Abstract
Background
In heart failure (HF), digoxin at low serum digoxin concentrations (SDC) reduces all-cause mortality and HF hospitalizations. However, the effects of digoxin on other cause-specific outcomes have not been studied in a propensity matched cohort.
Methods
The Digitalis Investigation Group trial, conducted during 1991–1993, enrolled 7788 ambulatory chronic HF patients. This analysis focuses on 4843 patients: 982 receiving digoxin with low (0.5–0.9 ng/ml) SDC at one month, and 3861 receiving placebo and alive at one month. Propensity scores for low SDC, calculated using a non-parsimonious multivariable logistic regression model, were used to match 982 low-SDC patients with 982 placebo patients. Matched Cox regression analyses were used to determine the effect of digoxin at low SDC on outcomes.
Results
All-cause mortality occurred in 315 placebo (rate, 1071/10000 person-years) and 288 low-SDC digoxin (rate, 871/10000 person-years) patients, respectively, during 2940 and 3305 years of follow up (hazard ratio {HR}, 0.81, 95% confidence interval {CI}, 0.68–0.98; p=0.028). Cardiovascular hospitalizations occurred in 493 placebo (2359/10,000 person-year) and 471 low-SDC digoxin (1963/10,000 person-year) patients, respectively during 2090 and 2399 years of follow up (HR, 0.82, 95% CI, 0.70–0.95; p=0.010). Low-SDC digoxin to placebo HR (95%CI) for HF mortality and HF hospitalizations were respectively, 0.65 (0.45–0.92; P=0.015) and 0.63 (0.52–0.77; P<0.0001). Low-dose digoxin (<=0.125 mg/day) was the strongest independent predictor of low SDC (adjusted odd ratio, 2.07, 95% CI 1.54–2.80).
Conclusions
Digoxin at low SDC significantly reduced mortality and hospitalizations in ambulatory chronic systolic and diastolic HF patients.
Keywords: Digoxin, Low Dose, Low Serum Concentration, Heart Failure, Mortality, Hospitalization
Introduction
Digoxin is the oldest and one of the least expensive heart failure drugs. It is approved by the United States Food and Drug Administration for use in heart failure. 1, 2 Digoxin reduces hospitalizations due to worsening heart failure without increasing mortality. 3–5 It is recommended by major national heart failure guidelines. 6–9 Yet, the use of digoxin is in decline, in part due to its lack of mortality benefit. 10–12 Therapeutic and toxic effects of digoxin are related to its dose and serum digoxin concentrations (SDC). 4, 13, 14 However, reports suggesting no survival benefit of digoxin or harmful effects of digoxin in women did not account for SDC. 3, 15 Digoxin at low SDC appears to reduce mortality in both men and women with heart failure. 4, 14 However, results of these post-hoc analyses were based on traditional multivariable risk adjustments. 16, 17
A recent comprehensive post-hoc analysis of the DIG trial demonstrated that compared with heart failure patients receiving placebo, those receiving digoxin at low (0.5–0.9 ng/ml) SDC had significant reduction in all-cause mortality and all-cause hospitalizations. 4 A propensity score analysis confirmed the effect of digoxin at low SDC on mortality and heart failure hospitalization. 4 However, the effects of digoxin at low SDC on other cause-specific outcomes have not been studied in a propensity-matched cohort. As in randomization, propensity score matching allows elimination of baseline covariate imbalance without access to outcomes data. 17–20 In addition, propensity score technique allows objective estimation of bias reduction. 20, 21 The purpose of this analysis is to examine the effect of digoxin at low SDC on various cause-specific outcomes in a propensity score-matched cohort of heart failure patients.
Materials and Methods
Study Design
Retrospective propensity matched analysis of the DIG trial, which was conducted in the U.S. (186 centers) and Canada (116 centers) in the early 1990’s. The design and the results of the DIG trial has been described previously. 3, 22
Patients
Of the 7788 heart failure patients with normal sinus rhythm in the DIG trial, 6,800 had left ventricular ejection fraction (LVEF) ≤45% and 988 had LVEF >45%. Most patients were receiving angiotensin-converting enzyme (ACE) inhibitors and diuretics. Data on beta-blockers were not collected. The current analysis was restricted to 982 patients who were receiving digoxin and had low (0.5–0.9 ng/ml) SDC at one month after randomization, and 3,861 patients receiving placebo, who were alive at one month. SDC 0.5–0.9 ng/ml has been shown to be therapeutic in prior studies. 4, 14 Specimens for SDC were analyzed in a central laboratory. 3
Outcomes
Primary outcomes were mortality and hospitalizations due to all causes, cardiovascular causes, and worsening heart failure. Data on vital status were 99% complete. 4 Secondary outcomes included other cause-specific deaths and hospitalizations.
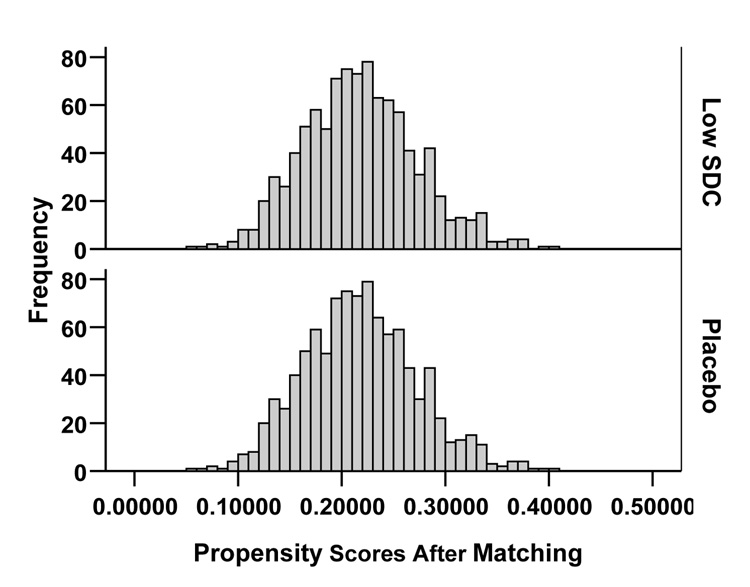
Bias Reduction by Propensity Score Matching
We compared baseline characteristics between treatment groups using Pearson chi-square and Wilcoxon rank-sum tests. Patients with low SDC were younger and less likely to have severe heart failure or to have chronic renal dysfunction (estimated glomerular filtration rate < 60 ml/min/1.73 square meter). 23 To achieve balance in baseline covariates, we matched all 982 low SDC patients to 982 unique patients in the placebo group, who had very similar propensities for low SDC (Figure 1). 24 We calculated propensity scores for low SDC, that is the conditional probability of developing low SDC, for all 4,843 patients using a non-parsimonious multivariable logistic regression model incorporating all measured baseline characteristics. 4, 20 To avoid inflated significance in baseline covariate imbalance in the pre-match cohort, we identified a random subset of 982 patients from the placebo group.
Figure 1.
Distribution of propensity score for the low serum digoxin concentrations, for patients receiving digoxin and placebo, before (a) and after (b) matching
Assessment of Bias Reduction: Absolute Standardized Differences
Covariate imbalance before and after propensity score matching was estimated using absolute standardized differences between the two treatment groups. 4, 20, 21, 25, 26 A standardized difference of less than 10% is taken to indicate a well-balanced covariate. 20, 21, 26 The standardized difference in propensity score between placebo and low SDC patients before and after matching were respectively 48% and 0.0%, indicating substantially improved covariate balance after matching. Placebo-low SDC absolute standardized differences for age, serum creatinine, and diuretic use were respectively 10%, 32%, and 12% before matching and 1%, 0%, and 2% after matching.
Statistical analysis
We used Kaplan-Meier analysis and matched Cox proportional hazards analyses to determine association between digoxin at low SDC and various outcomes. Proportional hazards assumptions were checked using log-minus-log scale survival plots for patients in the two treatment groups. To determine whether the effect of digoxin was homogeneous, we estimated the effects of low SDC (versus placebo) on all-cause mortality in various subgroups of patients. Finally, we identified predictors of low SDC among patients receiving digoxin using logistic regression analysis. 4
We conducted formal sensitivity analyses to describe the weight of our evidence by quantifying the degree of hidden bias that would need to be present to invalidate our main conclusions. All statistical tests were evaluated using two-tailed 95% confidence levels, and data analyses were performed using SPSS for Windows version 14. 27
Results
Patient Characteristics
Patients had a mean age of 63 years, 21% were women, 13% non-white, and 11% had LVEF >45%. Among the 982 low SDC patients, 17%, 73% and 11% respectively were receiving digoxin ≤0.125 mg, 0.25 mg and >0.25 mg per day, with a median dose of 0.25 mg/day. After matching, compared to placebo patients, those with low SDC were balanced in terms of all measured covariates (Table 1).
Table 1.
Baseline Patient Characteristics, Before and After Propensity Score Matching
| Before matching | After matching | ||||
|---|---|---|---|---|---|
| N (%) or mean (±SD) | Random Placebo (N=982) | P | Digoxin at SDC 0.5–0.9 (N=982) | P | Matched Placebo (N=982) |
| Age (years) | 64.2 (±10.8) | 0.004 | 62.8 (±10.6) | 0.834 | 62.7 (±11.2) |
| Age ≥65 years | 520 (53.0%) | 0.034 | 472 (48.1%) | 0.558 | 460(46.8%) |
| Female | 236 (24.0%) | 0.335 | 217 (22.1%) | 0.348 | 199 (20.3%) |
| Non-white | 162 (16.5%) | 0.025 | 126 (12.8%) | 1.000 | 126 (12.8%) |
| Body mass index, kg/square meter | 27.6 (±5.14) | 0.080 | 27.2 (±5.03) | 0.136 | 26.8 (±5.0) |
| Duration of HF (months) | 31.1 (±38.1) | 0.540 | 32.2 (±38.7) | 0.251 | 30 (±37) |
| Primary cause of HF | |||||
| Ischemic | 683 (69.6%) | 672 (68.4%) | 677 (68.9%) | ||
| Hypertensive | 98 (10.0%) | 98 (10.0%) | 90 (9.2%) | ||
| Idiopathic | 133 (13.5%) | 0.799 | 148 (15.1%) | 0.922 | 147 (15.0%) |
| Others | 68 (6.9%) | 64 (6.5%) | 68 (6.9%) | ||
| Comorbid conditions | |||||
| Prior myocardial infarction | 629 (64.1%) | 0.851 | 624 (63.5%) | 0.708 | 615 (62.6%) |
| Current angina | 277 (28.2%) | 0.479 | 262 (26.7%) | 0.759 | 256 (26.1%) |
| Hypertension | 485 (49.4%) | 0.021 | 433 (44.1%) | 1.000 | 432 (44.0%) |
| Diabetes | 294 (29.9%) | 0.147 | 265 (27.0%) | 0.235 | 241 (24.5%) |
| Chronic renal dysfunctioin | 460 (46.8%) | <0.0001 | 368 (37.5%) | 0.745 | 376 (38.3%) |
| Dose of study medication | 0.246 (±0.07) | 0.596 | 0.244 (±0.07) | 0.317 | 0.247 (±0.07) |
| Medications | |||||
| Pre-trial digoxin use | 409 (41.6%) | 0.033 | 457 (46.5%) | 0.104 | 494 (50.3%) |
| ACE inhibitors | 917 (93.4%) | 0.213 | 931 (94.8%) | 0.920 | 930 (94.7%) |
| Diuretics | 768 (78.2%) | 0.006 | 715 (72.8%) | 0.650 | 705 (71.8%) |
| Symptoms and signs of heart | |||||
| Dyspnea at rest | 225 (22.9%) | 0.077 | 192 (19.6%) | 0.820 | 188 (19.1%) |
| Dyspnea on exertion | 736 (74.9%) | 0.470 | 721 (73.4%) | 0.760 | 714 (72.7%) |
| Jugular venous distension | 133 (13.5%) | 0.172 | 112 (11.4%) | 1.000 | 113 (11.5%) |
| Third heart sound | 234 (23.8%) | 0.674 | 243 (24.7%) | 0.793 | 237 (24.1%) |
| Pulmonary râles | 149 (15.2%) | 0.368 | 134 (13.6%) | 0.791 | 129 (13.1%) |
| Lower extremity edema | 207 (21.1%) | 0.193 | 183 (18.6%) | 0.954 | 185 (18.8%) |
| NYHA functional class | |||||
| I | 141 (14.4%) | 173 (17.6%) | 181 (18.4%) | ||
| II | 558 (56.8%) | 0.273 | 536 (54.6%) | 0.845 | 524 (53.4%) |
| III | 268 (27.3%) | 259 (26.4%) | 259 (26.4%) | ||
| IV | 15 (0.8%) | 14 (1.4%) | 18 (1.8%) | ||
| Heart rate (/minute), | 78.3 (±12.4) | 0.067 | 77.2 (±12.7) | 0.329 | 77.8 (±12.5) |
| Blood pressure (mm Hg) | |||||
| Systolic | 127.8 (±20.0) | 0.171 | 126.6 (±19.8) | 0.926 | 126.5 (±20.0) |
| Diastolic | 75.2 (±11.3) | 0.543 | 75.6 (±10.8) | 0.753 | 75.4 (±11.1) |
| Chest radiograph findings | |||||
| Pulmonary congestion | 128 (13.0%) | 0.372 | 114 (11.6%) | 0.780 | 119 (12.1%) |
| Cardiothoracic ratio >0.5 | 586 (59.7%) | 0.217 | 558 (56.8%) | 0.891 | 554 (56.4%) |
| Serum creatinine (mg/dL) | 1.28 (±0.37) | <0.0001 | 1.21 (±0.32) | 0.675 | 1.22 (±0.32) |
| Ejection fraction (%) | 31.9 (±12.5) | 0.958 | 31.9 (±12.1) | 0.554 | 31.6 (±12.5) |
| Ejection fraction >45% | 130 (113.2%) | 0.146 | 108 (11.0%) | 0.670 | 115 (11.7%) |
Digoxin and Mortality
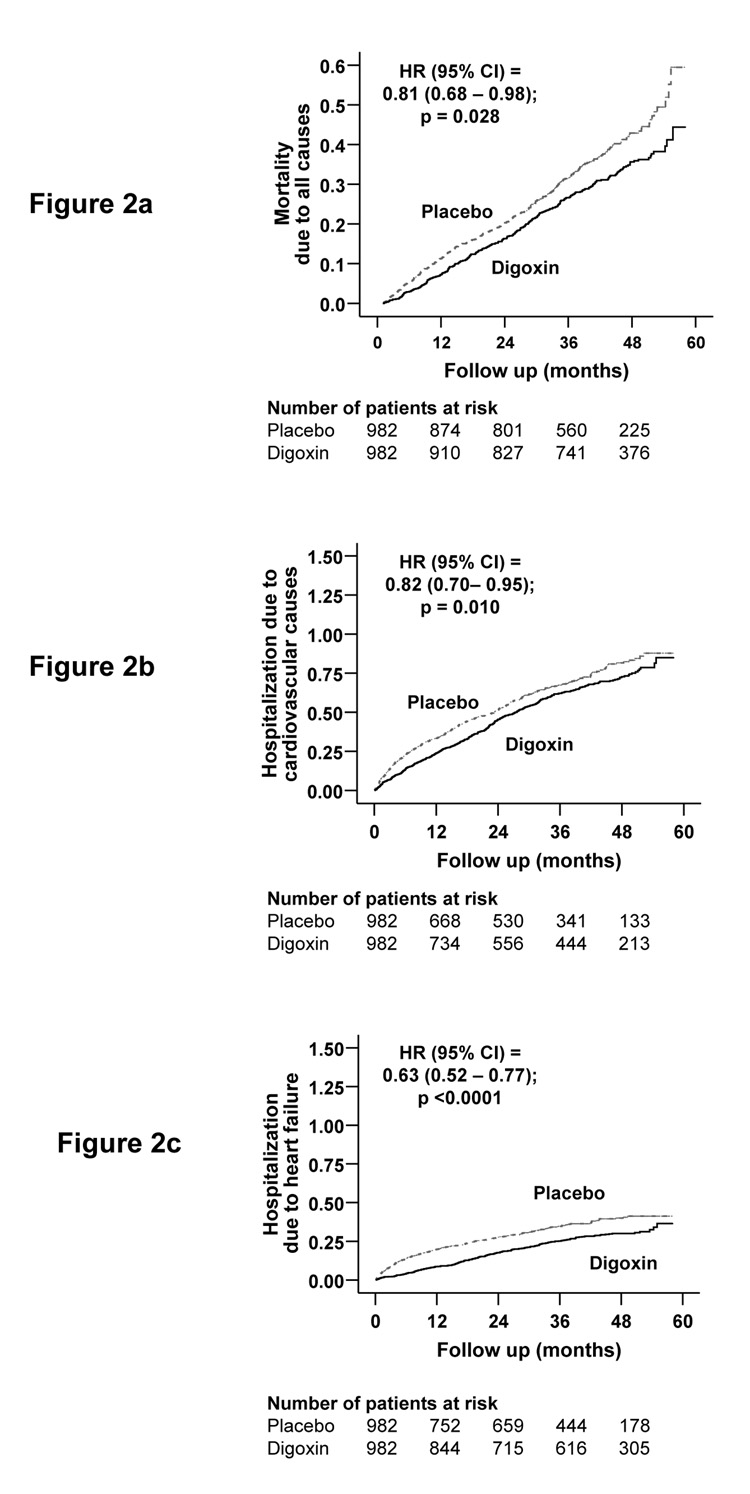
During 42 months of median follow-up, 31% patients died from all causes, including 24% from cardiovascular causes, and 10% from worsening heart failure. Kaplan-Meier plots for death due to all causes are displayed in Figure 2.
Figure 2.
Kaplan-Meier plots for (a) mortality due to all-causes, and hospitalizations due to (b) cardiovascular causes, and (c) worsening heart failure
Mortality due to all causes occurred in 315 patients receiving placebo during 2,940 years (1,071/10,000 person-year) and 288 patients receiving digoxin at low SDC during 3,305 years (871/10,000 person-year) of follow up (hazard ratio, 0.81, 95% confidence interval, 0.68–0.98; p=0.028; Table 2). This is consistent with our prior report of reduced all-cause mortality associated with low SDC (HR, 0.81; 95% CI, 0.67–0.97), using a somewhat different cohort of patients in the placebo group. 4
Table 2.
Effects of Digoxin at Low Serum Digoxin Concentrations (0.5 – 0.9 ng/ml) on Cause-Specific Mortalities
| Placebo (N=982) | Digoxin (N=982) | Absolute difference* (per 10,000 person-year) | Lives to be saved for ~5 million patients in one year | Hazard ratio (95% confidence interval)† | P value | |
|---|---|---|---|---|---|---|
| Death rates (per 10,000 person-year of follow up) | ||||||
| All-cause | 1,071 | 871 | − 200 | − 100,011 | 0.81 (0.68–0.98) | 0.028 |
| Cardiovascular | 813 | 717 | − 96 | − 47,915 | 0.90 (0.73–1.11) | 0.313 |
| Heart failure‡ | 354 | 260 | − 94 | − 46,765 | 0.65 (0.45–0.92) | 0.015 |
| Other cardiac§ | 415 | 421 | + 6 | + 2,804 | 1.09 (0.83–1.44) | 0.530 |
| Other vascular¶ | 44 | 36 | − 8 | − 3,955 | 1.00 (0.42–2.40) | 1.000 |
| Non-cardiovascular | 187 | 103 | − 84 | − 42,100 | 0.49 (0.30–0.81) | 0.005 |
| Unknown | 71 | 51 | − 20 | − 9,996 | 0.77 (0.37–1.57) | 0.467 |
Absolute differences in rates of mortality per 10,000 person-year of follow up were calculated by subtracting the death rates in the placebo group from the death rates in the digoxin group (before values were rounded)
Hazard ratios and confidence intervals (CI) were estimated from the Cox proportional-hazards models
This category includes patients who died from worsening heart failure, even if the final event was an arrhythmia
This category includes deaths presumed to result from arrhythmia without evidence of worsening heart failure and deaths due to atherosclerotic coronary disease, bradyarrhythmias, low-output states, and cardiac surgery
This category includes deaths due to stroke, embolism, peripheral vascular disease, vascular surgery, and carotid endarterectomy
When extrapolated to the US population, this represented a potential annual savings of about 100,000 lives if all of the estimated 5 million heart failure patients had similar characteristics to the DIG participants and were receiving digoxin at low SDC. Incidence rates and risks for cause-specific deaths in placebo and low SDC patients before and after matching are displayed in Table 2.
Our sensitivity analysis suggests that for an unmeasured binary covariate (unrelated to covariates in our propensity model) to explain away our results, that unmeasured covariate would need to increase the odds of developing low SDC by at least 48% and would also need to be a near-perfect predictor of all-cause mortality, at the p<0.05, suggesting that these results are at least somewhat resistant to hidden bias. An appropriate matched-samples comparison of hazard rates gave a Z-statistic of 2.20 (two-tailed P=0.028) for the comparison of low SDC to placebo.
Digoxin and Hospitalization
Overall 64% patients were hospitalized for all causes including 49% from cardiovascular causes and 26% from worsening heart failure. Kaplan-Meier plots for hospitalizations due cardiovascular causes, and heart failure are displayed in Figure 2.
Compared with 639 all-cause hospitalizations in placebo patients during 1,795 years (3,560/10,000 person-year), there were 625 all-cause hospitalizations in low SDC patients during 2,032 years (3,076/10,000 person-year) of follow up (HR, 0.92, 95% CI, 0.81–1.06; p=0.262; Table 3). Extrapolated to the US population, this would represent a potential annual reduction in total hospitalizations by over 240,000. The association between low SDC and all-cause hospitalization became significant (HR 0.83, 95% CI, 0.75–0.93; p=0.001) in a cohort with 1:3 matching (916 digoxin patients matched to 2,738 placebo patients; absolute standardized difference in propensity score=0.6%).
Table 3.
Effects of Digoxin at Low Serum Digoxin Concentrations (0.5 – 0.9 ng/ml) on Cause-Specific Hospitalizations
| Placebo (N=982) | Digoxin (N=982) | Absolute difference* (per 10,000 person-year) | Reduction (−) in hospitalizations for ~5 million heart failure patients in one year | Hazard ratio (95% confidence interval)† | P value | |
|---|---|---|---|---|---|---|
| Cause for hospitalization* | Hospitalization rates (per 10,000 person-year of follow up) | |||||
| All-cause | 3,560 | 3,076 | − 484 | − 242,051 | 0.92 (0.81–1.06) | 0.262 |
| Cardiovascular | 2,359 | 1,963 | − 396 | − 197,767 | 0.82 (0.70–0.95) | 0.010 |
| Worsening heart failure‡ | 1,158 | 781 | − 377 | − 188,610 | 0.63 (0.52–0.77) | <0.0001 |
| Ventricular arrhythmia, cardiac arrest | 128 | 145 | +18 | +8,896 | 1.06 (0.66–1.71) | 0.808 |
| SV arrhythmia§ | 150 | 120 | − 30 | − 14,947 | 0.80 (0.50–1.27) | 0.340 |
| AV block, bradyarrhythmias | 10 | 15 | +5 | +2,479 | 1.50 (0.25–8.98) | 0.657 |
| Suspected digoxin toxicity | 31 | 37 | +6 | +2,923 | 1.43 (0.54–3.75) | 0.469 |
| Myocardial infarction | 173 | 154 | − 19 | − 9,374 | 0.78 (0.49–1.24) | 0.293 |
| Unstable angina | 422 | 414 | − 8 | − 4,116 | 1.09 (0.82–1.46) | 0.551 |
| Stroke | 167 | 130 | − 37 | − 18,338 | 0.68 (0.42–1.13) | 0.136 |
| Coronary revascularization¶ | 51 | 86 | +35 | +17,347 | 2.08 (1.05–4.15) | 0.037 |
| Cardiac transplantation | 24 | 27 | +3 | +1,774 | 1.00 (0.35–2.85) | 1.000 |
| Other cardiovascular** | 408 | 427 | +18 | +9,247 | 1.10 (0.83–1.38) | 0.577 |
| Respiratory infection | 247 | 213 | − 33 | − 16,864 | 0.84 (0.59–1.21) | 0.358 |
| Other non-cardiovascular | 1220 | 1226 | +6 | +2,959 | 1.07 (0.89–1.28) | 0.484 |
| Unspecified | 20 | 15 | − 5 | − 2,659 | 0.67 (0.19–2.36) | 0.530 |
| Number of hospitalizations | 10,117 | 8,558 | − 1,559 | − 779,460 | ||
Data shown include the first hospitalization of each patient due to each cause.
Absolute differences were calculated by subtracting the percentage of patients hospitalized in the placebo group from the percentage of patients hospitalized in the digoxin group (before values were rounded).
Hazard ratios and confidence intervals (CI) were estimated from a Cox proportional-hazards models that used the first hospitalization of each patient for each reason.
Supraventricular (SV) arrhythmias include Atrioventricular (AV) block and bradyarrhythmias
This category includes coronary-artery bypass grafting and percutaneous transluminal coronary angioplasty
This category includes embolism, venous thrombosis, peripheral vascular disease, hypertension, other vascular surgery, cardiac catheterization, other types of catheterization, pacemaker implantation, installation of automatic implantable cardiac defibrillator, electrophysiologic testing, transplant-related evaluation, nonspecific chest pain, atherosclerotic heart disease, hypotension, orthostatic hypotension, and valve operation
Cardiovascular hospitalizations occurred in 493 placebo patients during 2,090 years (2,359/10,000 person-year) and 471 low SDC patients during 2,399 years (1,963/10,000 person-year) of follow up (HR, 0.82, 95% CI, 0.70–0.95; p=0.010; Table 3). Extrapolated to the US population, this would potentially prevent about 200,000 cardiovascular hospitalizations annually.
Hospitalizations due to worsening heart failure occurs in 287 placebo patients during 2,479 years (1,158/10,000 person-year) and 229 low SDC patients during 2,934 years (781/10,000 person-year) of follow up (HR, 0.63, 95% CI, 0.52–0.77; p<0.0001; Table 3. Extrapolated to the US population, this represented a potential reduction of about 190,000 heart failure hospitalizations in one year.
Incidence rates and risks for other cause-specific hospitalizations in patients receiving placebo and digoxin at low SDC in the propensity score-matched cohort are also displayed in Table 3.
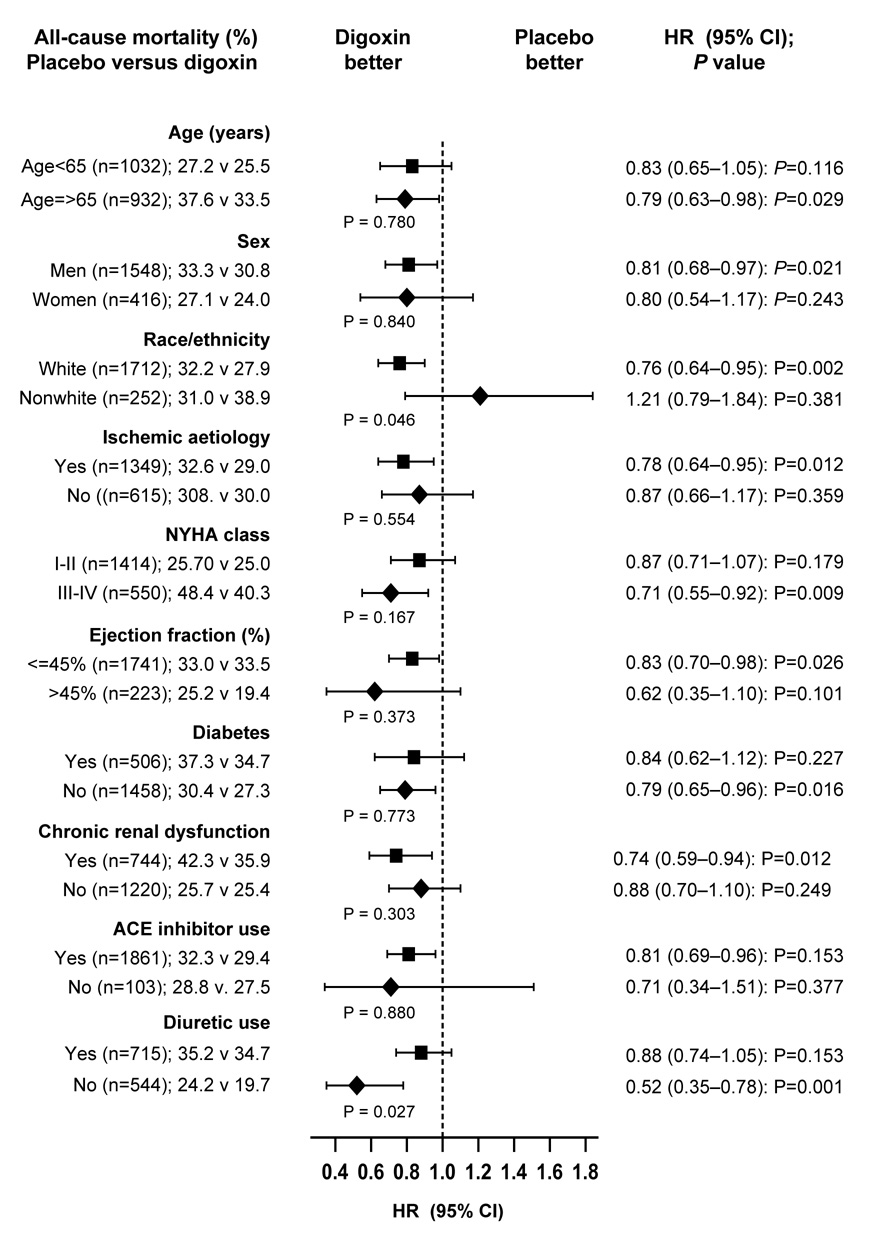
Subgroup Analysis
Reduction in mortality associated with use of digoxin at low SDC was noted in various subgroups of patients, including both sexes (p for interaction=0.840) and regardless of LVEF (p for interaction=0.373; Figure 3). The effects of digoxin among nonwhites (versus whites; p for interaction =0.046) and those receiving diuretics (versus not receiving; p for interaction=0.027; Figure 3) were significantly different.
Figure 3.
Effects of digoxin at low serum digoxin concentrations (0.5–0.9 ng/ml) on all-cause mortality in subgroups of propensity score matched heart failure patients (ACE=angiotensin-converting enzyme; CI= confidence interval; HR=hazard ratio; = NYHA=New York Heart Association)
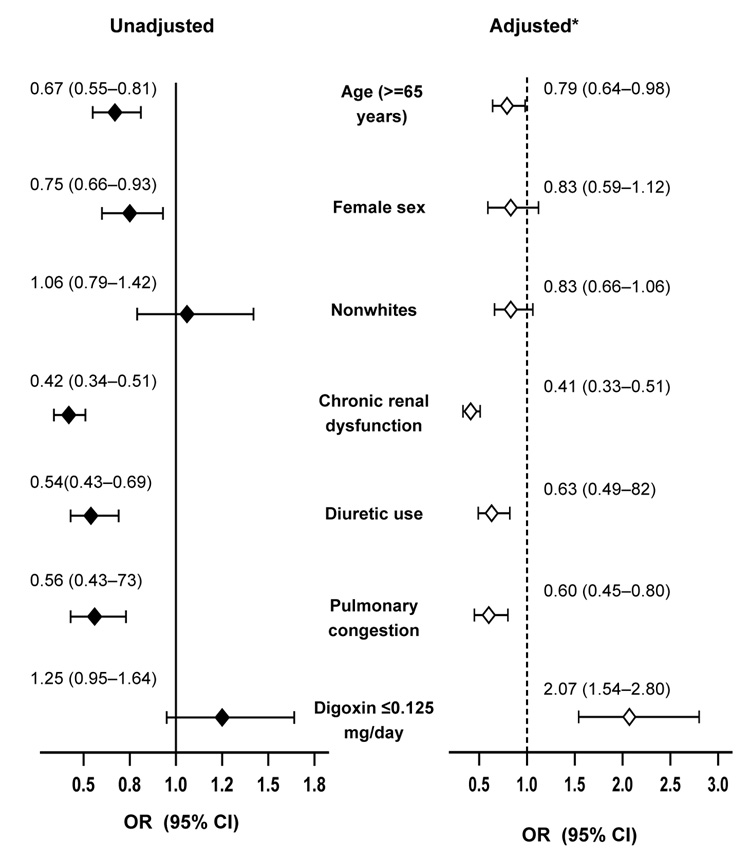
Predictors of Low SDC
Daily dose of digoxin was not a significant predictor of SDC in bivariate analysis. However, when adjusted for other predictors of SDC, low (≤0.125 mg/day) dose of digoxin was a significant predictor of low SDC (adjusted odds ratio, 2.07, 95% CI, 1.54–2.80; p<0.0001). Other independent predictors of SDC included age, chronic kidney disease, diuretic use, and pulmonary congestion, all of which lowered the odds of achieving a low SDC (Figure 4).
Figure 4.
Predictors of low (0.5–0.9 ng/ml) serum digoxin concentrations (SDC). An odds ratio >1 indicates increased odds of developing low SDC. For example, when adjusted for other predictors of SDC, presence of chronic renal dysfunction was associated with significant 59% lower odds of developing low SDC. Similarly, independent of other covariates, use of digoxin at ≤0.125 mg/day was associated with significant 107% higher odds of developing low SDC (*Adjusted for other covariates shown in the Figure, namely age, sex, race, chronic renal dysfunction, diuretic use, pulmonary congestion, and digoxin at ≤0.125 mg/day).
Chronic renal dysfunction was defined as estimated glomerular filtration rate <60 ml/m/1.73 sq. m. by Modification of Diet in Renal Disease methods; OR=odds ratio, CI=confidence interval
Discussion
The findings of the current analysis demonstrate that therapy with digoxin at low SDC (0.5–0.9 ng/ml) is associated with reduction in broader natural history endpoints such as all-cause mortality and cardiovascular hospitalizations in chronic heart failure. We also noted that low doses (≤0.125 mg/day) of digoxin are likely to achieve low SDC. Despite recent advances in therapy, heart failure is associated with high mortality and hospitalizations. Our data suggest that if used in low doses to achieve low SDC, digoxin can play a significant role in heart failure care.
Potential Mechanism of Action
Beneficial effects of digoxin at low SDC are primarily due to its effect on neurohormonal system. 6, 28 By inhibiting the sodium-potassium adenosine tri-phosphate pump in renal tubules and vagal afferent fibers, digoxin suppresses both the renin- angiotensin-aldosterone29–31 and the sympathetic nervous systems. 32, 33 This also explains the beneficial role of digoxin in diastolic heart failure. 5 It is believed that the inhibitory effect of digoxin on neurohormones in heart failure is optimum at low doses and low SDC. 2, 28 Low SDC also reduce the risk of digoxin toxicity and the morbidity and mortality associated with it. 34
Digoxin associated reduction in death due to non-cardiovascular causes may be due to misclassification of causes of death, which might also explain non-significance of its effects on cardiovascular mortality. Increased risk of coronary revascularizations among low SDC patients may be due to their longer survival. However, low SDC was not associated with myocardial infraction or unstable angina (Table 3). The less pronounced and non-significant benefit of low SDC in patients receiving (versus not receiving) diuretics may be associated with diuretic-associated subsequent increase in SDC (Figure 3).34 Our finding of no effect of digoxin in non-white patients lacks biological basis and could be due to chance. 4
Clinical Implications
Our findings support a more expanded role of digoxin in heart failure. Digoxin should be used in those who continue to remain symptomatic despite optimum therapy with ACE inhibitors or angiotensin receptor blocker and beta-blockers, or who cannot tolerate or afford these drugs. This is particularly important as about half of all heart failure patients do not receive therapy with ACE inhibitors or beta-blockers. 2, 10 Because most heart failure patients are elderly and many suffer from renal dysfunction, a starting dose of 0.125 mg/day of digoxin would be reasonable for most patients. If symptoms persist, dose may be increased in young, male patients with normal kidney function. About half of the patients in our analysis were <65 years and 73% of were receiving 0.25 mg/day of digoxin, yet achieved low SDC. In patients who are elderly, female, have chronic renal dysfunction, pulmonary congestion, or are receiving diuretics, any dose increase should be guided by SDC. Heart failure patients with multiple risk factors for high SDC should receive 0.125 mg of digoxin every other day. 4 We estimated that use of digoxin at low SDC in all 5 million heart failure patients in the US would prevent over 190,000 heart failure hospitalizations (Table 3). This will likely offset any cost associated with testing of SDC in select heart failure patients.
Comparison with Prior Studies
The effect of digoxin in reducing hospitalization due to worsening heart failure is now well recognized. 3, 5 Recent evidence suggests that digoxin-associated reduction in heart failure hospitalizations at low SDC (adjusted HR, 0.62; p<0.0001) is not further improved at high SDC (adjusted HR, 0.68; p<0.0001). 4 Therefore, the long-term benefit of digoxin seems to be maximized if a low SDC can be achieved. We observed that low daily doses are strong predictors of low SDC. The findings from the current analysis based on propensity score analysis provide more robust evidence that digoxin at low SDC reduces major natural history endpoints in heart failure. DIG participants were in general a decade younger than heart failure patients seen in clinical practice and the vast majority had NYHA class I–II symptoms. Therefore, the effects of digoxin at low SDC will probably be more pronounced in real-life heart failure patients who are older and have more advanced heart failure and comorbidity burden. 35
Strengths and Limitations
One of the strengths of our analysis is our use of propensity score matching. We assembled a cohort in which placebo and low SDC patients were balanced in all measured covariates. More importantly, our study cohort was assembled prior to occurrence of outcomes and without access to the outcomes data as would be used in a randomized trial. 17 Furthermore, propensity score technique allows objective estimation of pre-match imbalances and post-match balances in baseline covariates. When randomization is unethical or impractical, propensity score methods provide reliable, high-quality evidence using non-randomized designs. 19 A review of the 2005 ACC/AHA heart failure guidelines suggest of the 11 Class I recommendations for Stage C heart failure, 1 was based on level C evidence and 4 were based on level B evidence. Our data provide the strongest evidence to date of the benefit of low-dose digoxin at low SDC.
The key limitation of the propensity score analysis is that it cannot account for unmeasured confounders. Sensitivity analyses can determine the effect of such a potential confounder, however, it cannot determine if such a bias did in fact exist. 20, 36 Our sensitivity analysis suggest that the results of our study were fairly insensitive to potential hidden covariates. 36 Heart failure patients in the DIG trial were not receiving beta-blockers or aldosterone antagonists. However, data from the spironolactone and carvedilol trials in heart failure demonstrate that digoxin is effective when co-administered with these drugs. 37, 38 Results of our study are based on male and relatively younger patients with mild to moderate heart failure and normal sinus rhythm.
Conclusions
In conclusion, the results of our analysis based on a propensity-matched cohort of heart failure patients suggest that digoxin in low doses and at low SDC reduced major natural history endpoints including overall mortality and cardiovascular hospitalizations. Digoxin should be used in low doses to achieve low SDC in heart failure patients who are symptomatic despite therapy with ACE inhibitors or angiotensin receptor blockers, and beta-blockers, or who cannot tolerate or afford these drugs.
Acknowledgement
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through grants from the National Institute on Aging (1-K23-AG19211-04) and the National Heart, Lung, and Blood Institute (1-R01-HL085561-01 and P-50-HL077100).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Ali Ahmed conceived the study hypothesis and design, and wrote the first and the subsequent drafts of the paper. Ali Ahmed did the biostatistical analyses in consultation with Thomas Love. All authors interpreted the data, participated in critical revision of the paper for important intellectual content, and approved the final version of the article. Ali Ahmed had full access to the data.
Dedication
The authors wish to dedicate this article to the memories of Thomas W. Smith, MD (1936–1997) and Richard Gorlin, MD (1926–1997) who played a crucial role in enhancing our understanding of digoxin in heart failure and in the planning and conduct of the DIG trial.
Contributor Information
Ali Ahmed, University of Alabama at Birmingham, and VA Medical Center, Birmingham, AL, USA.
Bertram Pitt, University of Michigan, Ann Arbor, MI, USA.
Shahbudin H. Rahimtoola, University of Southern California, Los Angeles, CA, USA.
Finn Waagstein, Sahlgrenska University, Göteborg, Sweden.
Michel White, Montreal Heart Institute and University of Montreal, Montreal, Canada.
Thomas E. Love, Case Western Reserve University, Cleveland OH, USA.
Eugene Braunwald, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
References
- 1.Rahimtoola SH. Digitalis therapy for patients in clinical heart failure. Circulation. 2004;109:2942–2946. doi: 10.1161/01.CIR.0000132477.32438.03. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, van Veldhuisen DJ, Colucci WS. Contemporary use of digoxin in the management of cardiovascular disorders. Circulation. 2006;113:2556–2564. doi: 10.1161/CIRCULATIONAHA.105.560110. [DOI] [PubMed] [Google Scholar]
- 3.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) [Accessed on August 17, 2005];American College of Cardiology an Web Site. 2005 doi: 10.1016/j.jacc.2005.08.022. Available at: http://www.acc.org/clinical/guidelines/failure//index.pdf. [DOI] [PubMed]
- 7.Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Hear Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–1140. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 8.Adams K, Lindenfeld J, Arnold J, et al. Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Cardiac Failure. 2006;12:10–38. doi: 10.1016/j.cardfail.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Arnold JM, Liu P, Demers C, et al. Canadian Cardiovascular Society consensus conference recommendations on heart failure 2006: diagnosis and management. Can J Cardiol. 2006;22:23–45. doi: 10.1016/s0828-282x(06)70237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonarow GC, Yancy CW, Heywood JT. Adherence to heart failure quality-of-care indicators in US hospitals: analysis of the ADHERE Registry. Arch Intern Med. 2005;165:1469–1477. doi: 10.1001/archinte.165.13.1469. [DOI] [PubMed] [Google Scholar]
- 11.Brophy JM. Rehabilitating digoxin. Eur Heart J. 2006;27:127–129. doi: 10.1093/eurheartj/ehi686. [DOI] [PubMed] [Google Scholar]
- 12.Pitt B. Whither withering? The role of digoxin in patients with heart failure due to systolic left ventricular dysfunction in sinus rhythm. J Card Fail. 2006;12:347–348. doi: 10.1016/j.cardfail.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Smith TW, Butler VP, Jr, Haber E. Determination of therapeutic and toxic serum digoxin concentrations by radioimmunoassay. N Engl J Med. 1969;281:1212–1216. doi: 10.1056/NEJM196911272812203. [DOI] [PubMed] [Google Scholar]
- 14.Adams KF, Jr, Patterson JH, Gattis WA, et al. Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial: a retrospective analysis. J Am Coll Cardiol. 2005;46:497–504. doi: 10.1016/j.jacc.2005.02.091. [DOI] [PubMed] [Google Scholar]
- 15.Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med. 2002;347:1403–1411. doi: 10.1056/NEJMoa021266. [DOI] [PubMed] [Google Scholar]
- 16.Concato J, Feinstein AR, Holford TR. The risk of determining risk with multivariable models. Ann Intern Med. 1993;118:201–210. doi: 10.7326/0003-4819-118-3-199302010-00009. [DOI] [PubMed] [Google Scholar]
- 17.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 18.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 19.Michels KB, Braunwald E. Estimating treatment effects from observational data: dissonant and resonant notes from the SYMPHONY trials. JAMA. 2002;287:3130–3132. doi: 10.1001/jama.287.23.3130. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 22.The Digitalis Investigation Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 24.Levesque R Macro. In: SPSS® Programming and Data Management, 2nd Edition. A Guide for SPSS® and SAS® Users. 2nd Edition. Levesque R, editor. Chicago, IL: SPSS Inc.; [Last access date: June 4, 2005]. Available online at: http://www.spss.com/spss/data_management_book.htm. [Google Scholar]
- 25.Rosenbaum PR, Rubin DB. The bias due to incomplete matching. Biometriks. 1985;41:103–116. [PubMed] [Google Scholar]
- 26.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 27.SPSS for Windows, Rel. 14. Version. Chicago, IL: SPSS Inc., Chicago, IL; 2006. [Google Scholar]
- 28.Gheorghiade M, Ferguson D Digoxin. A neurohormonal modulator in heart failure? Circulation. 1991;84:2181–2186. doi: 10.1161/01.cir.84.5.2181. [DOI] [PubMed] [Google Scholar]
- 29.Torretti J, Hendler E, Weinstein E, Longnecker RE, Epstein FH. Functional significance of Na- K-ATPase in the kidney: effects of ouabain inhibition. Am J Physiol. 1972;222:1398–1405. doi: 10.1152/ajplegacy.1972.222.6.1398. [DOI] [PubMed] [Google Scholar]
- 30.Montanaro D, Antonello A, Baggio B, Finotti P, Melacini P, Ferrari M. Effects of digoxin on plasma renin activity in hypertensive patients. Int J Clin Pharmacol Ther Toxicol. 1980;18:322–323. [PubMed] [Google Scholar]
- 31.Covit AB, Schaer GL, Sealey JE, Laragh JH, Cody RJ. Suppression of the renin-angiotensin system by intravenous digoxin in chronic congestive heart failure. Am J Med. 1983;75:445–447. doi: 10.1016/0002-9343(83)90346-7. [DOI] [PubMed] [Google Scholar]
- 32.Thames MD. Acetylstrophanthidin-induced reflex inhibition of canine renal sympathetic nerve activity mediated by cardiac receptors with vagal afferents. Circ Res. 1979;44:8–15. doi: 10.1161/01.res.44.1.8. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson DW, Berg WJ, Sanders JS, Roach PJ, Kempf JS, Kienzle MG. Sympathoinhibitory responses to digitalis glycosides in heart failure patients. Direct evidence from sympathetic neural recordings. Circulation. 1989;80:65–77. doi: 10.1161/01.cir.80.1.65. [DOI] [PubMed] [Google Scholar]
- 34.Ruelaz RA, Rahimtoola SH. Was it digoxin toxicity?…very likely. J Card Fail. 2005;11:87–90. doi: 10.1016/j.cardfail.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–186. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 36.Rosenbaum PR. Sensitivity to Hidden Bias. In: Rosenbaum PR, editor. Observational Studies. 2 ed. New York: Springer-Verlag; 2002. pp. 110–124. [Google Scholar]
- 37.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 38.Eichhorn EJ, Lukas MA, Wu B, Shusterman N. Effect of concomitant digoxin and carvedilol therapy on mortality and morbidity in patients with chronic heart failure. Am J Cardiol. 2000;86:1032–1035. A1010–A1031. doi: 10.1016/s0002-9149(00)01146-2. [DOI] [PubMed] [Google Scholar]