Abstract
Stress begins in the brain and affects the brain, as well as the rest of the body. Acute stress responses promote adaptation and survival via responses of neural, cardiovascular, autonomic, immune and metabolic systems. Chronic stress can promote and exacerbate pathophysiology through the same systems that are dysregulated. The burden of chronic stress and accompanying changes in personal behaviors (smoking, eating too much, drinking, poor quality sleep; otherwise referred to as “lifestyle”) is called allostatic overload. Brain regions such as hippocampus, prefrontal cortex and amygdala respond to acute and chronic stress and show changes in morphology and chemistry that are largely reversible if the chronic stress lasts for weeks. However, it is not clear whether prolonged stress for many months or years may have irreversible effects on the brain. The adaptive plasticity of chronic stress involves many mediators, including glucocorticoids, excitatory amino acids, endogenous factors such as brain neurotrophic factor (BDNF), polysialated neural cell adhesion molecule (PSA-NCAM) and tissue plasminogen activator (tPA). The role of this stress-induced remodeling of neural circuitry is discussed in relation to psychiatric illnesses, as well as chronic stress and the concept of top-down regulation of cognitive, autonomic and neuroendocrine function. This concept leads to a different way of regarding more holistic manipulations, such as physical activity and social support as an important complement to pharmaceutical therapy in treatment of the common phenomenon of being “stressed out”. Policies of government and the private sector play an important role in this top-down view of minimizing the burden of chronic stress and related lifestyle (i.e. allostatic overload).
1. Introduction
The brain is the central organ of the stress response and determines what is stressful, as well as the behavioral and physiological responses to potential and actual stressors. The brain is also a target of stress and it changes structurally and chemically in response to both acute and chronic stressors. Glucocorticoids play a role in these changes, but there are other mediators as well. Although glucocorticoids and catecholamines are the two defining hormones of the “fight or flight” stress response, there are many other mediators, such as pro- and anti-inflammatory cytokines and the parasympathetic nervous system, that are also involved in the adaptation to stressors, as well as in the negative impact of chronic stress, known in every day language as being “stressed out”.
Indeed, there are important differences in the effects of acute and chronic stress, as well as differences in the consequences of acute and chronic treatment with glucocorticoids. This review explores these topics in the context of discussing the concepts of allostasis and allostatic load and overload and what they say about brain and body adaptation to acute and chronic stressors, as well as what can be done to reduce the negative aspects of allostatic overload. The principal theme of the discussion of interventions is how to take advantage of the central role that the brain plays in perceiving, responding to and adapting or not adapting efficiently to experiences and events throughout the lifecourse.
1.1 Defining stress, allostasis and allostatic load
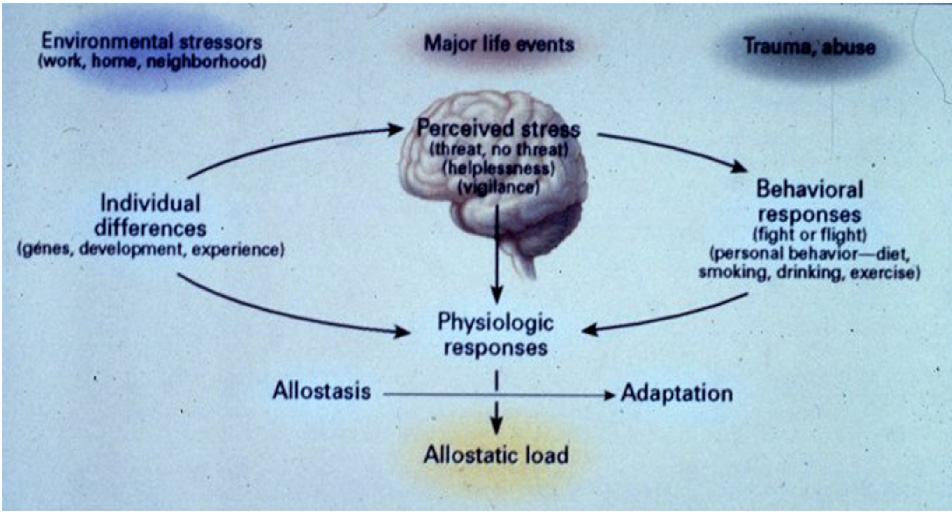
“Stress” is an ambiguous term and has connotations that make it less than useful in understanding how the body can adapt or fail to adapt efficiently to experiences in daily life, including both daily hassles as well as major life events and abuse or trauma (Fig. 1). Insight into the biological and behavioral processes can lead to a better understanding of ways to intervene, a topic that will be discussed at the end of this article. There are two sides to this story (McEwen, 1998): on the one hand, the body responds to almost any sudden, unexpected event by releasing chemical mediators – e.g. catecholamines that increase heart rate and blood pressure – and help the individual cope with the situation; on the other hand, chronic elevation of these same mediators - e.g. chronically increased heart rate and blood pressure – produce a chronic wear and tear on the cardiovascular system that can result, over time, in disorders such as stroke and heart attacks. For this reason, the term “allostasis” was introduced by Sterling and Eyer (Sterling and Eyer, 1988) to refer to the active process by which the body responds to daily events and maintains homeostasis (allostasis literally means “achieving stability through change” and is not intended to replace “homeostasis”).
Fig. 1. Brain is the central organ of the stress response.
The brain is the central organ of perceiving and responding to stressors and determines both the behavioral and physiological responses.
The response to acute stress is adaptive (allostasis) whereas the response to chronic stress can lead to dysregulaiton of the mediators and exacerbate pathophysiology (allostatic load or overload). Early life experiences and genetic constitution play a major role in determining both acute and chronic responses. Besides major life events and trauma and abuse, ordinary stressors from the family, neighborhood and work are major contributors to allostatic load. Reprinted from McEwen 1998, by permission.
Because chronically increased or dysregulated allostasis can lead to disease, we introduced the term “allostatic load or overload” to refer to the wear and tear that results from either too much stress or from inefficient management of allostasis, e.g. not turning off the response when it is no longer needed(McEwen and Stellar, 1993; McEwen, 1998; McEwen and Wingfield, 2003). Other forms of allostatic overload involve not turning on an adequate response in the first place or not habituating to the recurrence of the same stressor and thus dampening the allostatic response. The advantage of the terminology “allostatic load/overload” over terms such as “the burden of chronic stress” is that there are changes in behavior (poor sleep, eating or drinking too much, smoking, lack of physical activity) that are part of the allostatic load/overload concept which are not so obvious in the use of the word “stress”.
1.2 Multiple mediators and multiple systems
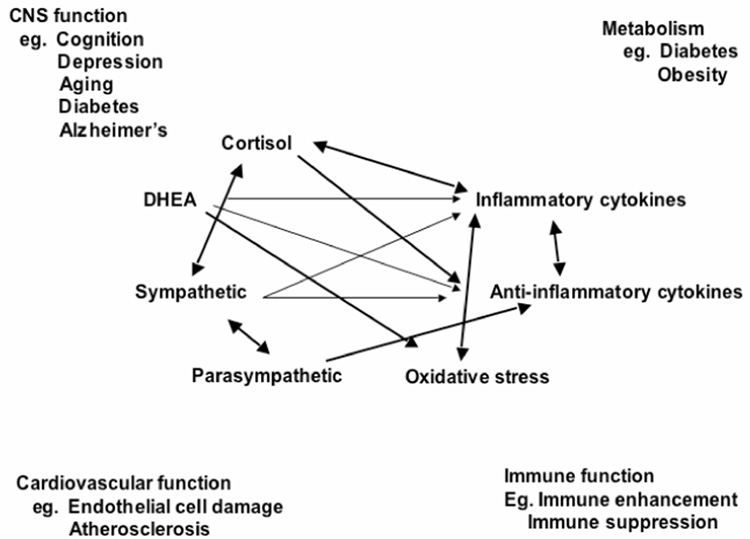
Protection and damage are the two contrasting sides of the physiology involved in defending the body against the challenges of daily life, whether or not we call them “stressors”. Glucocorticoids produced by the adrenal cortex in response to ACTH from the pituitary gland is the other major “stress hormone” besides adrenalin that we usually think of in connection with “stress”. However, there are other important hormones/mediators, as noted in Figure 2. Pro- and anti-inflammatory cytokines are produced by many cells in the body, and they regulate each other and are, in turn, regulated by glucocorticoids and catecholamines. Whereas catecholamines can increase pro-inflammatory cytokine production, glucocorticoids are known to inhibit this production (Sapolsky et al., 2000). Yet, there are exceptions – pro-inflammatory effects of glucocorticoids that depend on dose and cell or tissue type (Dinkel et al., 2003; MacPherson et al., 2005). The parasympathetic nervous system also plays an important regulatory role in this non-linear network of allostasis, since it generally opposes the sympathetic nervous system and slows the heart. Parasympathetic activity also has anti-inflammatory effects (Thayer and Lane, 2000; Borovikova et al., 2000).
Fig. 2. Mediators of stress and allostasis operate in a non-linear network.
Non-linear network of mediators of allostasis involved in the stress response. Arrows indicate that each system regulates the others, creating a non-linear network. Note that many body systems are influenced by the same mediators.
These interactions are non-linear and very complex. What this non-linearity means is that when any one mediator is increased or decreased, there are compensatory changes in the other mediators that depend on time course and level of change of each of the mediators. Unfortunately, we cannot measure all components of this system simultaneously and must rely on measurements of only a few of them in any one study. Yet the non-linearity must be kept in mind in interpreting the results of any investigation that measures the biomarkers of allostasis.
The concepts of allostasis and allostatic load/overload emphasize the existence of multiple interacting mediators and the almost inevitable consequences of wear and tear on the body and brain from adapting to demands of daily life over time. These concepts also include the behavioral and physiological consequences of how an individual responds to chronic stressors, large or small, in terms of eating, sleeping, drinking alcohol, smoking, physical activity, social interactions. This condition is often referred to as being “stressed out” and behavioral patterns (sometime called “lifestyle”) are a large part of allostatic overload.
The common experience of being “stressed out” has as its core the elevation of some of the key systems that lead to allostatic overload – e.g., cortisol, sympathetic activity and pro-inflammatory cytokines, with a decline in parasympathetic activity. Nowhere is this better illustrated than for sleep deprivation, which is a frequent result of being “stressed out”. Sleep deprivation produces an allostatic overload that can have deleterious consequences (McEwen, 2006; McEwen, 2007). The effects include elevated evening cortisol, insulin and blood glucose, elevated blood pressure, reduced parasympathetic activity and elevated levels of proinflammatory cytokines, as well as the gut hormone, ghrelin, which increases appetite. Hunger for comfort foods and increased caloric intake are one result, along with depressed mood and cognitive impairment (Dallman, 2003;McEwen, 2006). In contrast to these potentially maladaptive consequences, the same mediators are involved in the natural world in adaptation to environmental changes (McEwen and Wingfield, 2003).
1.3 Stress in the natural world
Allostasis and allostatic load are very important for animals in the natural world, which use these adaptive responses for their own benefit or for the benefit of the species. Here, the term allostatic load is used (as opposed to allostatic overload) to refer to cumulative effects of responding to environmental demands that have an adaptive value for the survival of the species. Regarding allostasis, in Spring, a sudden snowstorm is an acute stressor to birds and disrupts mating, and elevated cortisol is pivotal in directing the birds to suspend reproduction, to find a source of food and to relocate to a better mating site or at least to delay reproduction until the weather improves (Wingfield and Romero, 2000). For allostatic load, bears preparing to hibernate for the winter eat large quantities of food and put on body fat to act as an energy source during the winter (Nelson, 1980). This anticipatory accumulation of fat is used, then, to survive the winter and provide food for gestation of young.
This is in contrast to fat accumulation that occurs in bears that are captive in zoos and eating too much, partially out of boredom, while not exercising (McEwen and Wingfield, 2003). The accumulation of fat under these latter conditions can be called “allostatic overload” referring to a condition that is associated with pathophysiology. Yet, some types of allostatic overload can also have a useful purpose for the preservation of the species, such as in migrating salmon or the marsupial mouse, which die of excessive stress after mating – the stress, and allostatic load, being caused for salmon, in part, by the migration up the rapidly flowing rivers but also because of physiological changes that represent accelerated aging (Maule et al., 1989; Farrell, 2002; Gotz et al., 2005). The result is freeing up food and other resources for the next generation. In the case of the marsupial mouse, it is only the males that die after mating, due apparently to a response to mating that reduces the binding protein, corticosteroid binding globulin (CBG), for glucocorticoids and renders them much more active throughout the body (Cockburn and Lee, 1988).
2. Central role of the brain
The brain is the key organ of the stress response because it determines what is threatening and, therefore, potentially stressful, and also controls the behavioral and physiological responses, and resulting lifestyle, discussed earlier in this article, which are as important to development of allostatic load and overload as the stressful experiences themselves (Fig. 1).
There are enormous individual differences in the response to stress, based upon the experience of the individual early in life and in adult life. Positive or negative experiences in school, at work or in romantic and family interpersonal relationships can bias an individual towards either a positive or negative response in a new situation. For example, someone who has been treated badly in a job by a domineering and abusive supervisor and/or has been fired will approach a new job situation quite differently than someone who has had positive experiences in employment. How the individual reacts may carry over into habits such as smoking, drinking excessively, eating too much, poor sleep, lack of exercise and interaction with friends and family, all of which contribute to allostatic overload.
Early life experiences perhaps carry an even greater weight in terms of how an individual reacts to new situations. Early life physical and sexual abuse carry with it a life-long burden of behavioral and pathophysiological problems (Felitti et al., 1998; Heim and Nemeroff, 2001). Cold and uncaring families produce long-lasting emotional problems in children (Repetti et al., 2002). Some of these effects are seen on brain structure and function and in the risk for later depression and post-traumatic stress disorder (Kaufman and Charney, 1999; Kaufman et al., 2000; Vermetten et al., 2006).
Animal models have been useful in providing insights into behavioral and physiological mechanisms. Early life maternal care in rodents is a powerful determinant of life-long emotional reactivity and stress hormone reactivity and increases in both are associated with earlier cognitive decline and a shorter lifespan (Francis et al., 1999; Cavigelli and McClintock, 2003). Effects of early maternal care are transmitted across generations by the subsequent behavior of the female offspring as they become mothers, and methylation of DNA on key genes appears to play a role in this epigenetic transmission (Weaver et al., 2004). Furthermore, in rodents, abuse of the young is associated with an attachment, rather than an avoidance, of the abusive mother, an effect that increases the chances that the infant can continue to obtain food and other support until weaning (Sullivan et al., 2000). Moreover, other conditions that affect the rearing process can also affect emotionality in offspring. For example, uncertainty in the food supply for rhesus monkey mothers leads to increased emotionality in offspring and possibly an earlier onset of obesity and diabetes (Coplan et al., 2001).
So far, we have emphasized the important role of the environment and experiences of individuals in the health outcomes, but clearly genetic differences also play an important role. Different alleles of commonly occurring genes determine how individuals will respond to experiences. For example, the short form of the serotonin transporter is associated with a number of conditions such as alcoholism, and individuals who have this allele are more vulnerable to respond to stressful experiences by developing depressive illness (Caspi et al., 2003). In childhood, individuals with an allele of the monoamine oxidase A gene are more vulnerable to abuse in childhood and more likely to themselves become abusers, and to show antisocial behaviors compared to individuals with another commonly occurring allele (Caspi et al., 2002). Yet another example is the consequence of having the Val66Met allele of the BDNF gene on hippocampal volume, memory and mood disorders (Hariri et al., 2003; Pezawas et al., 2004; Jiang et al., 2005; Szeszko et al., 2005).
2.1 Stress and glucocorticoid effects on the hippocampus
2.1.1 Types of structural plasticity
One of the ways that stress hormones modulate function within the brain is by changing the structure of neurons. The hippocampus is one of the most sensitive and malleable regions of the brain and is also very important in cognitive function. Within the hippocampus, the input from the entorhinal cortex to the dentate gyrus is ramified by the connections between the dentate gyrus and the CA3 pyramidal neurons. One granule neuron innervates, on the average, 12 CA3 neurons, and each CA3 neuron innervates, on the average, 50 other CA3 neurons via axon collaterals, as well as 25 inhibitory cells via other axon collaterals. The net result is a 600-fold amplification of excitation, as well as a 300-fold amplification of inhibition, that provides some degree of control of the system (McEwen, 1999).
As to why this type of circuitry exists, the dentate gyrus-CA3 system is believed to play a role in the memory of sequences of events, although long-term storage of memory occurs in other brain regions (Lisman and Otmakhova, 2001). But, because the dentate gyrus-CA3 system is so delicately balanced in its function and vulnerability to damage, there is also adaptive structural plasticity, in that new neurons continue to be produced in the dentate gyrus throughout adult life, and CA3 pyramidal cells undergo a reversible remodeling of their dendrites in conditions such as hibernation and chronic stress (Popov et al., 1992; Popov and Bocharova, 1992; McEwen, 1999). The role of this plasticity may be to protect against permanent damage. As a result, the hippocampus undergoes a number of adaptive changes in response to acute and chronic stress.
One type of change involves replacement of neurons. The sub-granular layer of the dentate gyrus contains cells that have some properties of astrocytes (e.g. expression of glial fibrillary acidic protein) and which give rise to granule neurons (Kempermann and Gage, 1999; Seri et al., 2001). After bromodeoxy uridine administration to label DNA of dividing cells, these newly born cells appear as clusters in the inner part of the granule cell layer, where a substantial number of them will go on to differentiate into granule neurons within as little as 7 days. In the adult rat, 9000 new neurons are born per day and survive with a half-life of 28 days (Cameron and McKay, 2001). There are many hormonal, neurochemical and behavioral modulators of neurogenesis and cell survival in the dentate gyrus including estradiol, insulin-like growth factor-1, antidepressants, voluntary exercise and hippocampal-dependent learning (Aberg et al., 2000; Trejo, Carro et al., 2001; Czeh et al., 2001). With respect to stress, certain types of acute stress and many chronic stressors suppress neurogenesis or cell survival in the dentate gyrus, and the mediators of these inhibitory effects include excitatory amino acids acting via NMDA receptors and endogenous opioids (Gould et al., 1997).
Another form of structural plasticity is the remodeling of dendrites in the hippocampus. Chronic restraint stress causes retraction and simplification of dendrites in the CA3 region of the hippocampus (McEwen, 1999); Sousa et al., 2000). Such dendritic reorganization is found in both dominant and subordinate rats undergoing adaptation of psychosocial stress in the visible burrow system and it is independent of adrenal size (McKittrick et al., 2000).
What this result emphasizes is that it is not adrenal size or presumed amount of physiological stress per se that determines dendritic remodeling, but a complex set of other factors that modulate neuronal structure. Indeed, in species of mammals that hibernate, dendritic remodeling is a reversible process and occurs within hours of the onset of hibernation in European hamsters and ground squirrels, and it is also reversible within hours of wakening of the animals from torpor (Popov et al., 1992; Popov and Bocharova, 1992; Arendt et al., 2003). This implies that reorganization of the cytoskeleton is taking place rapidly and reversibly and that changes in dendrite length and branching are not “damage” but a form of structural plasticity.
2.1.2 Glucocorticoids do not work alone
Regarding the mechanism of structural remodeling, adrenal steroids are important mediators of remodeling of hippocampal neurons during repeated stress, and exogenous adrenal steroids can also cause remodeling in the absence of an external stressor. The role of adrenal steroids involve many interactions with neurochemical systems in the hippocampus, including serotonin, GABA and excitatory amino acids (McEwen, 1999; McEwen and Chattarji, 2004). Probably the most important interactions are those with excitatory amino acids such as glutamate. Excitatory amino acids released by the mossy fiber pathway play a key role in the remodeling of the CA3 region of the hippocampus, and regulation of glutamate release by adrenal steroids may play an important role (McEwen, 1999).
Among the consequences of restraint stress is the elevation of extracellular glutamate levels, leading to induction of glial glutamate transporters, as well as increased activation of the nuclear transcription factor, phosphoCREB (Wood et al., 2004). Moreover, 21d chronic restraint stress (21d chronic restraint stress) leads to depletion of clear vesicles from mossy fiber terminals and increased expression of presynaptic proteins involved in vesicle release (Magarinos et al., 1997) (Grillo et al., 2005). Taken together with the fact that vesicles that remain in the mossy fiber terminal are near active synaptic zones and that there are more mitochondria in the terminals of stressed rats, this suggests that chronic restraint stress increases the release of glutamate (Magarinos et al., 1997).
2.1.3 Variable glucocorticoid involvement in structural plasticity
Because glucocorticoids do not work alone, other mediators play a role in affecting the response to glucocorticoids. For neurogenesis in dentate gyrus, elevated glucocorticoid levels in an enriched environment or during physical activity are associated with increased neurogenesis and/or cell survival, even though there are other conditions in which glucocorticoids suppress neurogenesis (Mirescu and Gould, 2006). Chronicity of glucocorticoid elevation may play a role, with acute glucocorticoid elevation suppressing cell proliferation and prolonged glucocorticoid exposure ceasing to have this effect (Mirescu and Gould, 2006). Chronic restraint stress is known to reduce dentate gyrus proliferation whereas acute restraint does not have any measurable effect (Pham et al., 2003). In contrast, the ability of physical activity to elevate neurogenesis depends on the social housing environment: that is, individual housing of rats that results in elevated corticosterone levels prevented running from acutely increasing neurogenesis. Yet, reducing corticosterone levels by adrenalectomy and supplementation with corticosterone in the drinking water reinstated the positive effect of exercise on neurogenesis (Stranahan et al., 2006).
This implies a shift in glucocorticoid sensitivity and a possible factor may be excitatory neurotransmission. NMDA receptors play a role in regulation of neurogenesis, having both positive and negative effects in different experimental settings (Nacher and McEwen, 2006), and blocking NMDA receptors prevents acute glucocorticoid effects on neurogenesis (Cameron et al., 1998) indicating that the role of excitatory amino acids is a primary one. In this connection, it is important to recall the different effects of stress on memory that depend on the state of arousal and the timing with the learning situation (Joels et al., 2006). Moreover, the possible involvement of non-genomic effects of adrenal steroids must be considered (see below).
One of the consequences of the involvement of multiple mediators along with adrenal steroids in brain function is the conditional nature of adrenal steroid actions on memory. Emotional arousal for a rodent by being placed in a novel environment is required for adrenal steroids to enhance object recognition memory, that involves the hippocampus; the effects of adrenal steroids on this memory show an inverted U shaped dose response (Okuda et al., 2004). Moreover, spatial memory in a Morris water maze, a stressful behavioral task, is facilitated by adrenal steroids in wild type mice, but this facilitation is lacking in mice with a dimerization deficient glucocorticoid receptor (Oitzl et al., 1997). In the study involving novelty-induced emotional arousal, the dose range of corticosterone is such that both glucocorticoid receptor and mineralocorticoid occupancy are involved (Okuda et al., 2004). Yet, prior habituation to the novel environment, thus removing the emotional arouse of novelty, abolishes the facilitation (Okuda et al., 2004). Moreover, corticosterone doses that facilitate memory at 24h post training, inhibit memory retention at 1h post training (Okuda et al., 2004). The inhibition of memory retrieval by acute corticosteroid administration is a phenomenon that has also been reported (Newcomer et al., 1994; Newcomer et al., 1999; de Quervain et al., 2000; Roozendaal et al., 2003), and biphasic effects of corticosteroids on working memory have been described (Lupien et al., 2005). Joels and colleagues (Joels et al., 2006) propose a very plausible unifying theory that notes the importance of context and timing.
2.1.4 Effects of chronic glucocorticoid administration on morphology and memory
In spite of the fact that glucocorticoids do not work alone, chronic corticosterone treatment by injection or by passive administration in the drinking water are both able to cause dendrites to retract in CA3 hippocampus (Woolley et al., 1990; Magarinos et al., 1999; Sousa et al., 2000). Moreover, the effects of injected corticosterone are known to be blocked by Dilantin, an inhibitor of ion channels that has anti-epileptic effects, a result that is consistent with the evidence that glutamate is involved in remodelling (Watanabe et al., 1992). Yet, there is an important difference between the effects of repeated stress and chronic glucocorticoid exposure, in that chronic corticosterone treatment was reported to reduce the volume fraction occupied by mitochondria in the CA3 region (Coburn-Litvak et al., 2004) while, as noted earlier, 21d chronic restraint stress increases mitochondrial profiles in mossy fiber terminals (Magarinos et , 1997). This suggests that somewhat different mechanisms may be involved in effects of chronic restraint stress and corticosterone in hippocampus, a possibility that is supported by the finding that, while corticosterone treatment in the drinking water and 21d chronic restraint stress both caused CA3 remodeling when given alone, the combination of chronic restraint stress plus corticosterone treatment abolished the morphological change (Magarinos et al., 1998). These mechanistic differences remain to be determined.
In spite of the possible differences in mechanism, chronic corticosterone treatment and chronic restraint or immobilization stress both cause impairment of hippocampal dependent memory tasks, although there are differences in magnitude of effect that appear to be dependent on dose of corticosterone, duration of treatment, age of rat being treated and whether or not the cognitive task is a demanding one (Dachir et al., 1993; Arbel et al., 1994; Bardgett et al., 1994; Bodnoff et al., 1995; Endo et al., 1996; Bardgett et al., 1996; Mclay et al., 1998; Coburn-Litvak et al., 2003). These studies indicate that only more prolonged treatment by higher glucocorticoid doses are able to impair performance on more demanding tasks involving hippocampal function and that they do so under conditions in which there is no neuronal loss but there are reductions in volume of hippocampal neuropil that may be due to loss of glia cells or reduction of dendritic length and branching. Given these results with rodents, it is not so surprising that a relatively modest regimen of cortisol treatment for 12 months did not cause outright neuronal loss in the pigtail macaque hippocampus (Leverenz et al., 1999).
2.1.5 Role of genomic and non-genomic mechanisms
The hippocampus expresses both Type I (mineralocorticoid, mineralocorticoid receptor) and Type II (glucocorticoid, glucocorticoid receptor) receptors, and these receptors mediate a biphasic response to adrenal steroids in the CA1 region although not in the dentate gyrus (Joels, 2006), which, nevertheless, shows a diminished excitability in the absence of adrenal steroids (Margineanu et al., 1994). Other brain regions, such as the paraventricular nucleus, lacking in mineralocorticoid receptors but having glucocorticoid receptors, show a monophasic response (Joels, 2006). Adrenal steroids exert biphasic effects on excitability of hippocampal neurons in terms of long-term potentiation and primed burst potentiation (Diamond et al., 1992; Pavlides et al., 1994; Pavlides et al., 1995) and show parallel biphasic effects upon memory (Pugh et al., 1997).
In considering possible mechanisms for the biphasic responses, the co-expression of mineralocorticoid receptors and glucocorticoid receptors in the same neurons could give rise to heterodimer formation and a different genomic activation from that produced by either mineralocorticoid receptor or glucocorticoid receptor homodimers (Joels, 2006). In addition, deletion of the Type I (mineralocorticoid receptors) receptor by genetic means has revealed that MR are required for non-genomic regulation of glutamatergic transmission by glucocorticoids (Karst et al., 2005), a phenomenon that involved glucocorticoid enhancement of extracellular levels of glutamate (Venero and Borrell, 1999) that plays an important role in both modulatory and excitotoxic effects of glucocorticoids (see subsection below: “Mechanisms of structural remodeling”). Although beyond the scope of this review, the subject of non-genomic actions of adrenal steroids has taken on increasing importance in view of the discovery of adrenal steroid receptors that are G protein coupled in the amphibian brain (Orchinik et al., 1991), as well as glucocorticoid receptor immunoreactivity in post-synaptic and other non-nuclear regions of neurons in the rodent brain (Liposits and Bohn, 1993,; Johnson et al., 2005) and a large number of reported rapid, non-genomic actions of adrenal steroids (Borski, 2000; Makara and Haller, 2001). Hence it is perhaps not surprising that there are conditions involving neural transmission that favor either rapid positive or negative actions of adrenal steroids on processes such as learning and memory.
Although much of the work on mineralocorticoid receptors and glucocorticoid receptors has been done on rat and mouse brains, it is important to note that the rhesus monkey hippocampus has a predominance of mineralocorticoid receptors and relatively less glucocorticoid receptors compared to rodent species (Sanchez et al., 2000). This finding may have important implications for the effects of adrenal steroids on learning and vulnerability to stress and excitotoxicity, as well as age-related changes discussed earlier.
2.2 Prefrontal cortex and amygdala
Repeated stress also causes changes in other brain regions such as the prefrontal cortex and amygdala. Repeated stress causes dendritic shortening in medial prefrontal cortex (Sousa et al., 2000; Wellman, 2001; Vyas et al., 2002; Kreibich and Blendy, 2004; Cook and Wellman, 2004; Radley et al., 2004; Brown et al., 2005; Radley et al., 2005) but produces dendritic growth in neurons in amygdala (Vyas et al., 2002), as well as in orbitofrontal cortex (Liston et al., In Press). Along with many other brain regions, the amygdala and prefrontal cortex also contain adrenal steroid receptors; however, the role of adrenal steroids, excitatory amino acids and other mediators has not yet been studied in these brain regions. Nevertheless, in the amygdala, there is some evidence regarding mechanism, in that tissue plasminogen activator (tPA) is required for acute stress to activate not only indices of structural plasticity but also to enhance anxiety (Melchor et al., 2003). These effects occur in the medial and central amygdala and not in basolateral amygdala and the release of CRH acting via CRH1 receptors appears to be responsible (Matys et al., 2004).
Acute stress induces spine synapses in CA1 region of hippocampus (Shors et al., 2001) and both acute and chronic stress also increases spine synapse formation in amygdala (Vyas et al., 2002) but chronic stress decreases it in hippocampus (Pawlak et al., 2005). Moreover, chronic stress for 21 days or longer impairs hippocampal-dependent cognitive function (McEwen, 1999) and enhances amygdala-dependent unlearned fear and fear conditioning (Conrad et al., 1999), which are consistent with the opposite effects of stress on hippocampal and amygdala structure. Chronic stress also increases aggression between animals living in the same cage, and this is likely to reflect another aspect of hyperactivity of the amygdala (Wood et al., 2003). Behavioral correlates of remodeling in the prefrontal cortex include impairment in attention set shifting, possibly reflecting structural remodeling in the medial prefrontal cortex (Liston et al., In Press).
2.3 Role of other modulators in structural remodeling
Extracellular molecules play a role in remodeling in the hippocampus and amygdala. Neural cell adhesion molecule (NCAM) and its polysialated-NCAM (PSA-NCAM), as well as L1 are expressed in the dentate gyrus and CA3 region and the expression of both NCAM, L1, and PSA-NCAM are regulated by 21d chronic restraint stress (Sandi, 2004). Tissue plasminogen activator (tPA) is an extracellular protease and signalling molecule that is released with neural activity and is required for chronic stress-induced loss of spines and NMDA receptor subunits on CA1 neurons (Pawlak et al., 2005).
Within the neuronal cytoskeleton, the remodeling of hippocampal neurons by chronic stress and hibernation alters the acetylation of microtubule subunits that is consistent with a more stable cytoskeleton (Bianchi et al., 2003) and alters microtubule associated proteins, including the phosphorylation of a soluble form of tau, which is increased in hibernation and reversed when hibernation is terminated (Arendt et al., 2003).
Neurotrophic factors also play a role in dendritic branching and length in that BDNF +/− mice show a less branched dendritic tree and do not show a further reduction of CA3 dendrite length with chronic stress, whereas wild-type mice show reduced dendritic branching (Magarinos, McEwen, unpublished). However, there is contradictory information thus far concerning whether chronic restraint stress reduces BDNF mRNA levels, some reporting a decrease (Smith and Cizza, 1996) and other studies reporting no change (Kuroda and McEwen, 1998; Isgor et al., 2004). This may reflect the balance of two opposing forces, namely, that stress triggers increased BDNF synthesis to replace depletion of BDNF caused by stress (Marmigere et al., 2003). BDNF and corticosteroids appear to oppose each other – with BDNF reversing reduced excitability in hippocampal neurons induced by stress levels of corticosterone (Hansson et al., 2006).
Corticotrophin releasing factor (CRF) is a key mediator of many aspects related to stress (Koob, 1999). CRF in the paraventricular nucleus regulates ACTH (adrenocorticotrophic hormone) release from the anterior pituitary gland, whereas CRF in the central amygdala is involved in control of behavioral and autonomic responses to stress, including the release to tPA that is an essential part of stress-induced anxiety and structural plasticity in the medial amygdala (Matys et al., 2004). CRF in the hippocampus is expressed in a subset of GABA neurons (Cajal-Retzius cells) in the developing hippocampus, and early life stress produces a delayed effect that reduces cognitive function and the number of CA3 neurons as well as decreased branching of hippocampal pyramidal neurons (Brunson et al., 2001; Brunson et al., 2005). Indeed, CRF inhibits dendritic branching in hippocampal cultures in vitro (Chen et al., 2004).
3. Translation to the human brain
3.1 Depression, Cushing’s Disease and Anxiety Disorders
Much of the impetus for studying the effects of stress on the structure of the human brain has come from the animal studies summarized thus far. Although there is very little evidence regarding the effects of ordinary life stressors on brain structure, there are indications from functional imaging of individuals undergoing ordinary stressors, such as counting backwards that there are lasting changes in neural activity (Wang et al., 2005). Another study, using voxel-based morphometry, has uncovered a relationship between shrinkage of grey matter volume in the hippocampus and orbitofrontal cortex and prospective reports of chronic life stress over a 20 year period (Gianaros et al., 2007).
Moreover, the study of depressive illness and anxiety disorders has also provided some insights. Life events are known to precipitate depressive illness in individuals with certain genetic predispositions (Kessler, 1997; Kendler, 1998; Caspi et al., 2003). Moreover, brain regions such as the hippocampus, amygdala and prefrontal cortex show altered patterns of activity in PET (positron emission tomography) and fMRI (functional magnetic resonance imaging) and also demonstrate changes in volume of these structures with recurrent depression: decreased volume of hippocampus and prefrontal cortex and amygdala (Drevets et al., 1997; Sheline et al., 1999; Sheline et al., 2003).
Interestingly, amygdala volume has been reported to increase in the first episode of depression, whereas hippocampal volume is not decreased (MacQueen et al., 2003; Frodl et al., 2003). It has been known for some time that stress hormones, such as cortisol, are involved in psychopathology, reflecting emotional arousal and psychic disorganization rather than the specific disorder per se (Sachar et al., 1973). We now know that adrenocortical hormones enter the brain and produce a wide range of effects upon it.
In Cushing’s disease, there are depressive symptoms that can be relieved by surgical correction of the hypercortisolemia (Starkman and Schteingart, 1981; Murphy, 1991). Both major depression and Cushing’s disease are associated with chronic elevation of cortisol that results in gradual loss of minerals from bone and abdominal obesity. In major depressive illness, as well as in Cushing’s disease, the duration of the illness and not the age of the subjects predicts a progressive reduction in volume of the hippocampus, determined by structural magnetic resonance imaging (Starkman et al., 1992; Sheline et al., 1999). Moreover, there are a variety of other anxiety-related disorders, such as post-traumatic stress disorder (PTSD) (Bremner, 2002) (Pitman, 2001) and borderline personality disorder (Driessen et al., 2000), in which atrophy of the hippocampus has been reported, suggesting that this is a common process reflecting chronic imbalance in the activity of adaptive systems, such as the HPA (hypothalamo-pituitary-adrenal axis) axis, but also including endogenous neurotransmitters, such as glutamate.
Another important factor in hippocampal volume and function is glucose regulation. Outright Type 2 diabetes and poor glucose control as measured by glycosylated hemoglobin is associated with reduced hippocampal volume (Gold et al., 2007). Furthermore, poor glucose regulation is associated with smaller hippocampal volume and poorer memory function in individuals in their 60’s and 70’s who have “mild cognitive impairment” (mild cognitive impairment) (Convit et al., 2003), and both mild cognitive impairment and Type 2, as well as Type 1, diabetes are recognized as risk factors for dementia (Ott et al., 1996; de Leon et al., 2001; Haan, 2006).
3.2 Positive affect, self esteem and social support
Having a positive outlook on life and good self esteem appear to have long-lasting health consequences (Pressman and Cohen, 2005), and good social support is also a positive influence on the measures of allostatic load (Seeman et al., 2002). Positive affect, assessed by aggregating momentary experiences throughout a working or leisure day, was found to be associated with lower cortisol production and higher heart rate variability (showing higher parasympathetic activity), as well as a lower fibrinogen response to a mental stress test (Steptoe et al., 2005).
On the other hand, poor self esteem has been shown to cause recurrent increases in cortisol levels during a repetition of a public speaking challenge in which those individuals with good self esteem are able to habituate, i.e., attenuate their cortisol response after the first speech (Kirschbaum et al., 1995). Furthermore, poor self esteem and low internal locus of control have been related to 12–13% smaller volume of the hippocampus, as well as higher cortisol levels during a mental arithmetic stressor (Pruessner et al., 1999; Pruessner et al., 2005).
Related to both positive affect and self esteem is the role of friends and social interactions in maintaining a healthy outlook on life. Loneliness, often found in people with low self esteem, has been associated with larger cortisol responses to wakening in the morning, and higher fibrinogen and natural killer cell responses to a mental stress test, as well as sleep problems (Steptoe et al., 2004). On the other hand, having 3 or more regular social contacts, as opposed to 0 to 2 such contacts, is associated with lower allostatic load scores (Seeman et al., 2002).
4. Interventions: conventional vs top down
If being “stressed out” has such pervasive long-term effects on the brain as well as the body, what are the ways to reduce the negative consequences? The answers are simple and obvious but often difficult to achieve and they involve not only individual behaviors but also the ways in which people’s lives are shaped by policies of government and the private sector.
From the standpoint of the individual, it seems obvious that a major goal should be to try to improve sleep quality and quantity, to have good social support and a positive outlook on life, to have positive self-esteem, to maintain a healthy diet, to avoid smoking and to engage in regular moderate physical activity. Concerning physical activity, it is not necessary to become an extreme athlete, and seemingly any amount of moderate physical activity helps (Bernadet, 1995; Rovio et al., 2005).
From the standpoint of organization of society, the goal should be to create incentives at home and in work situations and build community services and opportunities that encourage the development of the beneficial individual lifestyle practices. The Acheson Report (Acheson, 1998) from the United Kingdom in 1998 recognized that no public policy should be enacted without considering the implications for health of all citizens. Thus basic education, housing, taxation, setting of a minimum wage, and addressing occupational health and safety and environmental pollution regulations are all likely to affect health via a myriad of mechanisms. At the same time, providing higher quality food and making it affordable and accessible in poor as well as affluent neighborhoods is necessary for people to eat better, providing they also learn what types of food to eat. Likewise, making neighborhood safer and more congenial and supportive (Sampson et al., 1997) can improve opportunities for positive social interactions and increased recreational physical activity. However, governmental policies are not the only way to reduce allostatic load. For example, businesses that encourage healthy lifestyle practices among their employees are likely to gain reduced health insurance costs and possibly a more loyal workforce (Aldana, 2001; Pelletier, 2001; Whitmer et al., 2003).
These are the “top down” strategies that are likely to work via higher cognitive brain areas and their downstream effects on many processes. As simple as the solutions seem to be, changing behavior and solving problems that cause stress at work and at home is often difficult and may require professional help on the personal level, or even a change of job or profession. Yet these are important goals because the prevention of later disease is very important for full enjoyment of life and also to reduce the financial burden on the individual and on society.
Nevertheless, many people often lack the proactive, long term view of themselves and/or feel that they must maintain a stressful lifestyle and, if they deal with these issues at all, they want to treat their problems with a pill. Are there any medications to treat being stressed out? In fact, there are many useful pharmaceutical agents: sleeping pills, anxiolytics, beta blockers and antidepressants are all drugs that are used to counteract some of the problems associated with being stressed out. Likewise, drugs that reduce oxidative stress or inflammation, block cholesterol synthesis or absorption and treat insulin resistance or chronic pain and can help deal with the metabolic and neurologic consequences of being stressed out. All are valuable to some degree, and yet each one has its side effects and limitations that are based in part on the fact that all of the systems that are dysregulated in allostatic overload are also systems that interact with each other and perform normal functions when properly regulated. Because of the non-linearity of the systems of allostasis, the consequences of any drug treatment may be either to inhibit the beneficial effects of the systems in question or to perturb other systems that interact with it in a direction that promotes an unwanted side effect. So the best solution would seem to be not to rely solely on such medications and find ways to change personal behaviors in a positive direction. Motivation and decision making and perseverance are all functions of the brain!
Acknowledgments
The author is indebted to many talented students and postdoctoral fellows and colleagues in many disciplines and in many parts of the world, including the MacArthur Research Network on Socioeconomic Status and Health. Research support: NIH Grants MH41256 and5P50 MH58911
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of insulin-like growth factor-1 selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson SD. Independent Inquiry into Inequalities in Health Report. London: The Stationary Office; 1998. [Google Scholar]
- Aldana SG. Financial impact of health promotion programs, A comprehensive review of the literature. Am. J. Health Promotion. 2001;15:296–320. doi: 10.4278/0890-1171-15.5.296. [DOI] [PubMed] [Google Scholar]
- Arbel I, Kadar T, Silbermann M, Levy A. The effects of long-term corticosterone administration on hippocampal morphology and cognitive performance of middle-age rats. Brain Res. 1994;657:227–235. doi: 10.1016/0006-8993(94)90972-5. [DOI] [PubMed] [Google Scholar]
- Arendt T, Stieler J, Strijkstra AM, Hut RA, Rudiger J, Van der Zee EA, Harkany T, Holzer M, Hartig W. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J. Neurosci. 2003;23:6972–6981. doi: 10.1523/JNEUROSCI.23-18-06972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett ME, Newcomer JW, Taylor GT. The effects of chronic corticosterone on memory performance in the platform maze task. Physiol. & Behav. 1996;59:1111–1115. doi: 10.1016/0031-9384(95)02172-8. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Taylor GT, Csernansky JG, Newcomer JW, Nock B. Chronic corticosterone treatment impairs spontaneous alternation behavior in rats. Behav. Neural Biol. 1994;61:186–190. doi: 10.1016/s0163-1047(05)80074-3. [DOI] [PubMed] [Google Scholar]
- Bernadet P. Benefits of physical activity in the prevention of cardiovascular disease. J. Cardiovasc. Pharmacol. 1995;25:S3–S8. doi: 10.1097/00005344-199525001-00003. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Heidbreder C, Crespi F. Cytoskeletal changes in the hippocampus following restraint stress. Role of serotonin and microtubules. Synapse. 2003;49:188–194. doi: 10.1002/syn.10230. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J. Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Borski RJ. Nongenomic membrane actions of glucocorticoids in vertebrates. TEM. 2000;11:427–436. doi: 10.1016/s1043-2760(00)00325-8. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Neuroimaging studies in post-traumatic stress disorder. Curr. Psychiat. Reports. 2002;4:254–263. doi: 10.1007/s11920-996-0044-9. [DOI] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cerebral Cortex. 2005;30:1–9. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc. Natl. Acad. Sci. USA. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RDG. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Tanapat P, Gould E. Adrenal steroids and N-methyl-D-aspartate recepor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience. 1998;82:349–354. doi: 10.1016/s0306-4522(97)00303-5. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA, McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proc. Natl. Acad. Sci. USA. 2003;100:16131–16136. doi: 10.1073/pnas.2535721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Brunson KL, Pomper JK, Grigoriadis DE, Durst W, Baram TZ. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc. Natl. Acad. Sci. USA. 2004;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn-Litvak PS, Pothakos K, Tata DA, McCloskey DP, Anderson BJ. Chronic administration of corticosterone impairs spatial reference memory before spatial working memory in rats. Neurobiol. Learning & Memory. 2003;80:11–23. doi: 10.1016/s1074-7427(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Coburn-Litvak PS, Tata DA, Gorby HE, McCloskey DP, Richardson G, Anderson BJ. Chronic corticosterone affects brain weight, and mitochondrial, but not glial volume fraction in hippocampal area CA3. Neuroscience. 2004;124:429–438. doi: 10.1016/j.neuroscience.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Cockburn A, Lee AK. Marsupial femmes fatales. Natural History. 1988;97:40–47. [Google Scholar]
- Conrad CD, Magarinos AM, LeDoux JE, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Convit A, Wolf OT, Tarshish C, de Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc. Natl. Acad. Sci. USA. 2003;100:2019–2022. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Smith ELP, Altemus M, Scharf BA, Owens MJ, Nemeroff CB, Gorman JM, Rosenblum LA. Variable foraging demand rearing: Sustained elevations in cisternal cerebrospinal fluid corticotropin-releasing factor concentrations in adult primates. Biol. Psychiat. 2001;50:200–204. doi: 10.1016/s0006-3223(01)01175-1. [DOI] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dachir S, Kadar T, Robinzon B, Levy A. Cognitive deficits induced in young rats by long-term corticosterone administration. Behav. & Neural Biol. 1993;60:103–109. doi: 10.1016/0163-1047(93)90173-f. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Chronic stress and obesity: A new view of 'comfort food'. Proc. Natl. Acad. Sci. USA. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J. Prediction of cognitive decline in normal elderly subjects with 2-[18F]fluoro-2-deoxy-D-glucose/positron-emission tomography FDG/PET. Proc. Natl. Acad. Sci. USA. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJF, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nature Neuroscience. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Dinkel K, MacPherson A, Sapolsky RM. Novel glucocorticoid effects on acute inflammation in the CNS. J. Neurochem. 2003;84:705–716. doi: 10.1046/j.1471-4159.2003.01604.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr., Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Driessen M, Hermann J, Stahl K, Zwaan M, Meier S, Hill A, Osterheider M, Petersen D. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch. Gen. Psychiat. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- Endo Y, Nishimura JI, Kimura F. Impairment of maze learning in rats following long-term glucocorticoid treatments. Neurosci. Lett. 1996;203:199–202. doi: 10.1016/0304-3940(95)12296-6. [DOI] [PubMed] [Google Scholar]
- Farrell AP. Coronary arteriosclerosis in salmon, growing old or growing fast? Comp. Biochem. & Physiol. Part A. 2002;132:723–735. doi: 10.1016/s1095-6433(02)00126-5. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am. J. Prev. Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, Bottlender R, Leinsinger G, Moller H-J. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol. Psychiat. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. NeuroImage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, Tsui W, Richardson S, Javier E, Convit A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–719. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- Gotz ME, Malz CR, Dirr A, Blum D, Gsell W, Schmidt S, Burger R, Pohli S, Riederer P. Brain aging phenomena in migrating sockeye salmon Oncorhynchus nerka nerka. J. Neural Transm. 2005;112:1177–1199. doi: 10.1007/s00702-004-0257-1. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LAM, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J.Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Wood GE, Reznikov LR, McEwen BS, Reagan LP. Immunocytochemical analysis of synaptic proteins provides new insights into diabetes-mediated plasticity in the rat hippocampus. Neuroscience. 2005;136:477–486. doi: 10.1016/j.neuroscience.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Haan MN. Therapy insight, type 2 diabetes mellitus and the risk of late-onset Alzheimer's disease. Nature Clin. Practice. Neurology. 2006;2:159–166. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Sommer WH, Metsis M, Stromberg I, Agnati LF, Fuxe K. Corticosterone actions on the hippocampal brain-derived neurotrophic factor expression are mediated by Exon IV promoter. J. Neuroendocrin. 2006;18:104–114. doi: 10.1111/j.1365-2826.2005.01390.x. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66 met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders, Preclinical and clinical studies. Biol. Psychiat. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Jiang X, Xu K, Hoberman J, Tian F, Marko AJ, Waheed JF, Harris CR, Marini AM, Enoch M-A, Lipsky RH. BDNF variation and mood disorders, A novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology. 2005;30:1353–1361. doi: 10.1038/sj.npp.1300703. [DOI] [PubMed] [Google Scholar]
- Joels M. Corticosteroid effects in the brain, U-shape it. Trends Pharmacol. Sci. 2006;27:244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Joels M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress, how does it work? Trends Cogn. Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Farb C, Morrison JH, McEwen BS, LeDoux JE. Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience. 2005;136:289–299. doi: 10.1016/j.neuroscience.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc. Natl. Acad. Sci. USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Charney DS. Neurobiological correlates of child abuse. Biol. Psychiat. 1999;45:1235–1236. doi: 10.1016/s0006-3223(99)00064-5. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function. Clinical implications. Biol. Psychiat. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. New nerve cells for the adult brain. Sci. Am. 1999;280:48–53. doi: 10.1038/scientificamerican0599-48. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Major depression and the environment: A psychiatric genetic perspective. Pharmacopsychiat. 1998;31:5–9. doi: 10.1055/s-2007-979287. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annu. Rev. Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosomatic Medicine. 1995;57:468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol. Psychiat. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Kreibich AS, Blendy JA. cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J. Neurosci. 2004;24:6686–6692. doi: 10.1523/JNEUROSCI.1706-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y, McEwen BS. Effect of chronic restraint stress and tianeptine on growth factors, GAP-43 and MAP2 mRNA expression in the rat hippocampus. Mol. Brain Res. 1998;59:35–39. doi: 10.1016/s0169-328x(98)00130-2. [DOI] [PubMed] [Google Scholar]
- Leverenz JB, Wilkinson CW, Wamble M, Corbin S, Grabber JE, Raskind MA, Peskind ER. Effect of chronic high-dose exogenous cortisol on hippocampal neuronal number in aged nonhuman primates. J. Neurosci. 1999;19:2356–2361. doi: 10.1523/JNEUROSCI.19-06-02356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liposits Z, Bohn MC. Association of glucocorticoid receptor immunoreactivity with cell membrane and transport vesicles in hippocampal and hypothalamic neurons of the rat. J. Neurosci. Res. 1993;35:14–19. doi: 10.1002/jnr.490350103. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus, Elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, Tu MT. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30:225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- MacPherson A, Dinkel K, Sapolsky R. Glucocorticoids worsen excitotoxin-induced expression of pro-inflammatory cytokines in hippocampal cultures. Exper. Neurol. 2005;194:376–383. doi: 10.1016/j.expneurol.2005.02.021. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc. Natl. Acad. Sci. USA. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur. J. Pharm. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Orchinik M, McEwen BS. Morphological changes in the hippocampal CA3 region induced by non-invasive glucocorticoid administration, a paradox. Brain Res. 1998;809:314–318. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Verdugo Garcia JM, McEwen BS. Chronic restraint stress alters synaptic terminal structure in hippocampus. Proc. Natl. Acad. Sci. USA. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makara GB, Haller J. Non-genomic effects of glucocorticoids in the neural system. Evidence, mechanisms and implications. Prog. Neurobiol. 2001;65:367–390. doi: 10.1016/s0301-0082(01)00012-0. [DOI] [PubMed] [Google Scholar]
- Margineanu D-G, Gower AJ, Gobert J, Wulfert E. Long-term adrenalectomy reduces hippocampal granule cell excitability in vivo. Brain Res.Bull. 1994;33:93–98. doi: 10.1016/0361-9230(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Marmigere F, Givalois L, Rage F, Arancibia S, Tapia-Arancibia L. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus. 2003;13:646–655. doi: 10.1002/hipo.10109. [DOI] [PubMed] [Google Scholar]
- Matys T, Pawlak R, Matys E, Pavlides C, McEwen BS, Strickland S. Tissue plasminogen activator promotes the effects of corticotropin releasing factor on the amygdala and anxiety-like behavior. Proc. Natl. Acad. Sci. USA. 2004;101:16345–16350. doi: 10.1073/pnas.0407355101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maule AG, Tripp RA, Kaattari SL, Schreck CB. Stress alters immune function and disease resistance in chinook salmon Oncorhynchus tshawytscha. J. Endocrin. 1989;120:135–142. doi: 10.1677/joe.0.1200135. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and Damaging Effects of Stress Mediators. New England J.Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu.Rev.Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor, allostasis and allostatic load. Metabolism. 2006;55:S20–S23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The physiology and neurobiology of stress and adaptation, Central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Chattarji S. Molecular mechanisms of neuroplasticity and pharmacological implications, the example of tianeptine. Eur. Neuropsychopharm. 2004;14:S497–S502. doi: 10.1016/j.euroneuro.2004.09.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the Individual, Mechanisms leading to disease. Archiv. Intern. Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm. & Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Mclay RN, Freeman SM, Zadina JE. Chronic corticosterone impairs memory performance in the Barnes maze. Physiol. & Behav. 1998;63:933–937. doi: 10.1016/s0031-9384(97)00529-5. [DOI] [PubMed] [Google Scholar]
- Melchor JP, Pawlak R, Strickland S. The tissue plasminogen activator - plasminogen proteolytic cascade accelerates amyloid-beta (Abeta) degradation and inhibits Abeta-induced neurodegeneration. J. Neurosci. 2003;23:8867–8871. doi: 10.1523/JNEUROSCI.23-26-08867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Murphy BEP. Treatment of major depression with steroid suppressive drugs. J. Steroid Biochem. Molec. Biol. 1991;39:239–244. doi: 10.1016/0960-0760(91)90069-h. [DOI] [PubMed] [Google Scholar]
- Nacher J, McEwen BS. The role of N-methyl-D-aspartate receptors in neurogenesis. Hippocampus. 2006;16:267–270. doi: 10.1002/hipo.20160. [DOI] [PubMed] [Google Scholar]
- Nelson RA. Protein and fat metabolism in hibernating bears. Federation Proc. 1980;39:2955–2958. [PubMed] [Google Scholar]
- Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME. Glucocorticoid-induced impairment in declarative memory performance in adult humans. J. Neurosci. 1994;14:2047–2053. doi: 10.1523/JNEUROSCI.14-04-02047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, Alderson AL. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch. Gen. Psychiat. 1999;56:527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, De Kloet ER, Joels M, Schmid W, Cole TJ. Spatial learning deficits in mice with a targeted glucocorticoid receptor gene disruption. Eur. J. Neurosci. 1997;9:2284–2296. doi: 10.1111/j.1460-9568.1997.tb01646.x. [DOI] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc. Natl. Acad. Sci. USA. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchinik M, Murray TF, Moore FL. A corticosteroid receptor in neuronal membranes. Science. 1991;252:1848. doi: 10.1126/science.2063198. [DOI] [PubMed] [Google Scholar]
- Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MMB. Association of diabetes mellitus and dementia, The Rotterdam study. Diabetologia. 1996;39:1392–1397. doi: 10.1007/s001250050588. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Kimura A, Magarinos AM, McEwen BS. Type I adrenal steroid receptors prolong hippocampal long-term potentiation. NeuroReport. 1994;5:2673–2677. doi: 10.1097/00001756-199412000-00067. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Watanabe Y, Magarinos AM, McEwen BS. Opposing role of adrenal steroid Type I and Type II receptors in hippocampal long-term potentiation. Neuroscience. 1995;68:387–394. doi: 10.1016/0306-4522(95)00151-8. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Rao BSS, Melchor JP, Chattarji S, McEwen B, Strickland S. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc. Natl. Acad. Sci. USA. 2005;102:18201–18206. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier KR. A review and analysis of the clinical- and cost-effectiveness studies of comprehensive health promotion and disease management programs at the worksite, 1998–2000 update. Am. J. Health Promotion. 2001;16:107–115. doi: 10.4278/0890-1171-16.2.107. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J. Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated, but not acute, restraint stress suppresses proliferation of neural precursor cells and increases PSA-NCAM expression in the adult rat dentate gyrus. J. Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Pitman RK. Hippocampal diminution in PTSD, More or less? than meets the eye. Hippocampus. 2001;11:73–74. doi: 10.1002/hipo.1022. [DOI] [PubMed] [Google Scholar]
- Popov VI, Bocharova LS. Hibernation-induced structural changes in synaptic contacts between mossy fibres and hippocampal pyramidal neurons. Neuroscience. 1992;48:53–62. doi: 10.1016/0306-4522(92)90337-2. [DOI] [PubMed] [Google Scholar]
- Popov VI, Bocharova LS, Bragin AG. Repeated changes of dendritic morphology in the hippocampus of ground squirrels in the course of hibernation. Neuroscience. 1992;48:45–51. doi: 10.1016/0306-4522(92)90336-z. [DOI] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychol. Bull. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Baldwin MW, Dedovic K, Renwick RMNK, Lord C, Meaney M, Lupien S. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. NeuroImage. 2005;28:815–826. doi: 10.1016/j.neuroimage.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Low self-esteem, induced failure and the adrenocortical stress response. Personality and Individual Differences. 1999;27:477–489. [Google Scholar]
- Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav. Neurosci. 1997;111:503–511. [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families, Family social environments and the mental and physical health of offspring. Psychol. Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- Roozendaal B, Griffith QK, Buranday J, de Quervain DJF, McGaugh JL. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval, Dependence on the basolateral amygdala. Proc. Natl. Acad. Sci. USA. 2003;100:1328–1333. doi: 10.1073/pnas.0337480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovio S, Kareholt I, Helkala E-L, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- Sachar EJ, Hellman L, Roffwarg HP, Halpern FS, Fukushima DK, Gallagher TF. Disrupted 24-hour patterns of cortisol secretion in psychotic depression. Arch Gen Psychiarty. 1973;28:19–24. doi: 10.1001/archpsyc.1973.01750310011002. [DOI] [PubMed] [Google Scholar]
- Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime, a multilevel study of collective effects. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain, Relative absence of glucocorticoid receptors in the hippocampal formation. J. Neurosci. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nature Rev. Neurosci. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Ryff CD, Dienberg G, Levy-Storms L. Social relationships, gender, and allostatic load across two age cohorts. Psychosom. Med. 2002;64:395–406. doi: 10.1097/00006842-200205000-00004. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am. J. Psychiat. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J. Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Cizza G. Stress-induced changes in brain-dervied neurotrophic factor expression are attenuated in aged Fischer 344/N rats. Neurobiol.Aging. 1996;17:859–864. doi: 10.1016/s0197-4580(96)00066-8. [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OFX, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in partiens with Cushing's syndrome. Biol. Psychiat. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Schteingart DE. Neuropsychiatric manifestations of patients with Cushing's syndrome. Arch. Intern. Med. 1981;141:215–219. [PubMed] [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Wardlaw J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc. Natl. Acad. Sci. USA. 2005;102:6508–6512. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis, A New Paradigm to Explain Arousal Pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. New York: John Wiley & Sons; 1988. pp. 629–649. [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nature Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, Ashtari M, Napolitano B, Bilder RM, Kane JM, Goldman D, Malhotra AK. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol. Psychiat. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus, a microdialysis study in freely moving rats. Eur. J. Neurosci. 1999;1:2465–2473. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD. Hippocampal and amygdalar volumes in dissociative identity disorder. Am. J. Psychiat. 2006;163:630–636. doi: 10.1176/appi.ajp.163.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc. Natl. Acad. Sci. USA. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992;2:431–436. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Whitmer RW, Pelletier KR, Anderson DR, Baase CM, Frost GJ. A wake-up call for corporate America. JOEM. 2003;45:916–925. doi: 10.1097/01.jom.0000086280.38338.83. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Romero LM. Coping with the Environment, Neural and Endocrine Mechanisms. New York: Oxford University Press; 2000. Adrenocortical responses to stress and their modulation in free-living vertebrates; pp. 211–234. [Google Scholar]
- Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus, Prevention by lithium treatment. Proc. Natl. Acad. Sci. USA. 2004;101:3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GE, Young LT, Reagan LP, McEwen BS. Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm. & Behav. 2003;43:205–213. doi: 10.1016/s0018-506x(02)00026-0. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]