Abstract
Pancreatic cholesterol esterase (CEase), which is secreted from the exocrine pancreas, is a serine hydrolase that aids in the bile salt-dependent hydrolysis of dietary cholesteryl esters and contributes to the hydrolysis of triglycerides and phospholipids. Additional roles for CEase in intestinal micelle formation and in transport of free cholesterol to the enterocyte have been suggested. There also are studies that point to a pathological role(s) for CEase in the circulation where CEase accumulates in atherosclerotic lesions and triggers proliferation of smooth muscle cells. Thus, there is interest in CEase as a potential drug target. 4-Chloro-3-alkoxyisocoumarins are a class of haloenol lactones that inhibit serine hydrolases and serine proteases and have the potential to be suicide inhibitors. In the present study, we have developed 3-alkoxychloroisocoumarins that are potent inhibitors of CEase. These inhibitors were designed to have a saturated cycloalkane ring incorporated into a 3-alkoxy substituent. The size of the ring as well as the length of the tether holding the ring were found to be important contributors to binding to CEase. 4-Chloro-3-(4-cyclohexylbutoxy)isocoumarin and 4-chloro-3-(3-cyclopentylpropoxy) isocoumarin were demonstrated to be potent reversible inhibitors of CEase, with dissociation constants of 11 nM and 19 nM, respectively. The kinetic results are consistent with predictions from molecular modeling.
Keywords: Inhibitors, cholesterol esterase, haloenolactones
1. Introduction
The well established link between plasma cholesterol levels and coronary artery disease and the contribution of elevated plasma cholesterol, specifically LDL-cholesterol, to other diseases including cancer, obesity and diabetes have made control of plasma cholesterol a major health aim. Because cholesterol is both the product of biosynthesis and of dietary intake, multiple approaches to control of plasma cholesterol have been initiated. The statins, inhibitors of the rate-limiting biosynthetic enzyme HMGCoA reductase in the pathway of cholesterol synthesis, have been highly successful in controlling plasma cholesterol levels.1 In addition, potent inhibitors of intestinal uptake of dietary cholesterol have been introduced.2 Ezetimibe, which is a member of this new class of drugs that limit the absorption of free cholesterol,3 appears to act directly at the enterocyte membrane to limit cholesterol uptake, possibly by blocking the Nieman-Pick C-1 like-1 protein (NPC1L1).4 Not all hypercholesterolemic individuals can tolerate statins.5 In addition, responsiveness of plasma cholesterol levels to dietary changes in cholesterol is highly variable.6 Therefore, there continues to be interest in other targets for control of plasma cholesterol.
Pancreatic cholesterol esterase (CEase; EC 3.1.1.13), also known as bile salt-stimulated lipase, bile salt-dependent lipase, carboxyl ester lipase, pancreatic lysophospholipase and nonspecific lipase, has been studied extensively as a potential target to prevent the absorption of dietary cholesterol. CEase, along with triglyceride lipase and pancreatic phospholipase A2, is secreted from the exocrine pancreas in response to a fat-containing meal where CEase aids in the bile salt-dependent hydrolysis of dietary cholesteryl esters, as well as contributes to the hydrolysis of triglycerides and phospholipids, consistent with the known broad substrate specificity of CEase.7 The major physiological role of CEase in determining the bioavailability of cholesterol that is derived from cholesteryl esters is supported by CEase gene knockout studies.8 A second role for CEase in transport of free cholesterol to the enterocyte has been suggested, although there are conflicting reports concerning this role for CEase.9,10 In addition, a role for CEase in intestinal micelle formation has been suggested, where CEase participates with phospholipase A2 in hydrolysis of lecithin to lysolecithin which is required for formation of intestinal micelles that efficiently deliver free cholesterol.11 In the absence of hydrolysis of lecithin to lysolecithin, the intestinal micelles that form do not deliver cholesterol efficiently.
There is another developing literature that points to a role(s) for CEase in the circulation. It has long been noted that CEase is detectable in blood. This CEase appears to be derived from pancreatic CEase that initially is secreted into the intestine. Part of this CEase, perhaps as a complex with chaperone GRP94, is internalized by enterocytes and is released into the circulation where it is associated with apolipoprotein B-containing lipoproteins. This circulating CEase accumulates in atherosclerotic lesions and triggers proliferation of smooth muscle cells.12,13 Recently, plaque-associated CEase has also been shown to exhibit pro-angiogenic properties by promoting proliferation, migration and capillary network formation by endothelial cells.14 Thus, CEase may be involved in pathophysiological angiogenesis, which adds to interest in CEase as a potential drug target.
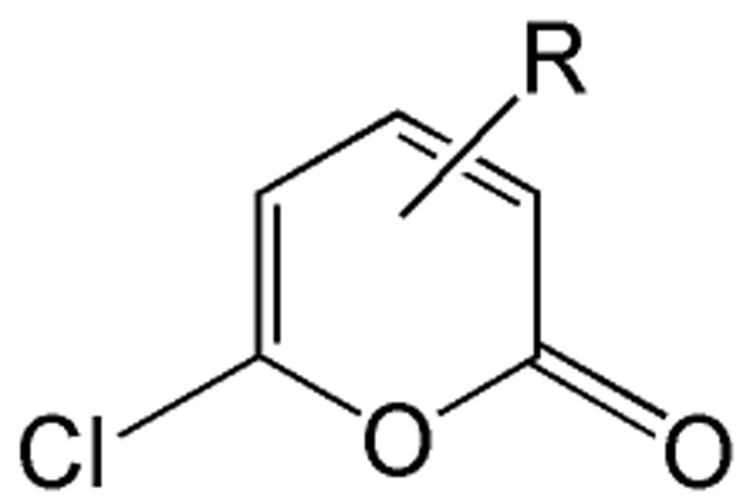
CEase is a serine hydrolase utilizing serine 194, histidine 435 and aspartate 320 as the catalytic triad and is part of the α/β hydrolase family of enzymes. Previously, we described the synthesis and testing of a series of 3- and 5- cycloalkyl-6-chloro-2-pyrones (Figure 1) that reversibly inhibited CEase and were effective at preventing the absorption of dietary cholesteryl esters.15–17 The chloropyrones are a class of haloenol lactones that inhibit serine hydrolases and serine proteases and have the potential to be irreversible inhibitors.
Figure 1.
Structure of pyrone inhibitors of CEase.
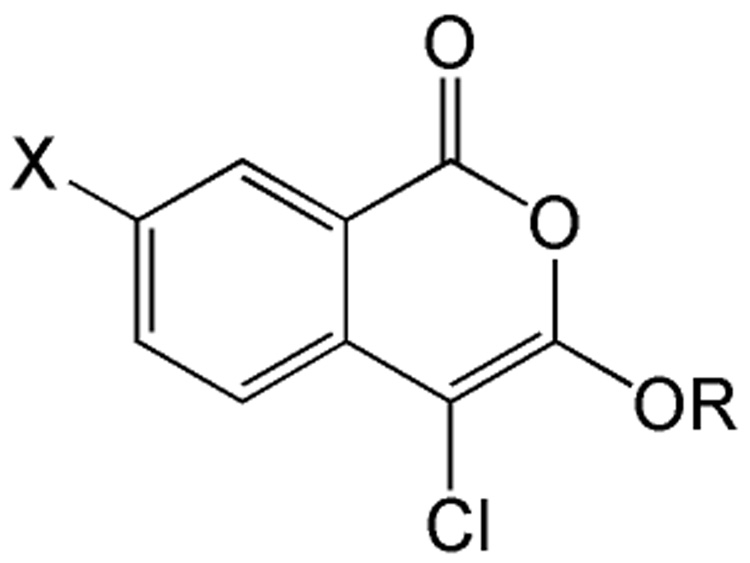
There is another class of haloenol lactones, the chloroisocoumarins, that also inhibit serine hydrolases and serine proteases.18 Recently we have described 3-alkoxy-4-chloroisocoumarins that are potent inhibitors of the serine protease urokinase plasminogen activator (uPA).19 uPA functions through a catalytic mechanism that is similar to CEase. In the present study, we have found that some 3-alkoxy-4-chloroisocoumarins are potent inhibitors of CEase. The general structure for these compounds is shown in Figure 2. In the majority of the compounds tested, the R group is an alkyl chain of zero to five carbons terminating in a saturated cyclopentyl or cyclohexyl ring or a bromine and X is hydrogen. In addition, in order to investigate the importance of the chlorine, compounds corresponding to one of the 3-alkoxy-4-chloroisocoumarins, but with a hydrogen or a trifluoroacetyl group replacing the chlorine were tested. Compounds where X is an amino or nitro group were to tested to investigate the effect of hydrogen bond donors and acceptors at the 7-position. We demonstrate that potent inhibitors of CEase with low nanomolar dissociation constants can be developed as lead compounds using the chloroisocoumarin scaffold.
Figure 2.
Structure of 3-alkoxy-4-chloroisocoumarin inhibitors of CEase.
2. Chemistry
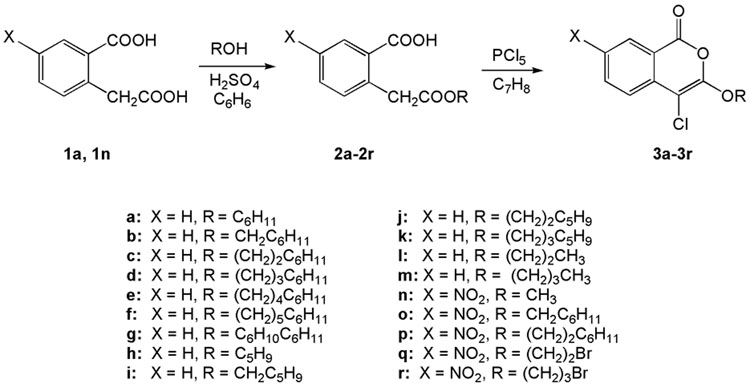
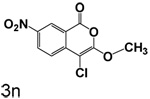
The synthesis of compounds 2q, 2r, 3q, 3r, 6a, 6b and 7a were reported by Heynekamp et al.19 The other compounds used in this study were prepared by similar methods. Scheme 1 outlines the synthesis of 3a–3r. Homophthalic acid (1a) or 5-nitrohomophthalic acid (1n) was esterified to give monoesters 2a–2r. Two equivalents of alcohol were used in these esterifications in order to minimize the amount of starting homophthalic acid in the product. The amount of diester byproduct is increased somewhat by using excess alcohol, but the diester is much easier to separate from the monoester product than is the starting diacid. Cyclization of esters 2a–2r with phosphorus pentachloride in toluene gave 3-alkoxy-4-chloroisocoumarins 3a–3r.
Scheme 1.
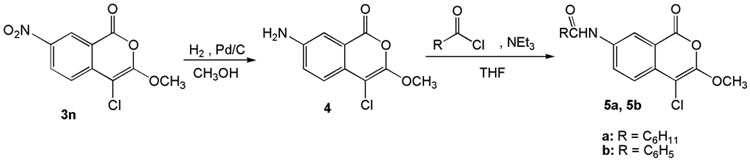
Compound 4, (7-amino-4-chloro-3-methoxyisocoumarin) was prepared by catalytic hydrogenation of the nitro group of 3n as shown in Scheme 2. The amides 5a and 5b were prepared by treating 4 with cyclohexanecarbonyl chloride and benzoyl chloride respectively in the presence of triethylamine (Scheme 2).
Scheme 2.
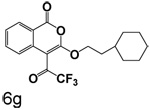
Scheme 3 shows the synthesis of compounds that lack chlorine in the four position. These compounds were prepared by cyclizing the corresponding monoesters with trifluoroacetic anhydride. Cyclization of esters with nitro groups on the ring gave the expected products unsubstituted at the 4-position (6a, 6b, 6d, 6e). Under the same conditions, esters without nitro groups on the ring gave products with trifluoroacetyl groups at the 4-position (6c, 6f, 6g).
Scheme 3.
Finally, reduction of the nitro group of compound 6b by catalytic hydrogenation provided amino substituted compound 7b.
3. Results and Discussion
Molecular modeling studies were conducted to determine the inhibition potential of the designed compounds by docking the inhibitors into the active site of CEase. The results of the flexible ligand docking were quantitatively and qualitatively analyzed to determine the predicted binding affinities. The results of the modeling studies suggested that 4-chloro-isocoumarins substituted at the 3-position with large lipophilic groups and longer tethers would be the best inhibitors. The active site of CEase contains a deep pocket to bind the long chain fatty acid portion of the native cholesterol ester substrates. The inhibitors were designed with the idea that lipophilic cycloalkyl groups would occupy the fatty acid site. Occupation of the fatty acid pocket by the lipophilic cycloalkyl groups would orient the reactive carbonyl of the isocoumarin backbone close to serine 194. This in turn would provide the proper orientation for potential irreversible binding. Structure activity relationships were determined by comparing predicted orientations and rankings with actual kinetic results. For the chloroisocoumarins, it was predicted that 3-alkoxy substituents with a terminal cyclopentyl group or cyclohexyl group would be good inhibitors, and that the length of the tether would be important. Figure 3shows a series of 4-chloro-3-cyclopentylalkoxyisocoumarins docked in the active site of CEase. The interaction energy that the cyclopentane ring achieves with the fatty acid pocket of cholesterol esterase dictates the location of the chloroisocoumarin. Thus the ability of the chloroisocoumarin to adopt an orientation close to serine 194 that might allow the inhibitor to participate as a suicide substrate appears to be critically dependent upon the substituent in the 3-position.
Figure 3.
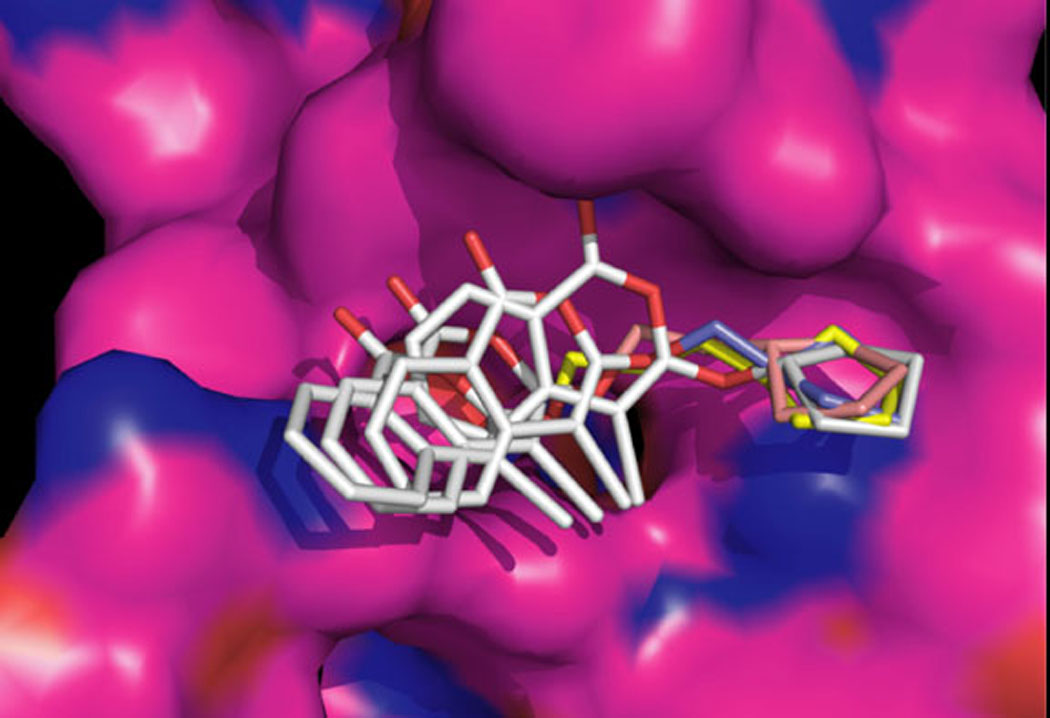
Molecular modeling of CEase with a series of 4-chloro-3-cyclopentylalkoxyisocoumarins where the cyclopentyl groups are attached with tethers of different lengths.
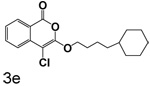
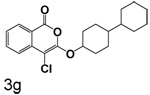
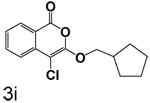
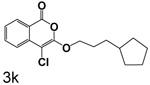
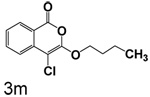
Table 1 shows the results from kinetic analysis of the isocoumarin-based compounds as inhibitors of CEase. As predicted, the length of the tether is important in determining the binding constants. For the cyclohexyl series, there is a marked improvement in binding going from a 3-methylene spacer to a 4-methylene spacer, after which further increase in the length of the tether markedly decreases binding. Compound 3e with a 4-methylene spacer shows a dissociation constant of 11 nM. Placing the 4-methylene spacer into a cyclohexane ring (compound 3g) is less effective. By comparison, increasing the spacer to 5-methylenes (compound 3f) raises the dissociation constant to 62 uM. For the cyclopentyl series, the length of the tether also is important, as shown by comparison of the dissociation constants for 3h–3k. Compound 3k shows a dissociation constant of 19 nM. Removal of the ring markedly affects binding, as shown with compounds 3l and 3m.
Table 1.
Dissociation constants for the reversible inhibition of CEase by 3-alkoxy isocoumarins.
| Structure | Ki (µM) | Structure | Ki (µM) |
|---|---|---|---|
 |
1.2 | 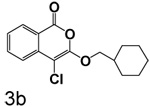 |
1.8 |
 |
1.2 | 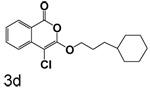 |
0.18 |
 |
0.011 | 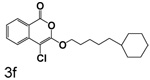 |
62 |
 |
0.19 | 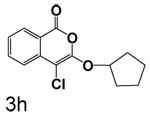 |
0.95 |
 |
0.36 | 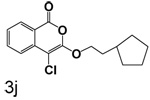 |
0.26 |
 |
0.019 | 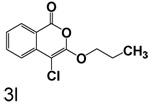 |
3.6 |
 |
6.5 | 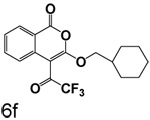 |
>100 |
 |
73 | 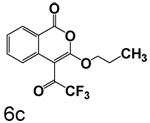 |
>25 |
 |
19 | 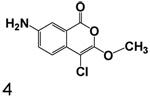 |
17 |
 |
5.7 | 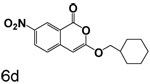 |
54 |
 |
0.7 | 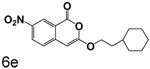 |
>100 |
 |
>25 | 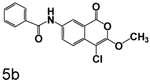 |
>250 |
 |
26 | 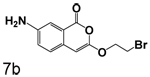 |
>100 |
 |
0.26 | 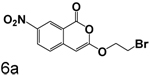 |
50 |
 |
1.6 | 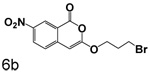 |
33 |
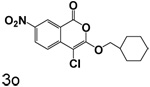
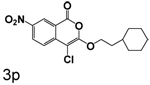
A chlorine or other group in the 4-position makes a significant but variable contribution. The increase in the size of the group in the 4-position going from chlorine to trifluoroacetyl is counterproductive. Compounds 6f, 6g and 6c, all of which have trifluoroacetyl groups at the 4-position, are poor inhibitors compared to 3b, 3c and 3l which have chlorine at the 4-position. The importance of chlorine to binding is shown by comparison of 3o and 6d, where the presence of the chlorine improved binding by an order of magnitude, and by comparison of 3p and 6e, where the presence of the chlorine improved binding more than two orders of magnitude.
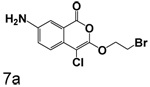
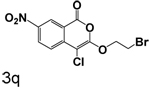
The effects of reduction of the 7-nitro group to an amino group are complex. Reduction of the 7-nitro group to an amino group had little effect when a small group was at the 3-position, as shown by comparison of 3n and 4. Addition of a large hydrophobic group to the amine at the 7-position (5a and 5b) did not improve binding, in marked distinction from our earlier studies of uPA. The effect of reduction of the 7-nitro group to an amino group was more pronounced when a larger group was in the 3-position, as shown by comparison of 3q and 7a where the reduction markedly increased the dissociation constant.
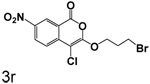
Replacment of the ring at the end of the 3-alkoxy substituent with a bromine demonstrated that the ring can be replaced. The importance of the chlorine in the 4-position to binding is also observed in the compounds where bromine is present in the 3-alkoxy group, as observed by comparison of 7a with 7b, 3q with 6a, and 3r with 6b. In all cases, the inhibition of CEase by the compounds in Table 1 was reversible, with no evidence of inactivation of the enzyme.
4. Conclusions
The isocoumarin scaffold with appropriate 3-alkoxy substituents can provide inhibitors of CEase with low nanomolar dissociation constants. The molecular modeling results suggest that the 3-alkoxy substituent anchors the inhibitors into the fatty acid binding pocket of CEase such that the lactone moiety of the isocoumarin ring is not optimally located for mechanism-based attack by serine 194. Consequently, these compounds are reversible active site inhibitors.
5. Experimental
5.1 Modeling
The X-ray crystal structure of CEase (pdb code 1F6W) was downloaded from the protein data bank. All compounds shown in Table 1 were docked to the enzyme using Autodock 3.0 on a cluster of Silicon Graphics workstations consisting of Octanes and O2s. The compounds were prepared using Sybyl 7.0 (Tripos Inc., St. Louis, MO). The molecules were drawn in, assigned partial charges using the included Gasteiger–Hückel method and energy minimized using the BFGS method. Energy minimizations were run for 10,000 iterations and the rotatable bonds defined to allow flexible ligand docking. The protein was prepared using Sybyl before docking by removing non-native substrates and water molecules. Polar hydrogens and Kollman Uni charges were added to the protein as well. The molecules were docked in an area around the active site serine defined by a cube of 60×60×60 Å using a Lamarckian genetic algorithm.
5.2 Kinetics
CEase (porcine) was from Sigma. CEase was assayed in 0.1 M Hepes, pH 7, containing 6 mM taurocholate and 1 mM p-nitrophenylbutyrate, at 405 nm. Inhibitors of CEase were generally analyzed by double reciprocal and Dixon plots and, for high-affinity inhibitors, by Straus-Goldstein plots. Kinetic analysis of Michaelis constants and kcat values was carried out by nonlinear regression analysis. Dissociation constants of inhibitors were determined by linear regression analysis of the double reciprocal, Dixon, and Straus-Goldstein plots.
5.3 Synthesis
Unless otherwise noted, all other reagents were obtained from commercial sources and used without further purification. Reagent quality solvents were used without purification with the exception of THF which was distilled from calcium hydride before use. Benzoyl chloride and cyclohexane carbonyl chloride were distilled before use. Alcohols that were not commercially available were prepared by the lithium aluminum hydride reduction of the corresponding carboxylic acids. Melting points were determined on a Thomas Hoover capillary melting point apparatus and are uncorrected. NMR spectra were recorded on a Bruker AC250 (250 MHz) NMR spectrometer in CDCl3 unless otherwise noted. Chemical shifts are reported in ppm (δ) relative to CHCl3 at 7.24 ppm for 1H NMR and 77.0 for 13C NMR. High resolution mass spectra were performed at the UNM Mass Spectrometry Facility, University of New Mexico, Albuquerque, NM. Analytical data was obtained from Galbraith Laboratories, Knoxville TN. Homophthalic acids were esterified to provide monoesters 2b–2r by the same general procedure described below for 2-[2-(cyclohexyloxy)-2-oxoethyl]benzoic acid (2a). The esters were cyclized with phosphorous pentachloride to form 4-chloro-3-alkyloxyisocoumarins 3b–3r by the general procedure described below for 4-chloro-3-cyclohexyloxyisocoumarin (3a). Amide 5b was synthesized by the same procedure described for 5a, substituting benzoyl chloride for cyclohexanecarbonyl chloride. Compounds 6a–6g were all synthesized by the same general procedure involving cyclization with trifluoroacetic anhydride which is described in detail for 6c. Compounds 2q, 2r, 3q, 3r, 6a, 6b and 7a were prepared as previously reported.19–21 Compound 4 was synthesized by a previously published procedure.19 Compounds 7b was synthesized by the same published procedure used for 7a.19 Spectral data are reported for new compounds and for compounds whose spectra are not previously reported in the literature.
2-[2-(Cyclohexyloxy)-2-oxoethyl]benzoic acid (2a)
A solution of homophthalic acid (1a, 4.0 g, 20 mmol), cyclohexanol (4.2 mL, 40 mmol) and 5 drops of concentrated sulfuric acid was refluxed in benzene (100 mL) for six hours with a water trap. The solution was cooled, washed with water (2 × 50 mL), brine (1 × 50 mL) and dried over magnesium sulfate. Filtration and evaporation of the solvent gave a pale yellow oil. Addition of hexane (125 mL) and chilling in an ice bath produced 3.45 g (63%) of compound 2a as white crystals: mp 115–116 °C; 1H NMR: δ 1.54 (m, 10H), 4.03 (s, 2H), 4.80 (m, 1H), 7.28 (d, 1H, J = 9.14 Hz), 7.39 (t, 1H, J = 7.55 Hz), 7.53 (t, 1H, J = 7.54 Hz), 8.12 (d, 1H, J = 7.74 Hz); 13C NMR: δ 23.67, 25.46, 31.53, 41.18, 73.03, 127.32, 128.65, 131.73, 132.26, 133.09, 137.07, 170.75, 172.46.
2-[2-(Cyclohexylmethoxy)-2-oxoethyl]benzoic acid (2b)
(60%) White crystals: mp 107–109 °C; 1H NMR: δ 1.31 (m, 11H), 3.90 (d, 2H, J = 6.36 Hz), 4.05 (s, 2H), 7.27 (d, 1H, J = 7.75 Hz), 7.38 (t, 1H, 7.65 Hz), 7.52 (dd, 1H, J = 1.29, 7.45 Hz), 8.11 (d, 1H, J = 7.75 Hz); 13C NMR: δ 25.27, 26.44, 29.67, 37.14, 40.74, 70.02, 127.40, 128.58, 131.80, 132.31, 133.16, 136.96, 171.43, 172.32.
2-[2-(2-Cyclohexylethoxy)-2-oxoethyl]benzoic acid (2c)
(61%) White crystals: mp 75–76 °C; 1H NMR: δ 1.27 (m, 13H), 4.06 (s, 2H), 4.14 (t, 2H, J = 6.85 Hz), 7.28 (d, 1H, J = 7.75 Hz), 7.39 (t, 1H, J = 7.65 Hz), 7.54 (td, 1H, J = 1.39 Hz, 7.58 Hz), 8.13 (dd, 1H, J = 1.19 Hz, 7.75 Hz); 13C NMR: δ 26.20 26.49, 33.12, 34.56, 35.90, 40.80, 63.16, 127.32, 128.52, 131.75, 132.25, 133.10, 136.91, 171.33, 172.33.
2-[2-(3-Cyclohexylpropoxy)-2-oxoethyl]benzoic acid (2d)
(75%) White crystals: mp 88–90 °C; 1H NMR: δ 0.83 (m, 15H), 4.07 (m, 4H), 7.27 (d, 1H, J = 7.94 Hz), 7.37 (t, 1H, J = 7.55 Hz), 7.52 (t, 1H, J = 7.35 Hz), 8.13 (d, 1H, J = 7.74 Hz); 13C NMR: δ 25.99, 26.33, 26.65, 33.26, 33.44, 37.30, 40.77, 65.30, 127.33, 128.52, 131.76, 132.26, 133.11, 136.92, 171.33, 172.43.
2-[2-(4-Cyclohexylbutoxy)-2-oxoethyl]benzoic acid (2e)
(42%) White crystals: mp 94–95 °C; 1H NMR: δ 1.14 (m, 17H), 4.04 (m, 4H), 7.27 (d, 1H, J = 7.54 Hz), 7.38 (t, 1H, J = 7.75 Hz), 7.52 (t, 1H, J = 7.15 Hz), 8.12 (d, 1H, J = 7.75 Hz), 13C NMR: δ 23.17, 26.44, 26.77, 28.94, 33.37, 37.06, 37.56, 40.81, 65.04, 127.38, 128.58, 131.79, 132.29, 133.13, 136.92, 171.40, 172.19.
2-[2-(5-Cyclohexylpentoxy)-2-oxoethyl]benzoic acid (2f)
(75%) Buff solid: mp 110–111 °C; 1H NMR: δ 1.26 (m, 19H), 4.05 (s, 2H), 4.09 (t, 2H, J = 6.76 Hz), 7.28 (d, 1H, J = 7.55 Hz), 7.39 (t, 1H, J = 7.76 Hz), 7.52 (t, 1H, J = 7.16 Hz), 8.12 (d, 1H, J = 7.76 Hz).
2-[2-(4-Cyclohexylcyclohexyloxy)-2-oxoethyl]benzoic acid (2g)
(60%) of cis/trans product. Recrystallization from ethyl acetate/hexane provided (21%) of the cis isomer. White crystals: mp 147–148 °C; 1H NMR: δ 1.37 (m, 20H), 4.04 (s, 2H), 4.99 (s, 1H), 7.27 (d, 1H, J = 7.55 Hz), 7.37 (t, 1H, J = 7.65 Hz), 7.52 (t, 1H, J = 7.35 Hz), 8.12 (d, 1H, J = 7.54 Hz); 13C NMR: δ 24.21, 26.80, 30.06, 30.17, 41.23, 42.20, 42.63, 70.49, 127.20, 128.45, 131.72, 132.23, 133.03, 137.21, 170.61, 171.54.
2-[2-(Cyclopentyloxy)-2-oxoethyl]benzoic acid (2h)
(72%) White crystals: mp 131–133 °C; 1H NMR: δ 1.66 (m, 8H), 4.00 (s, 2H), 5.16 (m, 1H), 7.26 (d, 1H, J = 7.15 Hz), 7.38 (td, 1H, J = 1.20, 7.65 Hz), 7.51 (td, 1H, J = 1.46, 7.40 Hz), 8.11 (dd, 1H, J = 1.30, 7.85 Hz), 11.61 (br s, 1H); 13C NMR: δ 23.67, 32.53, 41.04, 127.26, 128.57, 131.68, 132.21, 133.03, 136.97, 171.05, 172.45.
2-[2-(Cyclopentylmethoxy)-2-oxoethyl]benzoic acid (2i)
(65%)White crystals: mp 94–95 °C; 1H NMR δ 1.46 (m, 8H), 2.17 (m, 1H), 3.98 (d, 2H, J = 7.15 Hz), 4.05 (s, 2H), 7.27 (d, 1H, J = 7.55 Hz), 7.38 (t, 1H, J = 7.65 Hz), 7.52 (t, 1H, J = 7.35 Hz), 8.12 (d, 1H, J = 7.75 Hz); 13C NMR: δ 25.33, 29.28, 38.50, 40.76, 68.77, 127.34, 128.55, 131.74, 132.26, 133.09, 136.91, 171.41, 172.36.
2-[2-(2-Cyclopentylethoxy)-2-oxoethyl]benzoic acid (2j)
(47%) White crystals: mp 71–73 °C; 1H NMR: δ 1.40 (m, 11H), 4.06 (s, 2H), 4.12 (t, 2H, J = 6.85 Hz), 7.28 (d, 1H, J = 7.55 Hz), 7.39 (t, 1H, J = 7.65 Hz), 7.53 (t, 1H, J = 7.05 Hz), 8.13 (d, 1H, J = 7.75 Hz); 13C NMR: δ 25.08, 32.56, 34.70, 36.86, 40.80, 64.55, 127.36, 128.55, 131.77, 132.30, 133.13, 136.91, 171.39, 172.47.
2-[2-(3-Cyclopentylpropoxy)-2-oxoethyl]benzoic acid (2k)
(75%) White crystals: mp 72–73 °C; 1H NMR: δ 1.27 (m, 13H), 4.08 (m, 4H), 7.27 (d, 1H, J = 7.55 Hz), 7.37 (t, 1H, J = 7.75 Hz), 7.51 (t, 1H, J = 7.54 Hz), 8.12 (d, 1H, J = 7.74 Hz); 13C NMR: δ 25.20, 27.88, 32.26, 32.67, 39.80, 40.80, 65.23, 127.37, 128.57, 131.80, 132.30, 133.15, 136.95, 171.37, 172.40.
2-(2-Oxo-2-propoxyethyl)benzoic acid (2l)
(68%) White crystals: mp 103–104 °C; 1H NMR: δ 0.90 (t, 2H, J = 7.35 Hz), 1.63 (m, 2H), 4.05 (m, 4H), 7.27 (d, 1H, J = 7.54 Hz), 7.38 (t, 1H, J = 7.65 Hz), 7.52 (td, 1H, J = 1.39, 7.45 Hz), 8.12 (dd, 1H, J = 1.20, 7.74 Hz); 13C NMR: δ 10.39, 21.99, 40.78, 66.42, 127.40, 128.56, 131.78, 132.31, 133.15, 136.87, 171.42, 172.53.
2-(2-Butoxy-2-oxoethyl)benzoic acid (2m)
(70%) White crystals: mp 84–86 °C; 1H NMR: δ 0.89 (t, 2H, J = 7.25 Hz), 1.34 (m, 2H), 1.59 (m, 2H), 4.04 (s, 2H), 4.09 (t, 2H, J = 6.66 Hz), 7.27 (d, 1H, J = 7.55 Hz), 7.38 (t, 1H, J = 7.75 Hz), 7.52 (t, 1H, J = 7.35 Hz), 8.12 (d, 1H, J = 7.75 Hz); 13C NMR: δ 13.71, 19.92, 30.65, 40.79, 64.69, 127.39, 128.55, 131.78, 132.31, 133.16, 136.89, 171.43, 172.57.
2-(2-Methoxy-2-oxoethyl)-5-nitrobenzoic acid (2n)
(99%) Pale yellow crystals: mp 164–166 °C (lit.20,21 167–168 °C); 1H NMR: (DMSO-d6) δ 3.58 (s, 3H), 4.15 (s, 2H), 7.65 (d, 1H, J = 8.54 Hz), 8.33 (dd, 1H, J = 2.59, 8.35 Hz), 8.60 (d, 1H, J = 2.39 Hz); 13C NMR: (DMSO-d6) δ 51.65, 95.63, 124.92, 126.14, 131.71, 133.98, 143.08, 146.42, 166.22, 170.44.
2-(2-Cyclohexylmethoxy-2-oxoethyl)-5-nitrobenzoic acid (2o)
(80%) Buff crystals: mp 135–136.°C; 1H NMR: δ 1.35 (m, 11H), 3.94 (d, 2H, J = 6.16 Hz), 4.19 (s, 2H), 7.51 (d, 1H, J = 8.54 Hz), 8.39 (dd, 1H, J = 8.34, 2.58 Hz), 8.97 (d, 1H, J = 2.38 Hz), 11.33 (s, 1H).
2-(2-Cyclohexylethoxy-2-oxoethyl)-5-nitrobenzoic acid (2p)
(82%) Buff crystals: mp 126–127°C; 1H NMR: δ 1.29 (m, 13H), 4.17 (t, 2H, J = 6.95 Hz), 4.19 (s, 2H), 7.52 (d, 1H, J = 8.54 Hz), 8.39 (dd, 1H, J = 8.35, 2.39 Hz), 8.98 (d, 1H, J = 2.39 Hz), 11.34 (s, 1H).
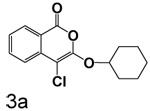
4-Chloro-3-(cyclohexyloxy)isocoumarin (3a)
A solution of 2a (1.5 g, 5.4 mmol) in benzene (125 mL) and phosphorous pentachloride (2.78 g, 13 mmol) was refluxed for sixteen hours. The solution was cooled and washed with water (2 × 25 mL), saturated sodium bicarbonate solution (2 × 50 mL) and brine (1 × 25 mL). The benzene layer was dried over magnesium sulfate, filtered and evaporated to give a dark red oil that was chromatographed (hexane/ethyl acetate). The resulting yellow oil was triturated with hexane (3 × 20 mL) to give 1.0 g (62%) of 3a as a pale yellow solid: mp 67–69 °C; 1H NMR: δ 1.60 (m, 10H), 4.79 (m, 1H), 7.35 (m, 1H), 7.68 (m, 2H), 8.16 (d, 1H, J = 7.95 Hz); 13C NMR: δ 23.31, 25.31, 31.93, 92.97, 117.62, 122.32, 126.16, 129.98, 135.43, 137.79, 152.59, 159.88; Anal. calcd for C15H15ClO3: C, 64.64; H, 5.42. Found: C, 64.33; H, 5.59.
4-Chloro-3-(cyclohexylmethoxy)isocoumarin (3b)
(60%) Pale yellow solid: mp 81–82 °C; 1H NMR: δ 1.60 (m, 11H), 4.17 (d, 2H, J = 6.16 Hz), 7.37 (m, 1H), 7.72 (m, 2H), 8.19 (d, 1H, J = 7.74 Hz); 13C NMR: δ 25.62, 26.32, 29.36, 37.68, 75.65, 90.85, 117.15, 122.07, 125.90, 129.92, 135.42, 137.85, 153.23, 159.47. Anal. calcd for C16H17ClO3: C, 65.64; H, 5.85. Found: C, 65.44; H, 5.96.
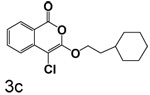
4-Chloro-3-(2-cyclohexylethoxy)isocoumarin (3c)
(55%) Pale yellow solid: mp 71–72 °C; 1H NMR: δ 1.28 (m, 13H), 4.41 (t, 2H, J = 6.75 Hz), 7.37 (m, 1H), 7.71 (m, 2H), 8.19 (d, 1H, J = 7.95 Hz); 13C NMR: δ 26.21, 26.49, 33.16, 34.32. 36.49, 69.12, 91.20, 117.27, 122.17, 126.01, 129.98, 135.48, 137.85,153.17, 159.54; Anal. calcd for C17H19ClO3: C, 66.56; H, 6.24. Found: C, 66.84; H, 6.42.
4-Chloro-3-(3-cyclohexylpropoxy)isocoumarin (3d)
(78%) Pale yellow solid: mp 55–56 °C; 1H NMR: δ 1.31 (m, 15H), 4.35 (t, 2H, J = 6.65 Hz), 7.37 (m, 1H), 7.71 (m, 2H), 8.18 (d, 1H, J = 7.74 Hz); 13C NMR: δ 26.32, 26.63, 33.18, 33.26, 37.25, 71.23, 91.16, 117.25, 122.15, 125.98, 129.95, 135.44, 137.83, 153.12, 159.51, 172.91. Anal. (C18H21ClO3).
4-Chloro-3-(4-cyclohexylbutoxy)isocoumarin (3e)
(73%) Pale yellow solid: mp 41–42 °C; 1H NMR: δ 1.31 (m, 17H), 4.36 (t, 2H, J = 6.56 Hz), 7.36 (m, 1H), 7.71 (m, 2H), 8.17 (d, 1H, J = 7.55 Hz); 13C NMR: δ 22.94, 26.44, 26.75, 29.55, 33.38, 37.00, 37.57, 70.95, 91.26, 117.30, 122.20, 126.04, 130.01, 135.50, 137.89, 153.16, 159.60. Anal. (C19H23ClO3) C, H.
4-Chloro-3-(5-cyclohexylpentoxy)isocoumarin (3f)
(84%) Pale yellow solid: mp °C; 1H NMR: δ 1.39 (m, 19H), 4.36 (t, 2H, J = 6.56 Hz), 7.37 (m, 1H), 7.71 (m, 2H), 8.18 (d, 1H, J = 7.55 Hz); 13C NMR: δ 25.98, 26.49, 26.81, 29.31, 33.48, 37.35, 37.62, 70.98, 91.27, 117.35, 122.23, 126.05, 130.04, 135.51, 137.92, 153.21, 159.59. Anal. (C20H25ClO3) C, H.
4-Chloro-3-(4-cyclohexylcyclohexyloxy)isocoumarin (3g)
(62%) Yellow solid: mp 125–126 °C; 1H NMR: δ 1.28 (m, 20H), 5.06 (s, 1H), 7.37 (m, 1H), 7.71 (m, 2H), 8.17 (d, 1H, J = 7.74 Hz); 13C NMR: δ 23.88, 26.83, 30.18, 30.43, 42.34, 42.79, 77.66, 92.94, 117.55, 122.20, 126.06, 129.94, 135.35, 137.80, 152.58, 159.81. Anal. (C21H25ClO3) C, H.
4-Chloro-3-(cyclopentyloxy)isocoumarin (3h)
(59%) Pale yellow crystals: mp 52–54 °C; 1H NMR: δ 1.76 (m, 8H), 5.27 (m, 1H), 7.36 (m, 1H), 7.67 (m, 2H), 8.17 (d, 1H, J = 7.94 Hz); 13C NMR: δ 23.49, 32.90, 84.52, 92.99, 117.53, 122.25, 126.13, 129.95, 135.38, 137.71, 152.65, 159.76. Anal. (C14H13ClO3) C, H.
4-Chloro-3-(cyclopentylmethoxy)isocoumarin (3i)
(59%) Pale yellow solid: mp 44–46 °C; 1H NMR: δ 1.60 (m, 8H), 2.36 (m, 1H), 4.24 (d, 2H, J = 7.15 Hz), 7.36 (m, 1H), 7.70 (m, 2H), 8.17 (d, 1H, J = 7.75 Hz); 13C NMR: δ 25.38, 29.18, 39.09, 74.61, 91.17, 117.27, 122.16, 125.97, 129.95, 135.44, 137.86, 153.20, 159.55. Anal. (C15H15ClO3) C, H.
4-Chloro-3-(2-cyclopentylethoxy)isocoumarin (3j)
(65%) Pale yellow crystals: mp 47–48 °C; 1H NMR: δ 1.42 (m, 11H), 4.38 (t, 2H, J = 6.75 Hz), 7.36 (m, 1H), 7.70 (m, 2H), 8.18 (d, 1H, J = 7.94 Hz); 13C NMR: δ 25.13, 32.65, 35.37, 36.61, 70.44, 91.13, 117.28, 122.20, 126.02, 130.02, 135.51, 137.93, 153.20, 159.58. Anal. (C16H17ClO3) C, H.
4-Chloro-3-(3-cyclopentylpropoxy)isocoumarin (3k)
(87%) Pale yellow oil: 1H NMR: δ 1.42 (m, 13H), 4.30 (t, 2H, J = 6.66 Hz), 7.30 (m, 1H), 7.63 (m, 2H), 8.11 (d, 1H, J = 7.34 Hz); 13C NMR: δ 25.20, 28.49, 31.97, 32.66, 39.71, 71.09, 91.13, 117.24, 122.13, 125.98, 129.95, 135.44, 137.82, 153.13, 159.48. Anal. (C17H19ClO3) C, H.
4-Chloro-3-propoxyisocoumarin (3l)
(68%) Yellow solid: mp 48–50 °C; 1H NMR: δ 1.04 (t, 2H, J = 7.45 Hz), 1.82 (m, 2H), 4.33 (t, 2H, J = 6.55 Hz), 7.39 (m, 1H), 7.70 (m, 2H), 8.17 (d, 1H, J = 7.74 Hz); 13C NMR: δ 10.25, 22.73, 72.34, 91.26, 117.32, 122.22, 126.07, 130.03, 135.52, 137.90, 153.17, 159.60. Exact mass calcd for C12H11ClO3: 238.0397, observed (M+H) 239.0475.
3-Butoxy-4-chloroisocoumarin (3m)
(75%) Yellow solid: mp 55–56 °C; 1H NMR: δ 0.96 (t, 2H, J = 7.35 Hz), 1.48 (m, 2H), 1.76 (m, 2H), 4.35 (t, 2H, J = 6.46 Hz), 7.33 (t, 1H, J = 7.35 Hz), 7.67 (m, 2H), 8.14 (d, 1H, J = 7.55 Hz); 13C NMR: δ 13.67, 18.91, 31.24, 70.55, 91.12, 117.21, 122.09, 125.95, 129.91, 135.40, 137.78, 153.11, 159.42.
4-Chloro-3-methoxy-7-nitroisocoumarin (3n)
(73%) Yellow solid: mp 129–132 °C (lit.20,21 128– 131°C) ; 1H NMR: δ 4.14 (s, 3H), 7.78 (d, 1H, J = 8.93 Hz), 8.48 (dd, 1H, J = 2.38, 8.94 Hz), 8.97 (d, 1H, J = 2.18 Hz); 13C NMR: δ 57.21, 89.50, 116.44, 123.26, 126.19, 129.62, 142.93, 144.94, 155.87, 157.15.
4-Chloro-3-cyclohexylmethoxy-7-nitroisocoumarin (3o)
(70%) Pale yellow solid: mp 106–108°C; 1H NMR: δ 1.45 (m, 11H), 4.28 (d, 2H, J = 6.16 Hz), 7.83 (d, 1H, J = 8.93 Hz), 8.53 (dd, 1H, J = 2.19, 8.93 Hz), 9.04 (d, 1H, J = 2.19 Hz). Exact mass calcd for C16H16ClNO5: 337.0717, observed (M+H) 338.0785.
4-Chloro-3-(2-cyclohexylethoxy)-7-nitroisocoumarin (3p)
(71%) Pale yellow solid: mp 120–121°C; 1H NMR: δ 1.38 (m, 13H), 4.52 (t, 2H, J = 6.56 Hz), 7.83 (d, 1H, J = 8.94 Hz), 8.52 (dd, 1H, J = 2.18, 8.94 Hz), 9.04 (d, 1H, J = 2.18 Hz). Exact mass calcd for C17H18ClNO5: 351.0874, observed (M+H) 352.0942.
7-Amino-4-chloro-3-methoxyisocoumarin (4)
(89%) Yellow crystals: mp 183–185 °C (lit.20,21 175–176 °C); 1H NMR: (DMSO-d6) δ 3.93 (s, 3H), 5.74 (br s, 2H), 7.14 (dd, 1H, J = 1.99, 8.54 Hz), 7.25 (d, 1H, J = 1.79 Hz), 7.40 (d, 1H, J = 8.54 Hz); 13C NMR: (DMSO-d6) δ 57.62, 91.12, 93.63, 110.79, 118.36, 122.86, 122.95, 125.07, 148.03, 150.22, 159.00.
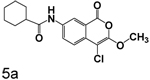
4-Chloro-7-cyclohexanecarboxamide-3-methoxyisocoumarin (5a)
To a solution of 4 (0.09 g, 0.39 mmol) in dry tetrahydrofuran (10 mL) was added cyclohexanecarbonyl chloride (65 uL, 0.47 mmol) and triethylamine (60 uL, 0.39 mmol). The solution was stirred at room temperature for six hours after which time the triethylamine hydrochloride was filtered off and washed with hot tetrahydrofuran (3 × 10 mL). The mother liquor was evaporated to give a pale yellow semi solid that was crystallized from tetrahydrofuran/hexane to afford 100 mg (76%) of a crude yellow solid. Recrystallization from ethanol provided 75 mg of 5a as a pale yellow solid: mp 213–214 °C; 1H NMR: (DMSO-d6) 1.33 (m, 6H), 1.78 (m, 4H), 2.31 (m, 1H), 4.00 (s, 3H), 7.58 (d, 1H, J = 8.54 Hz), 7.98 (d, 1H, J = 8.34 Hz), 8.49 (s, 1H), 10.17 (s, 1H); 13C NMR: (DMSO-d6) δ 25.16, 25.36, 29.01, 44.84, 57.27, 89.45, 117.17, 118.06, 122.23, 127.12, 131.80, 137.83, 152.35, 158.50, 174.49. Exact mass calcd for C17H18ClNO4: 335.0924, observed (M+H) 336.1003.
7-Benzamido-4-chloro-3-methoxyisocoumarin (5b)
(33%) Pale yellow solid: mp 220–221 °C; 1H NMR: (DMSO-d6) δ 4.02 (s, 3H), 7.56 (m, 3H), 7.68 (d, 1H, J = 8.74 Hz), 7.99 (m, 2H), 8.26 (dd, 1H, J = 2.19, 8.71 Hz), 8.68 (d, 1H, J = 2.19 Hz), 10.62 (s, 1H); 13C NMR: (DMSO-d6) δ 40.51, 89.34, 117.15, 119.35, 122.19, 127.55, 128.18, 128.30, 131.71, 132.44, 134.20, 137.55, 152.61, 158.51, 165.53. Exact mass calcd for C17H12ClNO4: 329.0455, observed (M+H) 330.0535.
3-Propoxy-4-(trifluoroacetyl)isocoumarin (6c)
A solution of 2l (0.5 g, 2 mmol) and trifluoroacetic anhydride (0.38 mL, 2.7 mmol) in dichloromethane (20 mL) was stirred for four hours at room temperature. The solution was evaporated and chromatographed (chloroform) to give 0.11 g (59% yield based on recovered starting material) of compound 6c as white crystals: mp 126–127 °C; 1H NMR: δ 1.03 (t, 3H, J = 7.45 Hz), 1.87 (m, 2H), 4.49 (t, 2H, J = 6.76 Hz), 7.39 (t, 1H, J = 7.65 Hz), 7.71 (m, 1H), 8.11 (d, 1H, J = 8.35 Hz), 8.19 (d, 1H, J = 7.94 Hz); 13C NMR: δ 10.03, 22.02, 73.13, 77.20, 90.70, 115.91, 115.96, 123.38, 126.51, 130.23, 136.25, 158.07, 162.63, 180.26. Anal. (C14H11F3O4) C, H.
3-Cyclohexylmethoxy-7-nitroisocoumarin (6d)
(60%) Yellow crystals: mp 122–123°C; 1H NMR: δ 1.04 (m, 11H), 4.00 (d, 2H, J = 5.96 Hz), 5.66 (s, 1H), 7.40 (d, 1H, J = 8.74 Hz), 8.40 (dd, 1H, J = 2.38, 8.74 Hz), 9.03 (d, 1H, J = 1.98 Hz). Exact mass calcd for C16H17NO5: 303.1107, observed (M+H) 304.1185.
3-(2-Cyclohexylethoxy)-7-nitroisocoumarin (6e)
(62%) Buff crystals: mp 143–144°C; 1H NMR: δ 1.33 (m, 13H), 4.25 (t, 2H, J = 6.55 Hz), 5.67 (s, 1H), 7.40 (d, 1H, J = 8.74 Hz), 8.39 (dd, 1H, J = 2.38, 8.74 Hz), 9.02 (d, 1H, J = 2.18 Hz). Exact mass calcd for C17H19NO5: 317.1263, observed (M+H) 318.1349.
3-Cyclohexylmethyl-4-(trifluoroacetyl)isocoumarin (6f)
(60%) White crystals: mp 112–113°C; 1H NMR: δ 1.45 (m, 11H), 4.36 (d, 2H, J = 6.36 Hz), 7.42 (t, 1H, J = 8.14 Hz), 7.74 (t, 1H, J = 7.15 Hz), 8.12 (d, 1H, J = 8.34 Hz), 8.23 (d, 1H, J = 7.95 Hz). Exact mass calcd for C18H17F3O4: 354.1079, observed (M+H) 355.1145.
3-(2-Cyclohexylethoxy)-4-trifluoroacetylisocoumarin (6g)
(60%) White crystals: mp 80–81°C. 1H NMR: δ 1.37 (m, 13H), 4.59 (t, 2H, J = 6.95 Hz), 7.42 (t, 1H, J = 8.14 Hz), 7.74 (t, 1H, J = 7.15 Hz), 8.15 (d, 1H, J = 8.34 Hz), 8.24 (d, 1H, J = 8.15 Hz). Exact mass calcd for C19H19F3O4: 368.1235, observed (M+H) 369.1245.
7-Amino-3-(2-bromoethoxy)isocoumarin (7b)
7-Amino-3-(2-bromoethoxy)isocoumarin (7b) (81%) Yellow crystals: mp> 280 °C; 1H NMR: (DMSO-d6) δ 3.79 (t, 2H, J = 5.17 Hz), 4.36 (t, 2H, J = 4.97 Hz), 5.50 (s, 2H), 7.03 (d, 1H, J = 8.54 Hz), 7.19 (m, 2H); 13C NMR: (DMSO-d6) δ 30.30, 68.71, 80.35, 110.20, 117.91, 123.17, 125.84, 128.03, 147.16, 154.71, 160.45. Exact mass calcd for C11H10BrNO3: 282.9844, observed (M+H) 283.9927.
Acknowledgements
This work was supported by a grant from the National Institutes of Health, HL68598.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Arterioscerl Throm Vasc Biol. 2004;24:e149–e161. doi: 10.1161/01.ATV.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 2.Murdoch D, Scott LJ. Ezetimibe/Simvastatin: a review of its use in the management of hypercholesterolemia. Am J Cardiovasc Drugs. 2004;4:405–422. doi: 10.2165/00129784-200404060-00009. [DOI] [PubMed] [Google Scholar]
- 3.Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 2005;44:467–494. doi: 10.2165/00003088-200544050-00002. [DOI] [PubMed] [Google Scholar]
- 4.Hui DY, Howles PN. Molecular mechanisms of cholesterol absorption and transport in the intestine. Sem Cell Develop Biol. 2005;16:183–192. doi: 10.1016/j.semcdb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Pearson TA. The epidemiologic basis for population-wide cholesterol reduction in the primary prevention of coronary artery disease. A J Cardiol. 2004;94:4F–8F. doi: 10.1016/j.amjcard.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 6.Wilson MM, Thomas DR. Dietary factors in atherogenesis. Curr Atheroscler Rep. 2003;5:324–330. doi: 10.1007/s11883-003-0056-4. [DOI] [PubMed] [Google Scholar]
- 7.Hui DY. Molecular biology of enzymes involved with cholesterol ester hydrolysis in mammalian tissues. Biochim Biophys Acta. 1996;1303:1–14. doi: 10.1016/0005-2760(96)00085-9. [DOI] [PubMed] [Google Scholar]
- 8.Howles PN, Carter CP, Hui DY. Dietary free and esterified cholesterol absorption in cholesterol esterase (bile salt-stimulated lipase) gene-targeted mice. J Biol Chem. 1996;271:7196–7202. doi: 10.1074/jbc.271.12.7196. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Candales A, Bosner MS, Spilburg CA, Lange LG. Cholesterol transport function of pancreatic cholesterol esterase: directed sterol uptake and esterification in enterocytes. Biochemistry. 1993;32:12085–12089. doi: 10.1021/bi00096a019. [DOI] [PubMed] [Google Scholar]
- 10.Shamir R, Johnson WJ, Zolfaghari R, Lee HS, Fisher EA. Role of bile salt-dependent cholesteryl ester hydrolase in the uptake of micellar cholesterol by intestinal cells. Biochemistry. 1995;34:6351–6358. doi: 10.1021/bi00019a013. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda I, Matsuoka R, Hamada T, Mitsui K, Imabayashi S, Uchino A, Sato M, Kuwano E, Itamura T, Yamada K, Tanaka K, Imaizumi K. Cholesterol esterase accelerates intestinal cholesterol absorption. Biochim Biophys Acta. 2002;1571:34–44. doi: 10.1016/s0304-4165(02)00204-0. [DOI] [PubMed] [Google Scholar]
- 12.Bruneau N, Bendayan M, Gingras D, Ghitescu L, Levy E, Lombardo D. Transcytosis of pancreatic bile salt-dependent lipase through human Int407 intestinal cells. Gastroenterology. 2003;124:470–480. doi: 10.1053/gast.2003.50051. [DOI] [PubMed] [Google Scholar]
- 13.Bruneau N, Richard S, Silvy F, Verine A, Lombardo D. Lectin-like Ox-LDL receptor is expressed in human INT-407 intestinal cells: involvement in the transcytosis of pancreatic bile salt-dependent lipase. Mol Biol Cell. 2003;14:2861–2875. doi: 10.1091/mbc.E02-08-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebai O, Le Petet-Thevenin J, Bruneau N, Lombardo D, Verine A. In vitro angiogenic effects of pancreatic bile salt-dependent lipase. Arterioscler Thromb Vasc Biol. 2005;25:359–364. doi: 10.1161/01.ATV.0000151618.49109.bd. [DOI] [PubMed] [Google Scholar]
- 15.Deck LM, Baca ML, Salas SL, Hunsaker LA, Vander Jagt DL. 3-Alkyl-6-chloro-2-pyrones: selective inhibitors of pancreatic cholesterol esterase. J Med Chem. 1999;42:4250–4256. doi: 10.1021/jm990309x. [DOI] [PubMed] [Google Scholar]
- 16.Stoddard-Hatch M, Brown WM, Deck JA, Hunsaker LA, Deck LM, Vander Jagt DL. Inhibition of yeast lipase (CRL1) and cholesterol esterase (CRL3) by 6-chloro-2-pyrones: comparison with porcine cholesterol esterase. Biochim Biophys Acta. 2002;1596:381–391. doi: 10.1016/s0167-4838(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 17.Heidrich JE, Contos LM, Hunsaker LA, Deck LM, Vander Jagt DL. Inhibition of pancreatic cholesterol esterase reduces cholesterol absorption in the hamster. BMC Pharmacology. 2004;4:5. doi: 10.1186/1471-2210-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kam C, Kerrigan J, Plaskon R, Duffy E, Lollar P, Suddath F, Powers J. Mechanism-based isocoumarin inhibitors for blood coagulation serine proteases. Effect of the 7-substituent in 7-imino-4-chloro-3-(isothioureidoalkoxy)isocoumarins on inhibitory and anticoagulant potency. J Med Chem. 1994;37:1298–1306. doi: 10.1021/jm00035a009. [DOI] [PubMed] [Google Scholar]
- 19.Heynekamp JJ, Hunsaker LA, Vander Jagt TA, Deck LM, Vander Jagt DL. Uncharged isocoumarin-based inhibitors of urokinase-type plasminogen activator. BMC Chem Biol. 2006;6:1. doi: 10.1186/1472-6769-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bihel F, Quelever G, Lelouard H, Petit A, Alves de Costa C, Pourquie O, Checler F, Thelland A, Pierre P, Karaus J. Synthesis of new 3-alkoxy-7-amino-4-isocoumarin derivatives as new beta-amyloid peptide production inhibitors and their activities on various classes of protease. Bioorg Med Chem. 2003;11:3141–3152. doi: 10.1016/s0968-0896(03)00235-9. [DOI] [PubMed] [Google Scholar]
- 21.Choksey I, Usagaonkar RN. Isocoumarins : Part XV. 7-Nitro- and aminoisocoumarins from 4-nitrohomophthalic acid. Indian J Chem Sect B. 1976;14B:596–598. [Google Scholar]