) neonatal rotavirus infections, (iii) rotavirus infection in the community, and (iv) nosocomial rotavirus infections. Studies in each group were then grouped by city for analysis.
For data on neonatal rotavirus infections and hospitalization due to rotavirus infection, studies published from 1990 to the present time were included. Since limited data are available on rotavirus in the community, all papers published on community rotavirus infections were included. For data on burden of rotavirus disease, all studies were included irrespective of detection technique used. To determine the G and P types circulating in India, only studies that used serotyping, hybridization assays or RT-PCR for G and P characterization of strains were included. Characterization data from studies using electropherotyping were not included, if it was not possible to also obtain data on G and P types from the studies.
Rotavirus diarrhoea in children hospitalized with acute gastroenteritis
A total of 29 studies carried out using samples obtained from children presenting with diarrhoea to a health care facility were included.
Rotavirus disease in hospitalized children
Data from 22 Indian cities are shown in Table I16-43. A total of 15,476 samples have been tested. Rates of rotavirus positivity ranged from 6 to 45 per cent (median 20.8%). The studies were carried out in various geographic locations in India. Most studies used ELISA and/or PAGE for the screening of rotavirus although latex agglutination assay, immunoblot and electron microscopy were also used in a limited number of studies.
Table I.
Burden of rotavirus disease in hospitalized children
| Centre | Year of study | Age (yr) | Testing method | Samples | ||
|---|---|---|---|---|---|---|
| Tested | Positive | |||||
| n | n | % | ||||
| North India: | ||||||
| Chandigarh16 | 1982 - 1985 | <5 | ELISA | 694 | 111 | 15.9 |
| Delhi17 | 1987 - 1989 | <5 | ELISA | 978 | 176 | 18.0 |
| Delhi18 | 1987 - 1988 | <5 | ELISA | 288 | 44 | 15.3 |
| Delhi19 | 1988 - 1990 | <5 | ELISA | 990 | 104 | 10.5 |
| Delhi20 | 1989 - 1990 | <3 | Immunodot | 400 | 23 | 6.0 |
| Delhi21 | 1990 - 1991 | <5 | ELISA/PAGE | 450 | 60 | 13.3 |
| Delhi22 | 1990 | <5 | ELISA | 157 | 71 | 45.0 |
| Delhi23 | 1998 - 2000 | <5 | ELISA/PAGE | 1172 | 158 | 13.5 |
| Delhi24 | 2000 - 2001 | <5 | ELISA | 584 | 137 | 23.5 |
| Delhi25 | 1998 - 2000 | <3 | ELISA/PAGE | 560 | 100 | 17.8 |
| North India26 | NK | NK | ELISA | 172 | 32 | 19.0 |
| Western India: | ||||||
| Bombay27 | 1984 - 1986 | NK | LA /EM / ELISA | 273 | 63 | 21.0 |
| Pune28 | 1990 - 1993 | children | ELISA | 722 | 188 | 26.0 |
| Pune29 | 1992 - 1996 | <5 | ELISA | 945 | 266 | 28.2 |
| Eastern India: | ||||||
| Dibrugarh30 | 1999 - 2000 | <5 | ELISA | 202 | 47 | 23.3 |
| South India: | ||||||
| Bangalore31 | 1988 - 1994 | NK | PAGE | 694 | 150 | 21.6 |
| Chennai32 | 1997 - 1999 | 0 - 2, >2 | ELISA | 245 | 51 | 20.8 |
| Chennai33 | 1995 - 1999 | <3 | ELISA | 745 | 168 | 22.6 |
| Hyderabad34 | 1998 - 1999 | <2 | PAGE | 352 | 57 | 16.2 |
| Manipal35 | NK | <5 | LA | 106 | 19 | 18 |
| Mysore31 | 1993 - 1994 | NK | PAGE | 447 | 50 | 11.2 |
| Tirupati36 | 1991 | <2 | ELISA | 170 | 40 | 23.5 |
| Vellore37 | 1995 - 1998 | <5 | ELISA / LA | 602 | 126 | 20.9 |
| Vellore38 | 2002 - 2004 | <5 | ELISA/ LA | 343 | 94 | 27.1 |
| Multiple**: | ||||||
| Multiple 139 | 1993 | 6m to <5 | ELISA | 458 | 63 | 13.7 |
| Multiple 240 | 1996 - 1998 | <5 | ELISA | 1502 | 313 | 20.8 |
| Multiple 341 | 1998 - 1999 | <5 | LA /EM / ELISA | 365 | 82 | 22.5 |
| Multiple 442@ | 1998 - 2000 | <4 | PAGE | 406 | 141 | 34.7 |
| Multiple 543# | 2001 | <4/all# | PAGE | 454 | 161 | 35.4 |
| Total | 15476 | 3095 | ||||
NK, not known
<4/all: Samples collected from two groups: children <4 yr, patients of all age groups
LA, latex agglutination; EM, electron microscopy; PAGE, polyacrylamide gel electrophoresis; ELISA, enzyme linked immunosorbent assay.
Multiple studies in which samples were collected from more than one city.
Multiple 1: Shimla, Lucknow, Bhopal, Nagpur, Davengere
Multiple 2: Shimla, Lucknow, Bhopal, Nagpur, Davengere, Delhi, Hyderabad
Multiple 3: Vellore, Mysore, Jalandhar, Yamunagar
Multiple 4: Kolkata, Imphal, @ Data on disease burden from Kolkata only
Multiple 5: Kolkata, Dibrugarh, Bhuvaneshwar, Chandigarh
Data from a study on the burden of infectious diseases in South Asia published in 2004 showed that an estimated 576,480 deaths occur due to diarrhoea in children in India44. Applying the above figure of 20.8 per cent of all diarrhoeas to be caused by rotavirus, it can be estimated that rotavirus is responsible for approximately 119,908 deaths annually. There are no published prospective studies that have examined rates of diarrhoea and hospitalizations in India. However, recent data from a birth cohort of 452 children in Vellore have shown that, in infancy rates of diarrhoeal episodes/child year (95% CI) is 3.6 (3.3 - 3.9), with a hospitalization rate/100 child years (95% CI) of 5.8 (3.8 - 8.8). Diarrhoea was the second most common cause for both outpatient visits and hospitalization after respiratory infections (unpublished data).
Rotavirus typing of strains obtained from hospitalized children
G- and P- typing was carried out using MAbs, genotyping primers or probes. A total of 1998 and 1108 samples were G- and P-typed respectively. The overall distribution of G and P types is shown in Figs 1 and 2. While most centres used MAbs or primers specific to G1- G4 and G9 types; G6, G8 and G10 were also included in some places. G1 and G2 were the most prevalent (24.7 and 23.4% respectively). However, the overall strain distribution varied from one location to another (Table II, Fig. 2). The commonest P type was P[8] (27%) followed by P[6] (21%) and P[4] (20%) (Table III, Fig. 3).
Fig. 2.
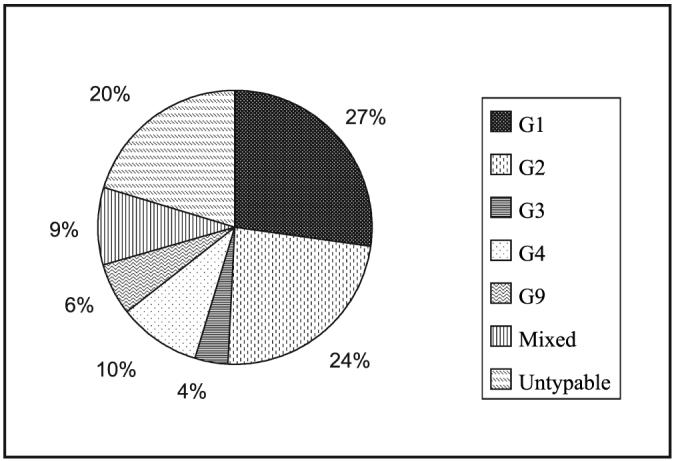
Overall distribution of G types in India (studies from 1990-2004). [Source: Table II].
Table II.
Distribution of G types in various geographic locations in India
| Centre | Year of study | Age (yr) | No. tested | G1 | G2 | G3 | G4 | G9 | Mixed | UT |
|---|---|---|---|---|---|---|---|---|---|---|
| North India: | ||||||||||
| Delhi21 | 1990 - 1991 | <5 | 51 | 17 | 13 | 5 | 4 | — | 5 | 7 |
| Delhi25 | 1998 - 2000 | <3 | 100 | 31 | 12 | 18 | 5 | — | — | 34 |
| Delhi24 | 2000 - 2001 | <5 | 135 | 32 | 18 | 8 | — | 21 | 10 | 46 |
| Western India: | ||||||||||
| Pune28 | 1990 - 1993 | children | 205 | 15 | 49 | 1 | 9 | — | 33 | 98 |
| Eastern India: | ||||||||||
| Calcutta45 | 1999 - 2000 | NK | 150 | 49 | 27 | — | 30 | — | 18 | 16 |
| South India: | ||||||||||
| Bangalore31 | 1988 - 1994 | NK | 150 | 15 | 20 | 53 | 7 | — | — | 55 |
| Chennai32 | 1997 - 1999 | 0 - 2, >2 | 48 | 7 | 33 | 1 | — | — | 7 | — |
| Chennai33 | 1995 - 1999 | <3 | 118 | 11 | 78 | 2 | 16 | — | 11 | — |
| Hyderabad34 | 1998 - 1999 | <2 | 46 | 16 | — | — | 8 | — | 5 | 17 |
| Mysore31 | 1993 - 1994 | NK | 50 | 23 | — | 12 | — | — | — | 15 |
| Vellore37 | 1995 - 1998 | <5 | 126 | 50 | 24 | 1 | 30 | 5 | 10 | 25 |
| Vellore38 | 2002 - 2004 | <5 | 94 | 44 | 8 | — | — | 18 | 4 | 19 |
| Multiple: | ||||||||||
| Multiple 139 | 1993 | 6 m to <5 | 63 | 7 | 14 | 7 | 6 | 15 | 7 | 7 |
| Multiple 240 | 1996 - 1998 | <5 | 287 | 51 | 99 | 2 | 32 | 50 | 31 | 22 |
| Multiple 341 | 1998 - 1999 | <5 | 82 | 33 | 7 | 9 | 12 | 7 | — | 14 |
| Multiple 442 | 1998 - 2000 | <4 | 159 | 61 | 38 | — | 20 | — | 11 | 29 |
| Multiple 543 | 2001 | <4/all | 126 | 62 | 17 | — | 6 | — | 26 | 15 |
| Total | 1998 | 524 | 457 | 119 | 185 | 116 | 178 | 419 |
UT, untypable; NK, not known
Table III.
Distribution of P types in various geographic locations in India
| Centre | Year of study | Age (yr) | No. tested | P[4] | P[6] | P[8] | P[11] | Mixed | UT |
|---|---|---|---|---|---|---|---|---|---|
| Delhi21 | 1990 - 1991 | <5 | 57 | 14 | 4 | 23 | — | 3 | 14 |
| Delhi24 | 2000 - 2001 | <5 | 135 | 16 | 29 | 32 | 1 | 7 | 50 |
| Multiple 139 | 1993 | 6m to <5 | 63 | 13 | 27 | 8 | — | 7 | 7 |
| Multiple 240 | 1996 - 1998 | <5 | 287 | 63 | 88 | 69 | 10 | 33 | 24 |
| Multiple 341 | 1998 - 1999 | <5 | 82 | 19 | 2 | 31 | — | — | 30 |
| Multiple 442 | 1998 - 2000 | <4 | 138 | 35 | 8 | 51 | — | 26 | 19 |
| Multiple 543 | 2001 | <4/all | 126 | 19 | 61 | — | — | 19 | 27 |
| Vellore37 | 1995 - 1998 | <5 | 126 | 32 | 7 | 39 | — | — | 48 |
| Vellore38 | 2002 - 2004 | <5 | 94 | 5 | 2 | 52 | 2 | — | 32 |
| 1108 | 216 | 228 | 305 | 13 | 95 | 251 |
UT, untypable
Fig. 3.
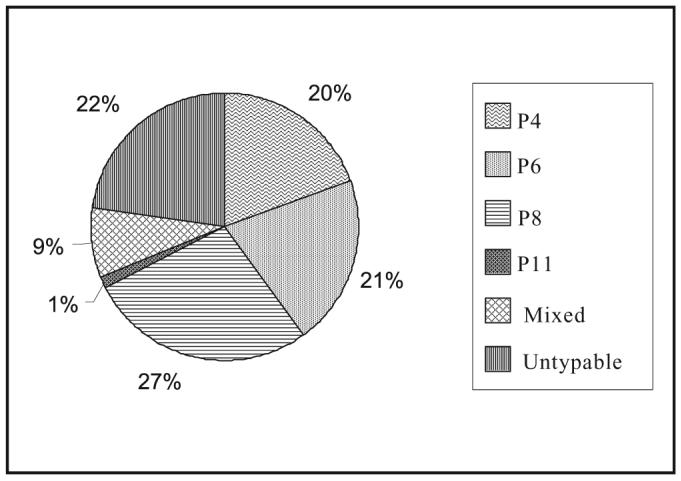
Overall distribution of P types in India (studies from 1990-2004). [Source: Table III].
Unusual strains of rotavirus
A number of unusual strains of rotavirus have been reported in the recent years. Characterization of strains that remained untypable by routine typing methods have resulted in the identification of these unusual strains including G6 strains from Pune46, G8 strains from Vellore37 and Mysore47, G12 from Calcutta (Kolkata)48, G3P[8] from western India49, G9P[19] strains from Manipur50 and G10P[11] strains from Vellore10. Most of these strains are of possible animal origin or animal-human reassortants, containing one or more genes that are highly identical to corresponding genes in animal rotaviruses.
The G6 genotype described from Pune showed >94 per cent identity of VP7 gene with bovine rotavirus RF isolated from France46. The G8 genotype described from Vellore and Mysore showed about 95 per cent similarity in the amino acid sequence of VP7 gene to bovine strain A537,47. The G3P[8] genotype described among tribals in western India showed 100 per cent identity of VP7 gene to simian rotaviruses and 99 per cent identity to human P[8]49. The G9P[19] genotype from Manipur appeared to be a porcine - human reassortant with human VP7 and VP4, VP6 and NSP4 closely related to porcine rotaviruses50. G10P[11] strains described from Vellore appear to be bovine human reassortants with all genes of bovine origin except NSP1 and NSP3 (unpublished data).
Comparison of rotavirus diarrhoea among outpatients and hospitalized patients
One study carried out between 1993 and 1996 in Pune was reviewed51. A total of 489 and 628 faecal samples were collected from inpatient and outpatient children <5 yr with diarrhoea respectively. The rate of rotavirus detection was higher among in-patients (28.3%) than outpatients (15.5%).
Neonatal rotavirus infections
Studies have reported the association of asymptomatic rotavirus infections in neonates with specific strains. The neonatal rotavirus strain is characterized by its endemicity and persistence in the neonatal ward. Maternal antibodies and physiologic immaturity of the neonatal gut may play a role in majority of neonatal infections being asymptomatic52. Neonatal rotavirus infections are believed to confer protection against subsequent infection and disease. Hence candidate vaccine strains have been derived from strains circulating in neonatal nurseries including two vaccine candidates currently in development in India53. However, symptomatic neonatal infections associated with necrotizing enterocolitis, acute gastroenteritis and feed intolerance have also been reported10.
Studies on neonatal rotavirus infections published before 1990 were reviewed in 200114. Subsequent studies using RT-PCR and genotyping of neonatal rotavirus strains have shown region specific asymptomatic infections by unusual strains (Table IV). This includes G9P[11] in Delhi54 and G10P[11] in Bangalore and Mysore55. Both symptomatic and asymptomatic infections by G10P[11] strain have been reported in Vellore10. Both neonatal strains appear to be bovine-human reassortants. G9P[11] is a human strain with a bovine VP4 while G10P[11] is composed mainly of bovine genes and has gene segments 5 and 7, encoding NSP1 and 3, of human origin53.
Table IV.
Neonatal rotavirus infections in India
| Centre | Year | Symptoms | Screening | No. Tested |
Positive |
Predominant strain | |
|---|---|---|---|---|---|---|---|
| n | n | % | |||||
| Delhi54 | 1993 | Asymptomatic | ELISA PAGE |
169 | 38 | 33 | G9P[11] |
| Bangalore55 | 1988 - 1997 | Asymptomatic | ELISA | 882 | 321 | 39 | G10P[11] |
| Vellore10 | 1999 - 2000 | Symptomatic/ asymptomatic |
ELISA | NK | 43 | NK | G10P[11] |
NK, not known; PAGE, polyacrylamide gel electrophoresis; ELISA, enzyme linked immunosorbent assay
Rotavirus infection in the community
Community based studies provide geographically representative information on the disease burden, strain prevalence and rates of rotavirus infection in the community. This is of considerable significance in understanding the natural course of infection and for comparison of symptomatic and asymptomatic infections.
Since no data on rotavirus infection in the community in India have been reviewed so far, all studies have been included. It must be noted that all studies except one from Vellore have been carried out around or before 1990, and hence may not represent the most recent scenario of community rotavirus infections. The studies have been carried out in various settings: rural, semi-urban and urban. All except one study have used ELISA for detection of rotavirus. The studies from four centres include samples from both symptomatic and asymptomatic infections while data from two centres are based only on symptomatic infections in the community (Table V). Rotavirus positivity rate ranges from 4 to 29.3 per cent in symptomatic cases and 2.4 to 12.3 per cent in asymptomatic cases.
Table V.
Rotavirus infection in the community
| Centre | Community | Year of study | Screening | Symptoms | No. Tested |
Positive | |
|---|---|---|---|---|---|---|---|
| n# | n# | % | |||||
| Delhi56 | Semi urban | NK | ELISA | Symptomatic | 212 | 45 | 21.2 |
| Asymptomatic | 82 | 2 | 2.4 | ||||
| North India57 | Urban/rural | 1982 - 1983 | ELISA | Symptomatic | 150 | 44 | 29.3 |
| Asymptomatic | 350* | 43 | 12.3 | ||||
| Chandigarh58 | Urban/peri-urban/rural | 1988 - 1991 | ELISA | Symptomatic | 218 | 25 | 11.5 |
| Haryana59 | Rural | 1985 - 1986 | ELISA | Symptomatic | 346 | 14 | 4 |
| Asymptomatic | 211 | 14 | 6.6 | ||||
| Varanasi60 | Urban | 1988 - 1989 | LA | Symptomatic | 17.7 | ||
| Asymptomatic | 4 | ||||||
| Vellore38 | Urban | 2002 - 2004 | ELISA | Symptomatic | 1152 | 82 | 7.1 |
NK, not known;
No. of samples;
No. of children tested positive for rotavirus; No. of samples collected per child not given LA, latex agglutination; ELISA, enzyme linked immunosorbent assay
Nosocomial rotavirus infections
Nosocomial enteric infections are defined as those occurring more than 72 h after admission to hospital for non diarrhoeal causes or shortly after discharge. Nosocomial rotavirus infection among children results in a significant economic burden, both by prolonging the hospitalization of the affected child and by also serving as a reservoir that propagates additional cases of rotavirus infection.
Three studies on nosocomial rotavirus infection were carried out in the paediatric wards of 2 hospitals in Calcutta (1985-1987) and one in Vellore (1990-1991) (Table VI). A total of 3530 children were screened for nosocomial enteric infections. The mean nosocomial enteric infection rate was 16.16 per cent. Of the nosocomial enteric infections, rotavirus accounted for about 22.5 per cent of cases.
Table VI.
Nosocomial rotavirus infections
| Centre | Year of study | Age (yr) | No. tested | % | Nosocomial infection no. | RV Positive | |
|---|---|---|---|---|---|---|---|
| No. | % | ||||||
| Vellore61 | 1990 - 1991 | <3 | 194 | 20.1 | 39 | 13 | 6.7 |
| Calcutta62 | 1985 - 1986 | <12 | 198 | 18.2 | 36 | 29 | 14.6 |
| Calcutta63 | 1987 | <5 | 3138 | 10.2 | 320 | 15* | 8.4 |
15 of 178 tested
Rotavirus is recognized as the major cause of diarrhoea hospitalizations among children in both developed and developing countries, and it is clear that improvements in hygiene and sanitation alone are not sufficient to decrease or eliminate disease. Based on studies that have shown promising results in prevention of severe gastroenteritis with vaccine candidates, several strains have been identified and tested as potential vaccines. Several vaccines are currently in various stages of development around the world. Two vaccines from the multinational manufacturers Merck and GlaxoSmithKline (GSK) have completed large scale trials and have been recently licensed. The Merck vaccine, Rotateq™ is composed of 5 rotavirus strains. Each strain is a single gene reassortant based on parent bovine strain WC3, containing outer capsid gene from a human strain. The vaccine induces immunity to G1- G4 and P[8] genotypes. The GSK vaccine, Rotarix™ is derived from a single human rotavirus strain G1P[8] that was attenuated by multiple passages in cell culture. Studies have shown both vaccines to be safe and effective5,6.
In India, candidate vaccines have been derived from strains causing asymptomatic infections in neonatal nurseries. The two India neonatal candidate vaccines are 116E, a G9P[11] rotavirus isolated from neonatal nurseries in Delhi53 and I321, a G10P[11] rotavirus described from Bangalore53. Both strains appear to be natural bovine-human reassortants. The vaccines are being manufactured by Bharat Biotech in India and are in their early phase trials. However, the efficacy of these vaccines has not been tested in developing countries, where previous rotavirus vaccine trials have been shown to evoke a lower seroresponse and be less effective64.
Summary
The rapid evolution of rotavirus strains and the emergence of new strains, possibly through the transmission of viruses across species or through reassortment between animal and human rotaviruses, makes it necessary to include intensive strain surveillance of as an important component of any vaccine implementation programme. This is particularly important in countries such as India where the mortality and economic burden associated with rotavirus is high. The data on the molecular epidemiology of rotaviruses have shown the emergence of interesting strains that may have animal origins. Based on VP7 type, alone G6, G8, G9, G10 and G12 strains have been newly identified in the past few years. The questions raised by these findings are whether this detection is because these strains are ‘emerging’ or because more sophisticated laboratory techniques are now being applied by researchers in India. Even if the latter is true, the collated data indicate that the diversity of rotaviruses in India is greater than found in most other developing countries, and that this will result in challenges for vaccine efficacy that have not been faced elsewhere.
The Cochrane Evidence Database analyses data from 64 trials with three kinds of vaccine, bovine, rhesus and human, and results indicate protection from rotavirus diarrhoea of any severity65. However, the important caveat to this update is that the majority of the trials were carried out in developed countries where the age of first rotavirus infection is later in childhood and where the degree of diversity of circulating strains is limited in comparison to developing countries. Rotavirus vaccines generally have yielded poor efficacy when tested in developing countries has led to concerns about the potential effectiveness of any future live, oral rotavirus vaccine in these settings64. Although rotavirus infection is universal early in childhood, the epidemiology of the disease is quite different in developed and developing countries. Differences in age of first infection, strain distribution, occurrence of mixed infections, seasonality and risk of mortality can affect decisions about vaccine composition and delivery. Higher doses of vaccine or additional doses may be required to overcome the inhibitory effects of competing intestinal flora, concomitant use of oral polio vaccine and high levels of humorally transferred maternal antibodies against rotavirus. Despite a recommendation by the World Health Organization that all rotavirus vaccines be tested early in developing countries, no data from the current vaccines are available in Africa or poor Asian countries. These data will be critical to determining the probability of success of the current rotavirus vaccines and to establish requirements for vaccines in pre-clinical and clinical development.
In summary, rotavirus epidemiology is complex as would be expected for a virus with multiple modes of transmission, and this complexity is amply illustrated in studies from India demonstrating the diversity of the virus in different settings. The new hope for prevention of morbidity and mortality due to this agent is the use of oral vaccines, which are effective in developed countries, but we need evidence from developing countries with the highest disease burden and virus diversity before accepting their efficacy in all settings.
Acknowledgment
The work in the authors' laboratory was supported by the Wellcome Trust, Indian Council for Medical Research and the Centers for Disease Control and Prevention.
References
- 1.Prasad BVV, Estes MK. Electron cryomicroscopy and computer image processing techniques: use in structure-function studies of rotavirus. In: Gray JJ, Desselberger U, editors. Rotavirus methods and protocols. New Jersey: Humana Press; 2000. pp. 9–32. [DOI] [PubMed] [Google Scholar]
- 2.Parashar UD, Gibson GJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–6. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344:564–72. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 4.Glass RI, Parashar UD. The promise of new rotavirus vaccines. N Engl J Med. 2006;354:75–7. doi: 10.1056/NEJMe058285. [DOI] [PubMed] [Google Scholar]
- 5.Vesikari T, Clark HF, Offit PA, Dallas MJ, Distefano DJ, Goveia MG, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 7.Estes M. Rotaviruses and their replication. In: Fields BN, Knipe DN, Howley PM, editors. Fields virology. 3rd ed. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 1625–55. [Google Scholar]
- 8.Greenberg HB, Flores J, Kalica AR, Wyatt RG, Jones R. Gene coding assignments for growth restriction, neutralization and subgroup specificities of the W and DS-1 strains of human rotavirus. J Gen Virol. 1983;64:313–20. doi: 10.1099/0022-1317-64-2-313. [DOI] [PubMed] [Google Scholar]
- 9.Kapikian AZ, Hoshino Y, Chanock RM. Rotaviruses. In: Knipe DM, Howley PM, editors. Fields virology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1787–833. [Google Scholar]
- 10.Iturriza Gomara M, Kang G, Mammen A, Jana AK, Abraham M, Desselberger U, et al. Characterization of G10P[11] rotaviruses causing acute gastroenteritis in neonates and infants in Vellore, India. J Clin Microbiol. 2004;42:2541–7. doi: 10.1128/JCM.42.6.2541-2547.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bresee J, Parashar UD, Holman R, Gentsch J, Glass R, Ivanoff B, et al. Generic protocol for hospital-based surveillance to estimate the burden of rotavirus gastroenteritis in children under 5 years of age. Generic protocols for (i) hospital-based surveillance to estimate the burden of gastroenteritis in children and (ii) A community-based survey on utilization of health care services for gastroenteritis in children; field test version (WHO/V&B/02.15) 2000. Available at http://www.who.int/vaccine_research/diseases/rotavirus/documents/en.
- 12.Iturriza Gomara M, Desselberger U, Gray J. Molecular epidemiology of rotaviruses: genetic mechanisms associated with diversity. In: Desselberger U, Gray J, editors. Viral gastroenteritis. Amsterdam: Elsevier Science; 2003. pp. 317–44. [Google Scholar]
- 13.Kang G, Kelkar SD, Chitambar SD, Ray P, Naik T. Epidemiological profile of rotaviral infection in India: challenges for the 21st century. J Infect Dis. 2005;192(Suppl):S120–6. doi: 10.1086/431496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain V, Parashar UD, Glass RI, Bhan MK. Epidemiology of rotavirus in India. Indian J Pediatr. 2001;68:855–62. doi: 10.1007/BF02762113. [DOI] [PubMed] [Google Scholar]
- 15.Broor S, Ghosh D, Mathur P. Molecular epidemiology of rotaviruses in India. Indian J Med Res. 2003;118:59–67. [PubMed] [Google Scholar]
- 16.Singh V, Broor S, Mehta S, Mehta SK. Molecular epidemiology of human rotavirus infections in Chandigarh (India) Indian J Med Res. 1990;91:9–14. [PubMed] [Google Scholar]
- 17.Chakravarthi A, Broor S, Natarajan R. Epidemiological and clinical characteristics of acute diarrhoea in children due to human rotavirus. J Trop Pediatr. 1992;38:192–3. doi: 10.1093/tropej/38.4.192. [DOI] [PubMed] [Google Scholar]
- 18.Chakravarti A, Kumar S, Mittal SK, Broor S. Clinical and epidemiological features of acute gastroenteritis caused by human rotavirus subgroups. J Diarrhoeal Dis Res. 1992;10:21–4. [PubMed] [Google Scholar]
- 19.Broor S, Husain M, Chatterjee B, Chakraborty A, Seth P. Temporal variation in the distribution of rotavirus electropherotypes in Delhi, India. J Diarrhoeal Dis Res. 1993;11:14–8. [PubMed] [Google Scholar]
- 20.Patwari AK, Srinivasan A, Diwan N, Aneja S, Anand VK, Peshin S. Rotavirus as an aetiological organism in acute watery diarrhoea in Delhi children: reappraisal of clinical and epidemiological characteristics. J Trop Pediatr. 1994;40:214–8. doi: 10.1093/tropej/40.4.214. [DOI] [PubMed] [Google Scholar]
- 21.Husain M, Seth P, Dar L, Broor S. Classification of rotavirus into G and P types with specimens from children with acute diarrhea in New Delhi, India. J Clin Microbiol. 1996;34:1592–4. doi: 10.1128/jcm.34.6.1592-1594.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatterjee B, Husain M, Kavita, Seth P, Broor S. Diversity of rotavirus strains infecting pediatric patients in New Delhi, India. J Trop Pediatr. 1996;42:207–10. doi: 10.1093/tropej/42.4.207. [DOI] [PubMed] [Google Scholar]
- 23.Chakravarti A, Rawat D, Chakravarti A. Molecular epidemiology of rotavirus in Delhi. Indian J Pathol Microbiol. 2004;47:90–3. [PubMed] [Google Scholar]
- 24.Bahl R, Ray P, Subodh S, Shambharkar P, Saxena M, Parashar U, et al. Delhi Rotavirus Study Group. Incidence of severe rotavirus diarrhea in New Delhi, India, and G and P types of the infecting rotavirus strains. J Infect Dis. 2005;192(Suppl):S114–9. doi: 10.1086/431497. [DOI] [PubMed] [Google Scholar]
- 25.Chakravarti A, Kumaria R, Chakravarti A. Prevalence of genotypes G1-G4 of human rotavirus in a hospital setting in New Delhi. Indian J Gastroenterol. 2005;24:127–8. [PubMed] [Google Scholar]
- 26.Sharma P, Sehgal R, Ganguly NK, Vaidyanathan U, Walia BNS. Serogroups of rotavirus in north India. J Trop Pediatr. 1994;40:58–9. doi: 10.1093/tropej/40.1.58. [DOI] [PubMed] [Google Scholar]
- 27.Desai HS, Banker DD. Rotavirus infection among children in Bombay. Indian J Med Sci. 1993;47:27–33. [PubMed] [Google Scholar]
- 28.Kelkar SD. Prevalence of human group A rotavirus serotypes in Pune, India (1990-1993) Indian J Med Res. 1997;106:508–12. [PubMed] [Google Scholar]
- 29.Kelkar SD, Purohit SG, Simha KV. Prevalence of rotavirus diarrhoea among hospitalized children in Pune, India. Indian J Med Res. 1999;109:131–5. [PubMed] [Google Scholar]
- 30.Phukan AC, Patgiri DK, Mahanta J. Rotavirus associated acute diarrhoea in hospitalized children in Dibrugarh, northeast India. Indian J Pathol Microbiol. 2003;46:274–8. [PubMed] [Google Scholar]
- 31.Aijaz S, Gowda K, Jagannath HV, Reddy RR, Maiya PP, Ward RL, et al. Epidemiology of symptomatic human rotaviruses in Bangalore and Mysore, India, from 1988 to 1994 as determined by electropherotype, subgroup and serotype analysis. Arch Virol. 1996;141:715–26. doi: 10.1007/BF01718329. [DOI] [PubMed] [Google Scholar]
- 32.Ananthan S, Saravanan P. Genomic diversity of group A rotavirus RNA from children with acute diarrhoea in Chennai, south India. Indian J Med Res. 2000;111:50–6. [PubMed] [Google Scholar]
- 33.Saravanan P, Ananthan S, Ananthasubramanium M. Rotavirus infection among infants and young children in Chennai, South India. Indian J Med Microbiol. 2004;22:212–21. [PubMed] [Google Scholar]
- 34.Anand T, Raju TA, Rao MV, Rao LV, Sharma G. Symptomatic human rotavirus subgroups, serotypes & electropherotypes in Hyderabad, India. Indian J Med Res. 2000;112:1–4. [PubMed] [Google Scholar]
- 35.Shetty M, Brown TA, Kotian M, Shivananda PG. Viral diarrhoea in a rural coastal region of Karnataka India. J Trop Pediatr. 1995;41:301–3. doi: 10.1093/tropej/41.5.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anand T, Lakshmi N, Kumar AG. Rotavirus diarrhea among infants and children at Tirupati. Indian Pediatr. 1994;31:46–8. [PubMed] [Google Scholar]
- 37.Kang G, Green J, Gallimore CI, Brown DWG. Molecular epidemiology of rotaviral infection in South Indian children with acute diarrhoea from 1995-1996 to 1998-1999. J Med Virol. 2002;67:101–5. doi: 10.1002/jmv.2197. [DOI] [PubMed] [Google Scholar]
- 38.Banerjee I, Ramani S, Primrose B, Moses P, Iturriza-Gomara M, Gray J, et al. Comparative study of rotavirus epidemiology in children from a community based birth cohort and a tertiary hospital in south India. J Clin Microbiol. 2006;44:2468–74. doi: 10.1128/JCM.01882-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramachandran M, Das BK, Vij A, Kumar R, Bhambal SS, Kesari N, et al. Unusual diversity of human rotavirus G and P genotypes in India. J Clin Microbiol. 1996;34:436–9. doi: 10.1128/jcm.34.2.436-439.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain V, Das BK, Bhan MK, Glass RI, Gentsch JR. Indian Strain Surveillance Collaborating Laboratories. Great diversity of group A rotavirus strains and high prevalence of mixed rotavirus infections in India. J Clin Microbiol. 2001;39:3524–9. doi: 10.1128/JCM.39.10.3524-3529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang G, Raman T, Green J, Gallimore CI, Brown DW. Distribution of rotavirus G and P types in north and south Indian children with acute diarrhoea in 1998-99. Trans R Soc Trop Med Hyg. 2001;95:491–2. doi: 10.1016/s0035-9203(01)90014-8. [DOI] [PubMed] [Google Scholar]
- 42.Das S, Sen A, Uma G, Varghese V, Chaudhuri S, Bhattacharya SK, et al. Genomic diversity of group A rotavirus strains infecting humans in eastern India. J Clin Microbiol. 2002;40:146–9. doi: 10.1128/JCM.40.1.146-149.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das S, Varghese V, Chaudhuri S, Barman P, Kojima K, Dutta P, et al. Genetic variability of human rotavirus strains isolated from Eastern and Northern India. J Med Virol. 2004;72:156–61. doi: 10.1002/jmv.10542. [DOI] [PubMed] [Google Scholar]
- 44.Zaidi A, Awasthi S, deSilva HJ. Burden of infectious diseases in South Asia. Br Med J. 2004;328:811–5. doi: 10.1136/bmj.328.7443.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khetawat D, Dutta P, Bhattacharya SK, Chakrabarti S. Distribution of rotavirus VP7 genotypes among children suffering from watery diarrhea in Kolkata, India. Virus Res. 2002;87:31–40. doi: 10.1016/s0168-1702(02)00081-3. [DOI] [PubMed] [Google Scholar]
- 46.Kelkar SD, Dindokar AR, Dhale GS, Zade JK, Ranshing SS. Culture adaptation of serotype G6 human rotavirus strains from hospitalized diarrhea patients in India. J Med Virol. 2004;74:650–5. doi: 10.1002/jmv.20226. [DOI] [PubMed] [Google Scholar]
- 47.Jagannath MR, Vethanayagam RR, Reddy BS, Raman S, Rao CD. Characterization of human symptomatic rotavirus isolates MP409 and MP480 having ‘long’ RNA electropherotype and subgroup I specificity, highly related to the P6[1], G8 type bovine rotavirus A5, from Mysore, India. Arch Virol. 2000;145:1339–57. doi: 10.1007/s007050070094. [DOI] [PubMed] [Google Scholar]
- 48.Das S, Varghese V, Chaudhury S, Barman P, Mahapatra S, Kojima K, et al. Emergence of novel human group A rotavirus G12 strains in India. J Clin Microbiol. 2003;41:2760–2. doi: 10.1128/JCM.41.6.2760-2762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Awachat PS, Kelkar SD. Unexpected detection of simian SA11-human reassortant strains of rotavirus G3P[8] genotype from diarrhea epidemic among tribal children of Western India. J Med Virol. 2005;77:128–35. doi: 10.1002/jmv.20425. [DOI] [PubMed] [Google Scholar]
- 50.Varghese V, Das S, Singh NB, Kojima K, Bhattacharya SK, Krishnan T, et al. Molecular characterization of a human rotavirus reveals porcine characteristics in most of the genes including VP6 and NSP4. Arch Virol. 2004;149:155–72. doi: 10.1007/s00705-003-0199-1. [DOI] [PubMed] [Google Scholar]
- 51.Kelkar SD, Purohit SG, Boralkar AN, Verma SP. Prevalence of rotavirus diarrhea among outpatients and hospitalized patients: a comparison. Southeast Asian J Trop Med Public Health. 2001;32:494–9. [PubMed] [Google Scholar]
- 52.Haffejee IE. Neonatal rotavirus infections. Rev Infect Dis. 1991;13:957–62. doi: 10.1093/clinids/13.5.957. [DOI] [PubMed] [Google Scholar]
- 53.Glass RI, Bhan MK, Ray P, Bahl R, Parashar UD, Greenberg H, et al. Development of candidate rotavirus vaccines derived from neonatal strains in India. J Infect Dis. 2005;192(Suppl):S30–5. doi: 10.1086/431498. [DOI] [PubMed] [Google Scholar]
- 54.Cicirello HG, Das BK, Gupta A, Bhan MK, Gentsch JR, Kumar R, et al. High prevalence of rotavirus infection among neonates born at hospitals in Delhi, India: predisposition of newborns for infection with unusual rotavirus. Pediatr Infect Dis J. 1994;13:720–4. doi: 10.1097/00006454-199408000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Vethanayagam RR, Ananda Babu M, Nagalaxmi KS, Maiya PP, Venkatesh HA, Purohit S, et al. Possible role of neonatal infection with the asymptomatic reassortant rotavirus (RV) strain I321 in the decrease in hospital admissions for RV diarrhea, Bangalore, India, 1988-1999. J Infect Dis. 2004;189:2282–9. doi: 10.1086/420889. [DOI] [PubMed] [Google Scholar]
- 56.Samantaray JC, Mohapatra LN, Bhan MK, Arora NK, Deb M, Ghai OP, et al. Study of rotavirus diarrhea in a north Indian community. Indian Pediatr. 1982;19:761–5. [PubMed] [Google Scholar]
- 57.Panigrahi D, Agarwal KC, Kaur T, Ayyagari A, Walia BN. A study of rotavirus diarrhoea in children in a north Indian community. J Diarrhoeal Dis Res. 1985;3:20–3. [PubMed] [Google Scholar]
- 58.Yachha SK, Singh V, Kanwar SS, Mehta S. Epidemiology, subgroups and serotypes of rotavirus diarrhea in north Indian communities. Indian Pediatr. 1994;31:27–33. [PubMed] [Google Scholar]
- 59.Raj P, Bhan MK, Prasad AK, Kumar R, Bhandari N, Jayashree S. Electrophoretic study of the genome of human rotavirus in rural Indian community. Indian J Med Res. 1989;89:65–8. [PubMed] [Google Scholar]
- 60.Nath G, Singh SP, Sanyal SC. Childhood diarrhoea due to rotavirus in a community. Indian J Med Res. 1992;95:259–62. [PubMed] [Google Scholar]
- 61.Desikan P, Daniel JD, Kamalarathnam CN, Mathan MM. Molecular epidemiology of nosocomial rotavirus infection. J Diarrhoeal Dis Res. 1996;14:12–5. [PubMed] [Google Scholar]
- 62.Dutta P, Bhattacharya SK, Saha MR, Dutta D, Bhattacharya MK, Mitra AK. Nosocomial rotavirus diarrhea in two medical wards of a pediatric hospital in Calcutta. Indian Pediatr. 1992;29:701–6. [PubMed] [Google Scholar]
- 63.Dutta P, Mitra U, Rasaily R, Bhattacharya SK, De SP, Sen D, et al. Prospective study of nosocomial enteric infections in a pediatric hospital, Calcutta. Indian Pediatr. 1993;30:187–94. [PubMed] [Google Scholar]
- 64.Hanlon P, Hanlon L, Marsh V, Byass P, Shenton F, Hassan-King M, et al. Trial of an attenuated bovine rotavirus vaccine (RIT 4237) in Gambian infants. Lancet. 1987;i:1342–5. doi: 10.1016/s0140-6736(87)90649-0. [DOI] [PubMed] [Google Scholar]
- 65.Soares-Weiser K, Goldberg E, Tamimi G, Pitan OC, Leibovici L. Rotavirus vaccine for preventing diarrhoea. The Cochrane Database of Systematic Rev. 2006;2:CD002848. doi: 10.1002/14651858.CD002848.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]