Summary
Oscillators in olfactory sensory neurons (OSNs) are both necessary and sufficient to sustain rhythms in electroanntenogram (EAG) responses, which suggests that odorant receptors (ORs) and/or OR-dependent processes are under clock control. Since EAGs have limited spatial resolution and do not necessarily reflect firing of action potentials, we measured single unit responses in different antennal sensillae from wild-type, clock mutant, odorant receptor mutant, and G-protein coupled receptor kinase 2 (Gprk2) mutant flies to examine the cellular and molecular mechanisms that drive rhythms in olfaction. Spontaneous spike amplitude, but not spontaneous or odor induced firing frequency, is under clock control in ab1 and ab3 basiconic sensillae and T2 trichoid sensillae. Mutants lacking odorant receptors in dendrites display constant low spike amplitudes, and reducing or increasing levels of GPRK2 in OSNs results in constant low or high spontaneous spike amplitudes, respectively, in ab1, ab3 and T2 sensillae. We conclude that spike amplitude is controlled by circadian clocks in basiconic and trichoid sensillae, and requires GPRK2 expression and the presence of functional ORs in dendrites. These results argue that rhythms in GPRK2 levels control OR localization and OR-dependent ion channel activity/composition to mediate rhythms in spontaneous spike amplitude.
Results and Discussion
Spontaneous spike amplitude of ab3A and ab3B neurons is under circadian clock control
Circadian changes in membrane properties have been observed in microbial systems, invertebrates and mammals. Rhythms in spontaneous firing frequency have been previously described both in the isolated Aplysia eye and in the hamster Suprachiasmatic nucleus [1, 2]. Rhythms in spontaneous activity recorded from the eye of the horseshoe crab, Limulus, cycles in opposite phase to the rhythm in activity in response to a stimulus [3]. Circadian changes in membrane potential have been documented in the mollusk Bulla gouldiana [4], the ciliate protozoan Paramecium, and in the dinoflagellate Gonyaulax [5]. Circadian fluctuations in resting membrane potential have also been described in PDF-positive clock neurons in the Drosophila brain [6]. Since circadian oscillators in OSNs from ab3 sensillae are necessary and sufficient to drive EAG rhythms [7, 8], we expected that OSNs in ab3 sensillae would also show rhythms in single unit responses.
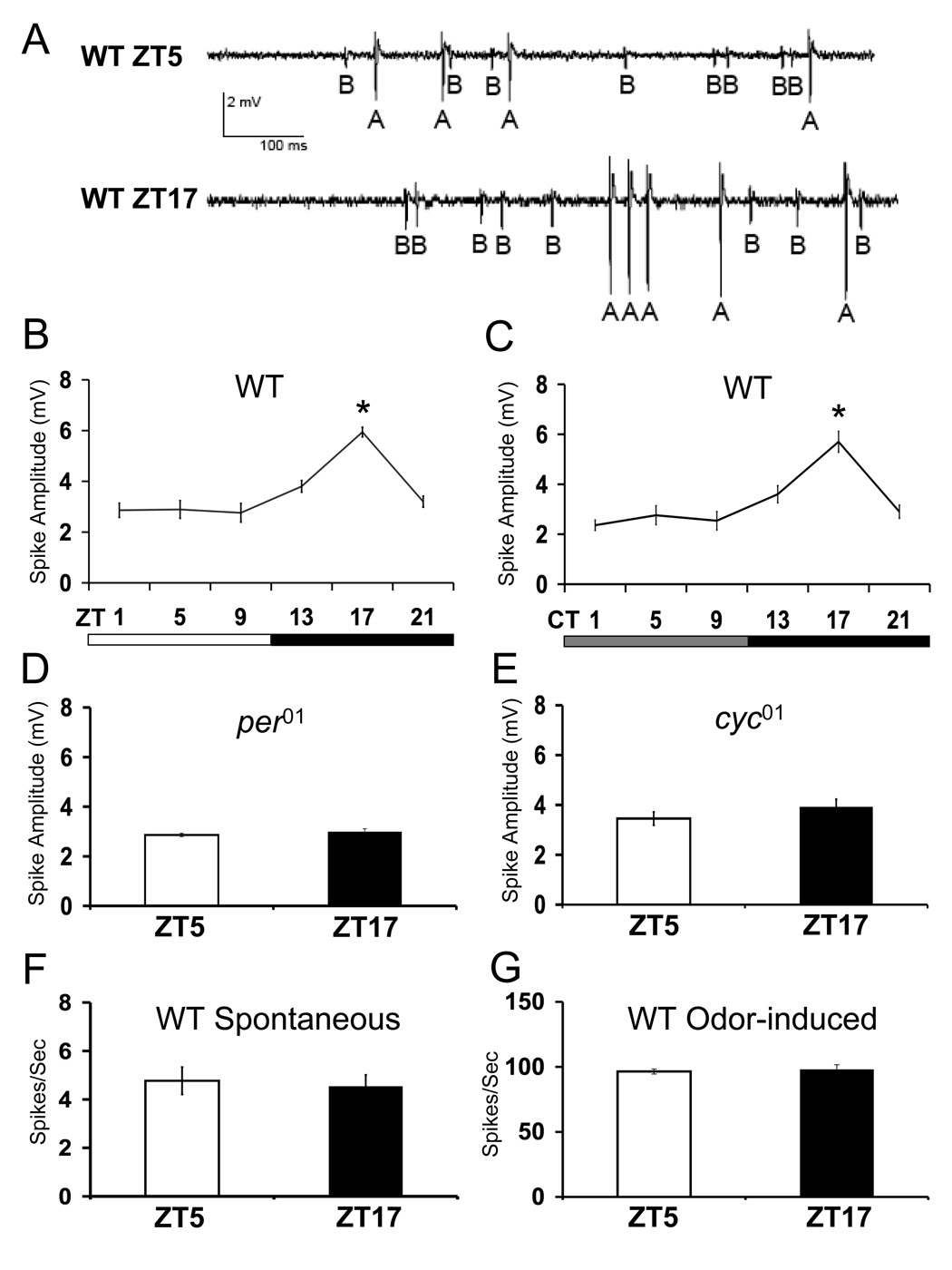
To determine if this was the case, spontaneous spikes were recorded from ab3 sensillae at times when EAG responses were at their peak (ZT17) or trough (ZT5) values in wild-type flies. At both time points, spontaneous spikes from ab3A and ab3B neurons were seen, but at ZT5 the amplitude of the response from these neurons was lower than at ZT17 (Fig. 1A). To determine if this difference represented a diurnal rhythm, spontaneous spike amplitudes from larger and more easily quantifiable ab3A neurons were measured every four hours during a 12h light: 12h dark (LD) cycle. A significant (P<0.001) 2-fold rhythm in ab3A spike amplitude was detected, with a peak at ZT17 and a trough at ZT5 (Fig. 1B). This rhythm in spike amplitude persisted in DD conditions (Fig. 1C) and was absent in clock mutants per01 and cyc01 (Fig. 1D, E), thus demonstrating that this phenomenon is indeed circadian in nature. Similar results were seen when the ab3B neuron was analyzed (data not shown), thus demonstrating that there is a bona fide circadian rhythm of spontaneous spike amplitude in ab3 sensillae with a phase identical to that in EAG responses.
Figure 1.
Spontaneous spike amplitudes are under circadian clock control in the ab3 sensillae. Single unit recordings of ab3 sensillae were made from flies entrained for at least 3 days in LD cycles. Spike amplitudes were quantified from the A neuron. WT denotes Canton-S flies. Error bars denote SEM. (A) A ~1second recording of spontaneous activity from an ab3 sensillum in wild-type (WT) flies at ZT5 or ZT17. A and B denote spontaneous spikes from the two neurons housed in ab3 sensillae. (B) Spontaneous spike amplitudes in wild-type flies during LD cycles or (C) constant darkness. The overall effects of time of day is significant by one way ANOVA (P<0.001) in panels B and C. Asterisk indicates significant (P<0.01) increase in EAG responses at ZT17 (panel B) or CT17 (panel C) compared with responses at all other times of day. (D) Spontaneous spike amplitudes in per01 and (E) cyc01 flies at ZT 5 and ZT 17. The difference in mean amplitudes of spontaneous spikes at ZT 5 and ZT 17 is not statistically significant (P>0.4) in panels D and E. Each time point represents amplitudes calculated from a minimum of 70 individual spikes in panels B–E. (F) Spontaneous firing frequency is not rhythmic in WT flies at ZT5 and ZT17 (P>0.5). (G) Odor induced firing frequency in response to a 10 −4 dilution of ethyl butyrate is not rhythmic in WT flies at ZT5 and ZT17 (P>0.4). Responses from a minimum of 6 OSNs were used to compute firing rate.
The extent of circadian influence on neuronal activity in ab3 sensillae was determined by recording the rate of spontaneous firing and the response to a quantified pulse (~500ms) of ethyl butyrate (10−4 dilution) from ab3A neurons. No changes in either spontaneous firing frequency (Fig. 1F) or odor induced firing frequency (Fig. 1G) were detected between ZT5 and ZT17, indicating that the circadian clock does not influence firing rate in ab3 neurons. We wished to determine if the clock also controlled odor-induced spike amplitude, but continuous changes in shape of the spike waveform in response to odors precluded quantification of odor-induced spike amplitude [9, 10].
Spontaneous spike amplitude of ab1A neuron is under circadian clock control
Single unit recordings from the A neuron of ab1 sensillae were made in wild-type flies collected during LD to determine if circadian control of spike amplitude extends to other classes of basiconic sensillae. It has been shown that ab1 sensillae are maximally sensitive to ethyl acetate [11], which is the primary odorant used in previous EAG rhythm experiments [7, 8]. We found a rhythm in ab1A spike amplitude similar to that seen in ab3 sensillae in that both peak in the middle of the night and have a ~2-fold peak to trough change (Supplemental Fig. 1A). This rhythm persists in constant DD conditions and is absent in clock mutants per01 and cyc01, thereby demonstrating circadian clock control (Supplemental Fig. 1B–D). When spike amplitudes of the ab1B neuron were analyzed, a similar rhythm in phase and fold-change compared to the ab1A neuron was seen (data not shown), but the responses from ab1C and ab1D neurons were too small to be accurately or reliably measured. As in ab3A neurons, neither the spontaneous rate nor the ethyl acetate (10−4 dilution) induced rate of ab1A neuron spike firing were rhythmic in LD (Supplemental Fig. 1E, F). These experiments demonstrate that spontaneous and odor induced frequency of firing is not influenced by the circadian clock in ab1 and ab3 sensillae, and spike amplitude is the only parameter we measured that was under circadian clock control.
Single unit recordings from trichoid sensillae display rhythms in spike amplitude
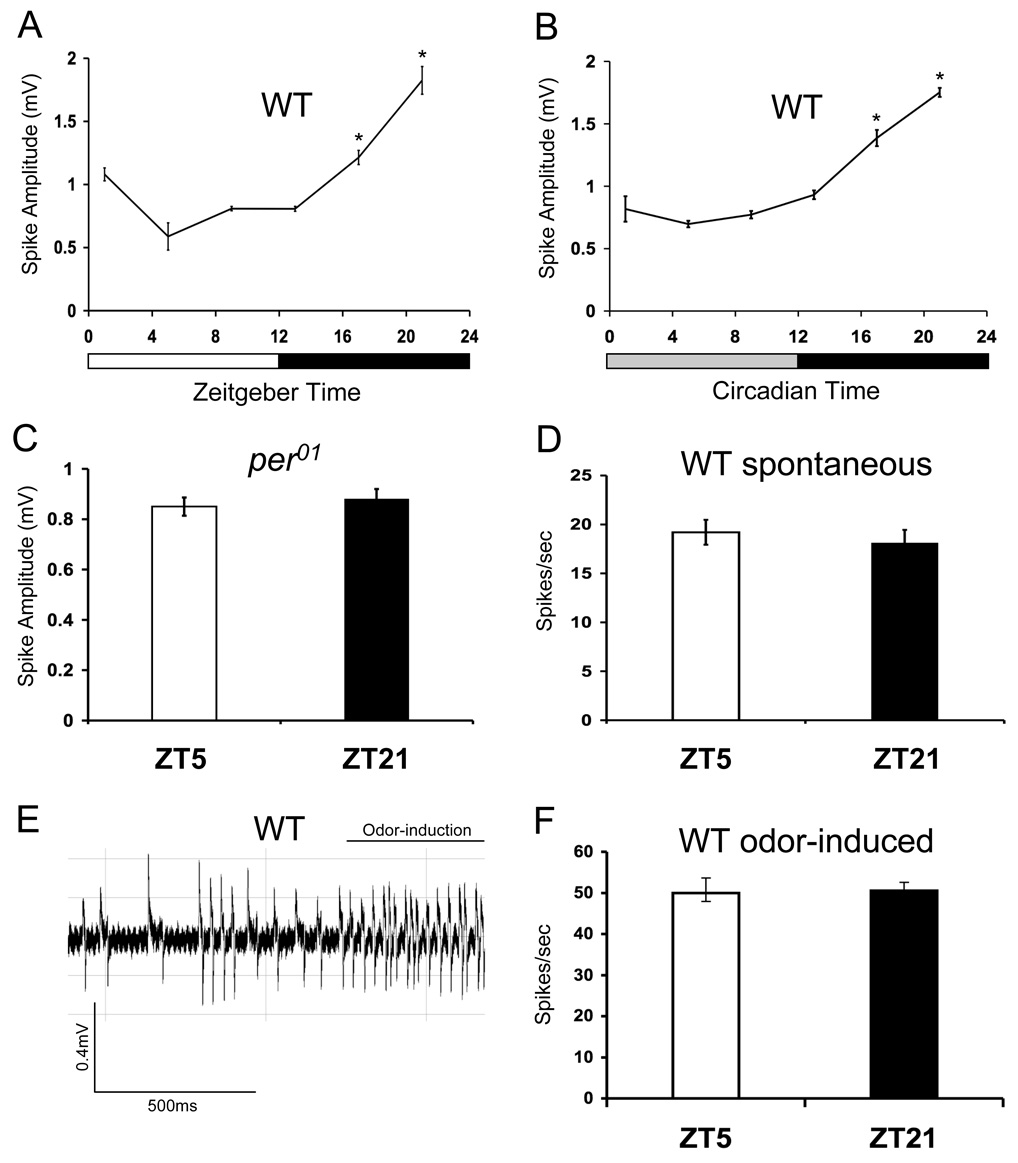
To determine if other classes of sensillae exhibit rhythms in spike amplitude, we measured single unit responses from trichoid sensillae, which are thought to mediate responses to pheromones [12]. When spike amplitude of the A neuron from T2 sensillae was quantified, we found a ~3-fold circadian change which peaked at ZT21, four hours later than the peak in ab1 and ab3 basiconics (Fig. 2A). This rhythm persisted in DD conditions, and was absent in per01 flies (Fig. 2B, C). The later peak phase of spike amplitude rhythms in trichoid sensillae could be due to differences in circadian oscillator phase between trichoid and basiconic sensillae, but the core circadian oscillator component TIMELESS cycled in the same phase in trichoid and basiconic sensillae (Supplemental Fig. 2). Like the basiconic sensillae, no daily changes in spontaneous firing frequency were seen in T2 sensillae (Fig. 2D). Although odorants that produce robust responses in T2 sensillae have not been identified [13], trans-2-hexanal produces a modest yet reliable response [9](Fig. 2E). The frequency of trans-2-hexanal evoked spikes in T2 sensillae remained constant at ZT5 and ZT21 (Fig. 2F), thus demonstrating that odor-evoked spike frequency in T2 sensillae doesn’t vary over a diurnal cycle.
Figure 2.
Spontaneous spike amplitudes are under circadian clock control in T2 sensillae. Single unit recordings of T2 sensillae were made from flies entrained for at least 3 days in LD cycles. Spike amplitudes were quantified from the A neuron. Mean amplitude was calculated from a minimum of 30 spikes at each timepoint. WT denotes Canton-S flies. Error bars denote SEM. Spontaneous spike amplitudes in wild-type flies during LD cycles (A) or constant darkness (B). The overall effects of time of day is significant by one-way ANOVA (P <0.001) in panels A and B. Asterisk indicates signficant (P<0.05) increase in EAG responses at ZT17 and ZT21 (panel A) or CT17 and CT21 (panel B) compared with responses at all other times of day. (C) Spontaneous spike amplitudes in per01 flies at ZT5 and ZT17. The difference in mean amplitudes is not statistically significant (P>0.6). (D) Spontaneous firing frequency is not rhythmic in WT flies at ZT 5 and ZT21 (P>0.5). Responses from a minimum of 6 OSNs were used to compute firing rate. (E) A ~1.5 second recording of trans-2-hexanal induced activity from a T2 sensillum in WT flies at ZT5. The bar indicates when a 10 −1 dilution of trans-2-hexanal was applied. (F) Odor-induced firing frequency in response to trans-2-hexanal is not rhythmic in WT flies (P>0.9). Responses from a minimum of 6 OSNs were used to compute firing rate.
The T1 subset of trichoid sensillae are uniquely involved in the perception of the only volatile pheromone known in flies – 11-cis vaccenyl acetate (cVA), a male specific lipid that mediates aggregation behavior [14–16]. Spontaneous spike amplitude in T1 sensillae was rhythmic with a ~2.5-fold higher amplitude at ZT21 than at ZT5, whereas spontaneous spike frequency in T1 sensillae was constant (Supplemental Fig. 3). Unfortunately we could not measure rhythms in cVA-evoked activity in T1 sensillae (see Supplemental Materials). These experiments show that the circadian clock controls spontaneous spike amplitude, but not spike frequency, in ab3, ab1, T2 and T1 sensillae.
Rhythms in spike amplitude are dependent on odorant receptors in dendrites
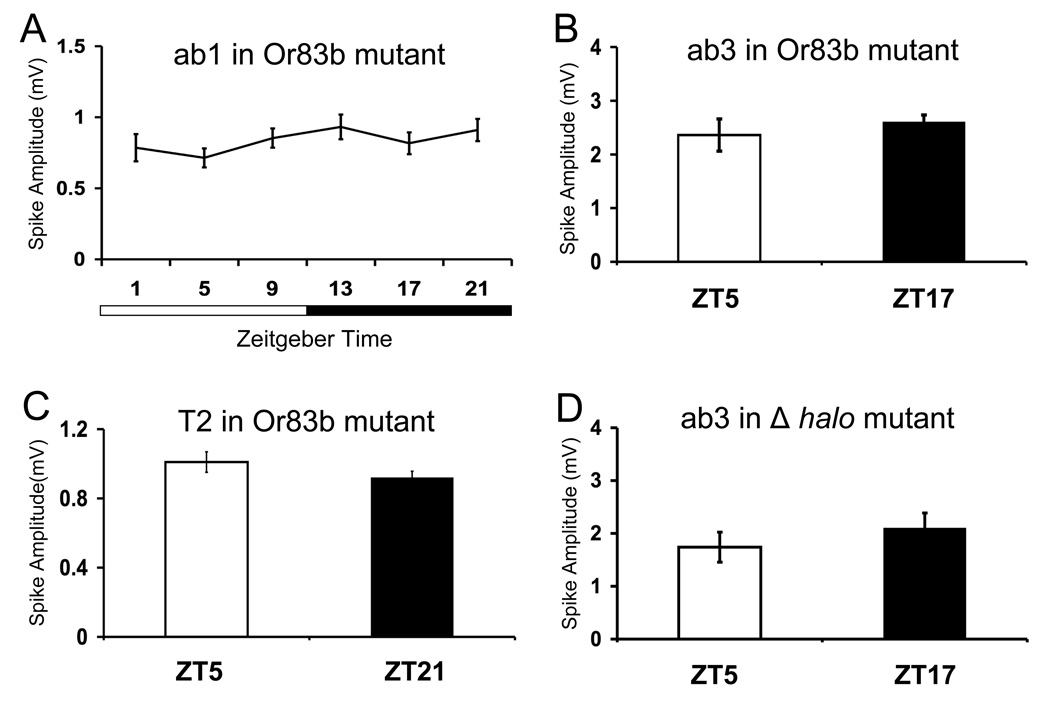
An Or83b deletion mutant that lacks Or83b mRNA and protein is anosmic because Or83b protein is necessary for the localization and function of odor-dependent cation channels in the OSN dendritic membrane in flies [17–20]. The Or83b deletion mutant shows spontaneous activity, albeit at lower levels, but no odor induced responses [18]. Since we detect rhythms in spontaneous activity from ab1, ab3 and T2 sensillae, we hypothesized that rhythms in spike amplitude will persist in the Or83b mutant. However, spontaneous spike amplitude did not show a significant rhythm in ab1, ab3, and T2 sensillae from Or83b mutant flies recorded during LD cycles (Fig. 3A–C). Since Or83b mutant flies lack ORs in the dendrites of OSNs, mutants that lack ORs would also be expected to lack rhythms in spontaneous spike amplitude. The Δ halo mutant deletes the Or22a and Or22b genes, thereby eliminating odor-induced responses in ab3 sensillae [21]. When single unit responses of the ab3A neuron were quantified, we found no circadian change in spike amplitude from the Δ halo mutant (Fig. 3D). These results argue that ORs and/or OR-dependent processes are controlled by circadian clocks in OSNs.
Figure 3.
Or83b and Δhalo mutants show no rhythm in spike amplitude. Single unit recordings were made from flies entrained for at least 3 days in LD cycles. Spike amplitudes were quantified from the A neuron of ab1 (A), T2 (C), and ab3 (B, D) sensillae. Mean amplitude was calculated from a minimum of 40 spikes at each time point. Error bars denote SEM. (A) Spontaneous spike amplitudes of ab1 sensillae from OR83b null mutant flies during LD cycles. One-way ANOVA shows no significant effect of time of day (P>0.6) on spike amplitude of ab1 sensillae in the Or83b null mutant flies. Spontaneous spike amplitudes of ab3 (B) and T2 (C) sensillae from OR83b null mutant flies at their respective peak and trough time points. No significant differences in the spike amplitudes of ab3A neurons (P> 0.7) or T2A neurons (P>0.2) were seen in Or83b mutant flies. (D) Spontaneous spike amplitudes of ab3 sensillae from Δhalo mutant flies at ZT5 and ZT17. No significant (P>0.4) differences in the mean spike amplitudes of ab3 sensillae were seen in Δhalo mutant flies.
GPRK-2 levels determine spike amplitudes
We recently showed that the abundance of Drosophila Gprk2 mRNA and protein cycle in antennae with a peak during the middle of the night, and that GPRK2 levels determine the amplitude of EAG responses [22]. Moreover, EAG amplitude and GPRK2 levels peak when ORs are localized predominantly in dendrites (e.g. ZT17), and GPRK overexpression enhances OR localization to dendrites at times when ORs are normally at low levels in dendrites (e.g. ZT5) [22]. These results are consistent with those showing that ORs must be present in the dendrite to produce rhythms in spike amplitude (Fig. 3), and suggest that GPRK2 levels may control the spike amplitude of single unit responses.
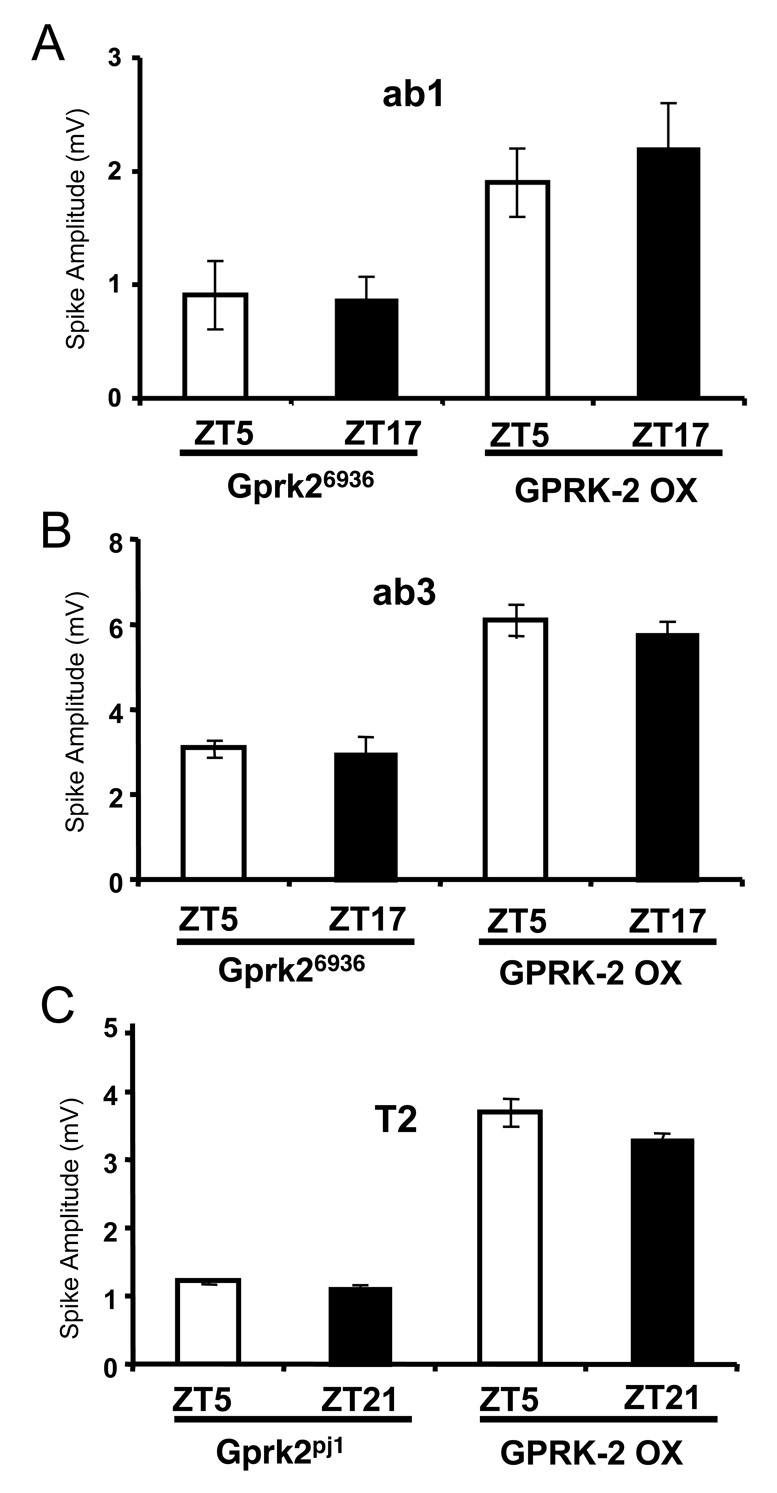
Single unit responses were measured in different sensillar classes from gprk2 mutants and flies that overexpress GPRK2 in OSNs to determine whether GPRK2 levels control spike amplitude. The amplitude of the A neuron spike is constantly high when GPRK-2 is overexpressed in OSNs from ab1, ab3, and T2 sensillae (Fig. 4A–C). In contrast, spike amplitudes in the A neurons of ab1 and ab3 basiconic sensillae and T2 trichoid sensillae were always close to the wild-type trough in Gprk26936 and Gprk2pj1 mutant flies, respectively (Fig. 4A–C). These experiments, along with those of Tanoue et al. [22], demonstrate that the levels of GPRK2 regulate EAG and spike amplitude rhythms in different classes of sensillae.
Figure 4.
Rhythms in spike amplitude are abolished in Gprk2 mutant and overexpression flies. Single unit recordings were made from flies entrained for at least 3 days in LD cycles. Spike amplitudes were quantified from the A neuron of ab1 (A), ab3 (B), and T2 (C) sensillae. Mean amplitude was calculated from a minimum of 40 spikes at each timepoint. Error bars denote SEM. Spontaneous spike amplitudes of ab1 (A) or ab3 (B) sensillae from Gprk26936 and GPRK2 overexpression (GPRK2 OE) flies during LD cycles. Spontaneous spike amplitudes of T2 (C) sensillae from Gprk2pj1 (left) and GPRK2 OE flies during LD cycles. Mean spike amplitudes at ZT5 and ZT17 for ab1 and ab3 sensillae and ZT5 and ZT21 for T2 sensillae were not significantly different in Gprk2 mutants and GPRK2 OE flies (P> 0.1). Mean spike amplitudes between Gprk2 mutants and GPRK2 OE flies at peak and trough time points was significant in all three sensillar classes (P< 0.0001).
One hypothesis to explain rhythms in the amplitude of spontaneous spikes and EAGs is that ion channel activity/composition is under circadian control. Drosophila ORs were recently found to form heteromeric odor-gated and cyclic-nucleotide activated cation channels [19, 20]. Tanoue et al. demonstrate that ORs accumulate in OSN dendrites in a circadian fashion, where OR abundance peaks near mid-night and is low during the day [22]. These rhythms are dependent on the levels of G-protein coupled Receptor Kinase 2 (GPRK2), and coincide with rhythms in the amplitude of both EAGs and spontaneous spikes [22]. Taken together, these results suggest a model whereby GPRK2 controls the abundance and/or activity of OR-dependent odor-gated cation channels in OSN dendrites, which in turn alter membrane potential to generate rhythms in the amplitude of spontaneous spikes and EAG responses. We can’t exclude the possibility that the clock modulates other molecular or cellular targets to generate rhythms in EAG and spike amplitude such as other ion channels expressed in OSNs [23–25], the composition of sensillar lymph [26], and/or the size and shape of OSNs.
Though a clear ecological explanation for rhythms in spike amplitude is not understood, one could hypothesize that the circadian clock could tune the olfactory system to a higher gain level (higher signal to noise ratio) by modulating spike amplitude irrespective of the stimulus preferentially in the subjective night. For instance, rhythms in OSN spike amplitude might produce increased activity in downstream neurons during the night and decreased activity during the day in response to the same stimulus. Recent evidence demonstrates that locomotor activity of paired male and female Drosophila is increased during the subjective night, and is dependant on an intact olfactory system [27]. In addition, behavioral responses to odors in Drosophila are lower during the day than at night, and are controlled by the circadian clock [28]. These phenomena may represent behavioral consequences of this electrophysiological rhythm that could provide an advantage in courtship, in food acquisition, or in predator avoidance. These data are consistent with circadian clock-dependent rhythms in mating activity [29]. The phase of the peak of trichoids could translate to heightened behavioral activity associated with mating during the subjective night. Thus, our results suggest that spike amplitude, in addition to firing frequency, can also encode meaningful information in the peripheral OSNs, which is transmitted to higher processing centers of CNS that mediate behavioral responses to odors.
Supplementary Material
Acknowledgements
We thank W. van der Goes van Naters from J. Carlson’s lab at Yale University for teaching PK to perform single unit recordings, L. Vosshall for providing Or83b-Gal4 and Or83b null mutant flies, J. Carlson for providing Δ halo mutant flies, T. S. Ha from D. Smith’s lab for teaching AC to perform single unit recordings on T1 sensillae, S. Dryer for his insightful comments, and M. Zoran for use of lab equipment. This research was supported by NIH grant DC04857to PH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jacklet JW. Circadian rhythm of optic nerve impulses recorded in darkness from isolated eye of Aplysia. Science. 1969;164:562–563. doi: 10.1126/science.164.3879.562. [DOI] [PubMed] [Google Scholar]
- 2.Yamazaki S, Kerbeshian MC, Hocker CG, Block GD, Menaker M. Rhythmic properties of the hamster suprachiasmatic nucleus in vivo. J Neurosci. 1998;18:10709–10723. doi: 10.1523/JNEUROSCI.18-24-10709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlow RB., Jr Circadian rhythms in the Limulus visual system. J Neurosci. 1983;3:856–870. doi: 10.1523/JNEUROSCI.03-04-00856.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michel S, Geusz ME, Zaritsky JJ, Block GD. Circadian rhythm in membrane conductance expressed in isolated neurons. Science. 1993;259:239–241. doi: 10.1126/science.8421785. [DOI] [PubMed] [Google Scholar]
- 5.Adamich M, Laris PC, Sweeney BM. In vivo evidence for a circadian rhythm in membranes of Gonyaulax. Nature. 1976;261:583–585. doi: 10.1038/261583a0. [DOI] [PubMed] [Google Scholar]
- 6.Park D, Griffith LC. Electrophysiological and anatomical characterization of PDF-positive clock neurons in the intact adult Drosophila brain. J Neurophysiol. 2006;95:3955–3960. doi: 10.1152/jn.00117.2006. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan B, Dryer SE, Hardin PE. Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature. 1999;400:375–378. doi: 10.1038/22566. [DOI] [PubMed] [Google Scholar]
- 8.Tanoue S, Krishnan P, Krishnan B, Dryer SE, Hardin PE. Circadian clocks in antennal neurons are necessary and sufficient for olfaction rhythms in Drosophila. Curr Biol. 2004;14:638–649. doi: 10.1016/j.cub.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Clyne P, Grant A, O'Connell R, Carlson JR. Odorant response of individual sensilla on the Drosophila antenna. Invert Neurosci. 1997;3:127–135. doi: 10.1007/BF02480367. [DOI] [PubMed] [Google Scholar]
- 10.Guillet J, Bernard J. Shape and amplitude of the spikes induced by natural or electrical stimulation in insect receptors. J. Insect Physiol. 1972;18:2155–2171. [Google Scholar]
- 11.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 12.Benton R. Sensitivity and specificity in Drosophila pheromone perception. Trends Neurosci. 2007;30:512–519. doi: 10.1016/j.tins.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 13.van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ejima A, Smith BP, Lucas C, van der Goes van Naters W, Miller CJ, Carlson JR, Levine JD, Griffith LC. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr Biol. 2007;17:599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 17.Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008 doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 20.Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hannsson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008 doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 21.Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37:827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 22.Tanoue S, Krishnan P, Chatterjee A, Hardin PE. G-protein coupled Receptor Kinase 2 is required for rhythmic olfactory responses in Drosophila. Curr Biol. 2008 doi: 10.1016/j.cub.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubin AE, Liles MM, Seligman F, Le T, Tolli J, Harris GL. Involvement of genes encoding a K+ channel (ether a go-go) and a Na+ channel (smellblind) in Drosophila olfaction. Ann N Y Acad Sci. 1998;855:212–222. doi: 10.1111/j.1749-6632.1998.tb10569.x. [DOI] [PubMed] [Google Scholar]
- 24.Kulkarni NH, Yamamoto AH, Robinson KO, Mackay TF, Anholt RR. The DSC1 channel, encoded by the smi60E locus, contributes to odor-guided behavior in Drosophila melanogaster. Genetics. 2002;161:1507–1516. doi: 10.1093/genetics/161.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin H, Mann KJ, Starostina E, Kinser RD, Pikielny CW. A Drosophila DEG/ENaC channel subunit is required for male response to female pheromones. Proc Natl Acad Sci U S A. 2005;102:12831–12836. doi: 10.1073/pnas.0506420102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dolzer J, Krannich S, Fischer K, Stengl M. Oscillations of the transepithelial potential of moth olfactory sensilla are influenced by octopamine and serotonin. J Exp Biol. 2001;204:2781–2794. doi: 10.1242/jeb.204.16.2781. [DOI] [PubMed] [Google Scholar]
- 27.Fujii S, Krishnan P, Hardin P, Amrein H. Nocturnal male sex drive in Drosophila. Curr Biol. 2007;17:244–251. doi: 10.1016/j.cub.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou X, Yuan C, Guo A. Drosophila olfactory response rhythms require clock genes but not pigment dispersing factor or lateral neurons. J Biol Rhythms. 2005;20:237–244. doi: 10.1177/0748730405274451. [DOI] [PubMed] [Google Scholar]
- 29.Sakai T, Ishida N. Circadian rhythms of female mating activity governed by clock genes in Drosophila. Proc Natl Acad Sci U S A. 2001;98:9221–9225. doi: 10.1073/pnas.151443298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.