Abstract
Male sex, obesity, and age are risk factors for obstructive sleep apnea, although the mechanisms by which these factors increase sleep apnea susceptibility are not entirely understood. This study examined the interrelationships between sleep apnea risk factors, upper airway mechanics, and sleep apnea susceptibility. In 164 (86 men, 78 women) participants with and without sleep apnea, upper airway pressure-flow relationships were characterized to determine their mechanical properties [pharyngeal critical pressure under hypotonic conditions (passive Pcrit)] during non-rapid eye movement sleep. In multiple linear regression analyses, the effects of body mass index and age on passive Pcrit were determined in each sex. A subset of men and women matched by body mass index, age, and disease severity was used to determine the sex effect on passive Pcrit. The passive Pcrit was 1.9 cmH2O [95% confidence interval (CI): 0.1-3.6 cmH2O] lower in women than men after matching for body mass index, age, and disease severity. The relationship between passive Pcrit and sleep apnea status and severity was examined. Sleep apnea was largely absent in those individuals with a passive Pcrit less than -5 cmH2O and increased markedly in severity when passive Pcrit rose above -5 cmH2O. Passive Pcrit had a predictive power of 0.73 (95% CI: 0.65-0.82) in predicting sleep apnea status. Upper airway mechanics are differentially controlled by sex, obesity, and age, and partly mediate the relationship between these sleep apnea risk factors and obstructive sleep apnea.
Keywords: physiopathology, pharynx, obstructive sleep apnea
OBSTRUCTIVE SLEEP APNEA (OSA) is a complex multifactorial disorder characterized by recurrent pharyngeal obstruction during sleep. It is well recognized that sex, obesity, and age are risk factors for this disorder. Obesity and specifically central adiposity increase both the prevalence and severity of sleep apnea (38). Men have a three- to fourfold increased prevalence of sleep apnea than premenopausal women (11, 61), although the prevalence of sleep apnea in postmenopausal women approaches that of men (3, 42, 58). Age is also a known risk factor for sleep apnea in cross-sectional and longitudinal studies (35, 57). Nevertheless, the mechanisms by which these risk factors increase sleep apnea susceptibility are not known.
Upper airway obstruction during sleep can result from alterations in either passive mechanical pharyngeal properties or disturbances in neuromuscular control. Anatomic alterations, including tonsillar hypertrophy, craniofacial abnormalities, and central adiposity, may increase the propensity toward pharyngeal collapse. Specifically, fatty deposits around the neck and pharyngeal lumen may increase upper airway collapsibility (26, 27, 46, 47, 59). In addition, central adiposity reduces lung volume (1, 16, 24), which predisposes to upper airway collapse and increases sleep apnea severity (17, 18). These anatomic factors may account for observed increases in pharyngeal collapsibility in sleep apnea compared with normal individuals (12, 14, 23, 37, 55, 60). A recent study suggests that alterations in upper airway structural properties may determine sleep apnea severity independent of obesity, sex, and age; however, the passive upper airway mechanical properties were not precisely assessed (60). In previous work, differences in upper airway collapsibility between men and women have been reported, although the small sample size did not allow investigators to account for influences of obesity and disease severity simultaneously (25).
To assess the independent effects of multiple known major risk factors for sleep apnea (obesity, sex, and age), the present study examined the relationship between these risk factors and passive upper airway properties in a large cohort of normal subjects and sleep apnea patients. The passive mechanical properties of the upper airway were assessed by determining the pharyngeal critical pressure under hypotonic conditions (passive Pcrit) (6, 36, 37, 51). We hypothesized that male sex, age, and obesity were associated with increased mechanical instability of the upper airway. We further hypothesized that the relationship between sleep apnea risk factors and OSA would be mediated, in part, by elevations in passive Pcrit. We tested our hypotheses in a sample of subjects with and without sleep apnea and found marked differences in the modulation of passive Pcrit by sex, obesity, and age. Some of the results of this study have been presented in abstract form (29, 30).
METHODS
Subjects
We conducted an analysis of a sample of 108 sleep apnea patients (67 men, 41 women) and 56 normal subjects (19 men, 37 women) previously recruited for other protocols from the Johns Hopkins Sleep Disorders Center and the general community, respectively. Subjects with a history of a concurrent sleep disorder were excluded. OSA was defined as a non-rapid eye movement (NREM) respiratory disturbance index (RDI) greater than 10 events/h. Informed written consent was obtained for the protocols, which were approved by the Johns Hopkins Institutional Review Board.
Measurements
Polysomnography
Subjects were evaluated by full-montage inlaboratory nocturnal polysomnography. Sleep staging, respiratory events, and arousals were scored using standard criteria (41). Respiratory arousals were identified according to American Academy of Sleep Medicine criteria (2) and respiratory events using previously reported criteria from our laboratory (40). An apnea was defined as cessation of airflow for 10 s or more. A hypopnea was defined as a discernible reduction in airflow in association with a ≥4% desaturation and/or arousal.
Upper airway assessment of nasal pressure and airflow
Patients returned for a second polysomnography night to determine their passive Pcrit during NREM sleep as previously described (6, 36, 37, 51). Airflow was monitored with a pneumotachograph (no. 5, Hans Rudolph, Kansas City, MO) attached to a differential pressure transducer placed between a tight-fitting nasal mask (Comfort Classic, Respironics, Murrysville, PA) and a continuous positive airway pressure (CPAP) unit designed to apply pressures between -20 and +20 cmH2O. Respiratory effort was monitored via a Hyatt-type esophageal balloon (Ackrad Laboratories, Cranford, NJ) placed via a pernasal approach for monitoring esophageal pressure and/or a piezoelectrode abdominal and thoracic strain gauge. Patients slept in the supine position with one pillow during measurement periods. All monitored parameters on this night were recorded directly on a computer software system (Windaq, Dataq, Akron, OH; or Somnologica, Medcare, Buffalo, NY).
Protocol
Passive Pcrit
During stable NREM sleep, the nasal mask pressure was increased until flow-limited breathing was eliminated (holding pressure) (6, 36, 37, 51). Thereafter, nasal pressure was rapidly reduced to specific levels for five breaths and returned to holding pressure for at least 1 min of stable NREM sleep at holding pressure. At least two series of stepwise reductions in nasal pressure to at least three distinct nasal pressure levels separated by 1-2 cmH2O were collected in each subject.
Analyses
Upper airway collapsibility components of the passive pressure-flow relationship
The passive pressure-flow relationship of the upper airway was determined by plotting the maximal inspiratory airflow (Vimax) from breaths 2-5 during the pressure drop against nasal pressure during stable NREM sleep, as previously described (36, 37). The passive Pcrit was determined as the zero-flow intercept from the linear regression of Vimax vs. nasal pressure. To ensure that adequate quality pressure-flow relationships were used to derive the passive Pcrit, we required at least three distinct nasal pressure levels, and that the lowest nasal pressure level obtained was within 3 cmH2O of the calculated passive Pcrit.
Statistical Analyses
Descriptive statistics were used to characterize the patient sample, using means with SDs unless otherwise stated. Passive Pcrit was found to be normally distributed in men (P = 0.46) and women (P = 0.14) by the Shapiro-Wilkes test, a test for normality. The bivariate relationships between sleep apnea risk factors [age, sex, and body mass index (BMI)] and passive Pcrit was initially examined using the Pearson product-moment correlation. Multivariable linear regression was used to examine the relationship between the passive Pcrit and predictors (age, sex, BMI). To account for sex-related differences in BMI and sleep apnea severity, analyses were stratified by sex to examine the associations between passive Pcrit, age, and BMI. Further subanalyses were performed in younger women to examine the potential impact of menopause on BMI and age relationships to passive Pcrit. Specific subgroups were analyzed for women up to the median age of menopause (51 yr old) and for those who had reached the 10th percentile for menopause (45 yr old) (8, 15, 32). Multivariable linear regression models were then constructed in men and women to examine whether BMI and age were independently associated with passive Pcrit as continuous and categorical variables.
In examining the independent effects of sex on passive Pcrit, we recognized that differences in BMI and RDI distribution could be due to selection bias and might explain observed differences in passive Pcrit in men and women. To account for differences in BMI and RDI severity between men and women (see Table 1) and minimize the effects of selection bias, a subsample of men and women were matched (Table 2) on RDI and BMI, based on the smallest Euclidian distance (53).
Table 1.
Anthropometric data for entire study sample
| Women (n = 78) |
Men (n = 86) |
All (n = 164) |
|||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | *P Value | Mean ± SD | Range | |
| Age, yr | 42.2±10.5 | 19.0-70.0 | 42.4±10.1 | 21.0-68.0 | 0.91 | 42.3±10.3 | 19.0-70.0 |
| BMI, kg/m2 | 43.0±10.3 | 23.0-71.5 | 36.9±11.8 | 19.3-70.4 | 0.0004 | 39.8±11.5 | 19.3-71.5 |
| RDI, events/h | 27.3±34.8 | 0-138.9 | 52.1±35.7 | 0-126.9 | 0.0001 | 40.3±37.3 | 0-138.9 |
BMI, body mass index; RDI, respiratory disturbance index.
Comparison of men and women.
Table 2.
Anthropometric data for matched subset
| Women (n = 30) |
Men (n = 30) |
|||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| Age, yr | 42.3±11.5 | 19.0-70.0 | 41.8±9.4 | 21.0-68.0 |
| BMI, kg/m2 | 39.9±12.2 | 23.1-71.5 | 39.9±11.1 | 23.1-60.3 |
| RDI, events/h | 41.5±41.9 | 0.4-138.9 | 44.2±42.3 | 0-123.9 |
To examine whether upper airway mechanics mediate the relationship between known sleep apnea risk factors, sleep apnea status, and sleep apnea severity, we determined the relationship between passive Pcrit and sleep apnea status and severity. Pearson product-moment correlation and logistic regression (odds ratio; OR) were used to quantify the relationship between passive Pcrit and sleep apnea status (RDI > 10 events/h) and severity for NREM, REM, and total sleep. Receiver operating characteristics of passive Pcrit in predicting sleep apnea status were determined to obtain predictive power, sensitivity, specificity, and the percent correctly classified [(true positives + true negatives)/total subjects]. In particular, we determined the sensitivity and specificity for a passive Pcrit threshold of -5 cmH2O as previous literature has demonstrated this threshold to predict sleep apnea status (37, 48, 50).
Linear and logistic regression models were checked to ensure that the residuals were normally distributed and met the assumptions of regression analysis. The bivariate and multivariable data are displayed as means ± standard error of the mean (SE) with 95% confidence intervals (95% CI). All statistical analyses were performed using STATA 9 (Stata, College Station, TX).
RESULTS
Anthropometric and sleep study characteristics for the entire study population and stratified by sex are shown in Table 1. The study sample was comprised of an approximately equal number of men (n = 86) and women (n = 78) and encompassed a broad range of age, BMI, and sleep apnea disease severity. The prevalence of obesity (BMI ≥ 30 kg/m2) for the entire sample was 75.6% (87.1% and 65.1% in women and men, respectively). The women were of similar age to the men but were more obese and had less severe OSA. There were 108 subjects with obstructive sleep apnea (41 women vs. 67 men; age 44.2 ± 10.0 yr, range 19-62 yr; BMI 41.1 ± 11.7 kg/m2, range 23-58 kg/m2; NREM RDI 59.4 ± 32.1, range 10.2-138.9 events/h) and 56 subjects without obstructive sleep apnea (37 women vs. 19 men; age 38.8 ± 9.8 yr, range 23-70 yr; BMI 37.3 ± 10.7 kg/m2, range 19-71 kg/m2; NREM RDI 3.4 ± 2.6, range 0-9.9 events/h).
Passive Pcrit and Sleep Apnea Risk Factors in the Entire Sample (Men and Women)
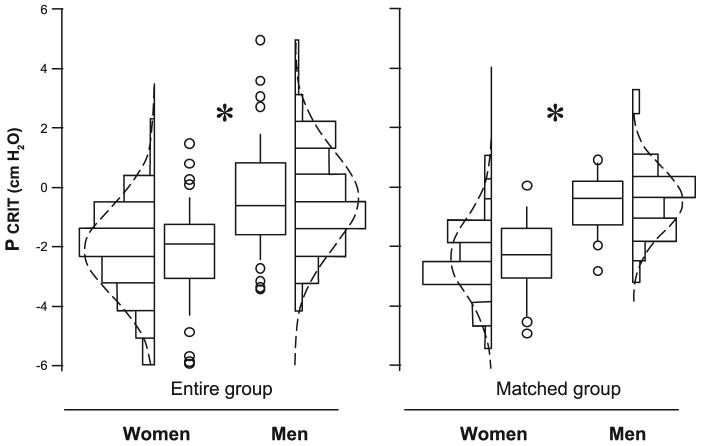
The passive Pcrit significantly correlated with BMI (r = 0.37; 95% CI: 0.23-0.50; P < 0.0001) but not age (r = 0.12; 95% CI: -0.03-0.27; P = 0.12) for the entire group (n = 164). In addition, the passive Pcrit was significantly higher in men (-0.4 ± 3.2 cmH2O) than women (-2.2 ± 3.1 cmH2O) with a difference of 1.8 ± 0.5 cmH2O (P < 0.001; Fig. 1, left).
Fig. 1.
Women have decreased pharyngeal critical pressure under hypotonic conditions (passive Pcrit) compared with men. A ∼2.0-cmH2O increase in passive Pcrit was observed in men compared with women (P < 0.02) in the entire group (left) and the male and female subgroups matched for respiratory disturbance index (RDI) and body mass index (BMI) (right). Values were adjusted for age and BMI. Line = median; box = 25th-50th percentiles; whiskers and cap = 95th percentile. Additional data points are represented that were outside the 5th and 95th percentiles. Bars = frequency histogram; dotted line = normal distribution plot; *P < 0.05.
In multivariable analyses, we confirmed that male sex, BMI, and age were independently associated with the passive Pcrit (see Table 3, model 1). In particular, the passive Pcrit was considerably higher in men than women after adjusting for age and BMI (2.63 cmH2O; 95% CI: 1.75-3.51 cmH2O; P < 0.0001, Table 3, model 1). The passive Pcrit also increased with BMI independent of sex and age [1.40 cmH2O higher per 10 kg/m2 increase in BMI (95% CI: 1.01-1.78 cmH2O per 10 kg/m2; P < 0.001)]. As BMI increased, however, the passive Pcrit increased more in men than women (0.78 cmH2O per 10 kg/m2 increase in BMI; see Table 3, model 2, interaction term, P = 0.048). Finally, the sex- and BMI-adjusted passive Pcrit increased with age [0.52 cmH2O higher per decade (95% CI: 0.10-0.93 cmH2O per decade; P = 0.015)]. Because of differences in the distribution of BMI and sleep apnea severity between the men and women, the associations between the passive Pcrit and sleep risk factors (age and BMI) were further analyzed in each sex separately.
Table 3.
Multivariable regression models for passive Pcrit in entire study sample
|
Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| β | 95 % CI | P Value | β | 95 % CI | P Value | |
| Sex | 2.63 | 1.75-3.51 | <0.001 | 1.81 | 0.61-3.00 | 0.003 |
| Age (per 10 yr) | 0.52 | 0.10-0.93 | 0.015 | 0.54 | 0.13-0.95 | 0.011 |
| BMI (per 10 kg/m2) | 1.40 | 1.01-1.78 | <0.001 | 0.93 | 0.34-1.53 | 0.002 |
| Sex × BMI | 0.78 | 0.00-1.56 | 0.048 | |||
Passive Pcrit, pharyngeal critical pressure under hypotonic conditions; CI, confidence interval.
BMI and Age Effects on Passive Pcrit in Sex-Stratified Samples
Multivariable analyses were performed to examine whether BMI and age were independent predictors of passive Pcrit in men and women. In men, the passive Pcrit varied directly with BMI (Fig. 2, right). In sex-stratified models, men continued to demonstrate a trend toward greater increases in passive Pcrit per change in BMI compared with women (P = 0.067; data below).The age-adjusted passive Pcrit was 1.67 cmH2O higher per 10 kg/m2 increase in BMI (95% CI: 1.20-2.15 cmH2O per 10 kg/m2, P =0.0001). In contrast to BMI, age was not associated with passive Pcrit in the men (Fig. 3, right) (P = 0.58).
Fig. 2.
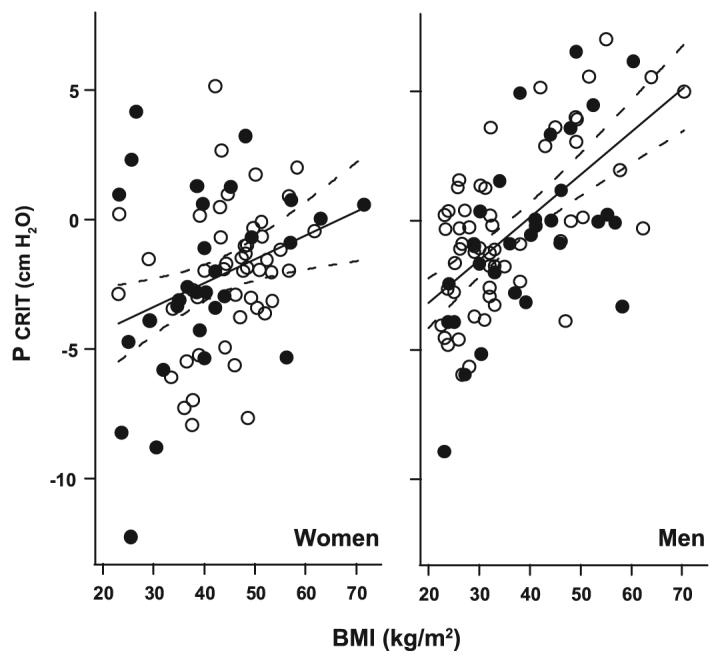
Passive Pcrit vs. BMI in men and women. In men and women, there was a significant correlation between the passive Pcrit and BMI (adjusted for age) for the entire sample of men and women. The magnitude of the change in Pcrit (solid lines) was 1.67 cmH2O per 10 kg/m2 change in BMI [95% confidence interval (CI): 1.20-2.15 cmH2O per 10 kg/m2] in men (right) vs. 0.95 cmH2O per 10 kg/m2 change in BMI (95% CI: 0.32-1.57 cmH2O per 10 kg/m2) in women (left). Note that the distribution of the subjects in the subset matched for RDI and BMI (closed symbols) did not differ from the distribution in the entire subject groups (open and closed symbols) for both men and women. Dashed lines represent 95% CIs.
Fig. 3.
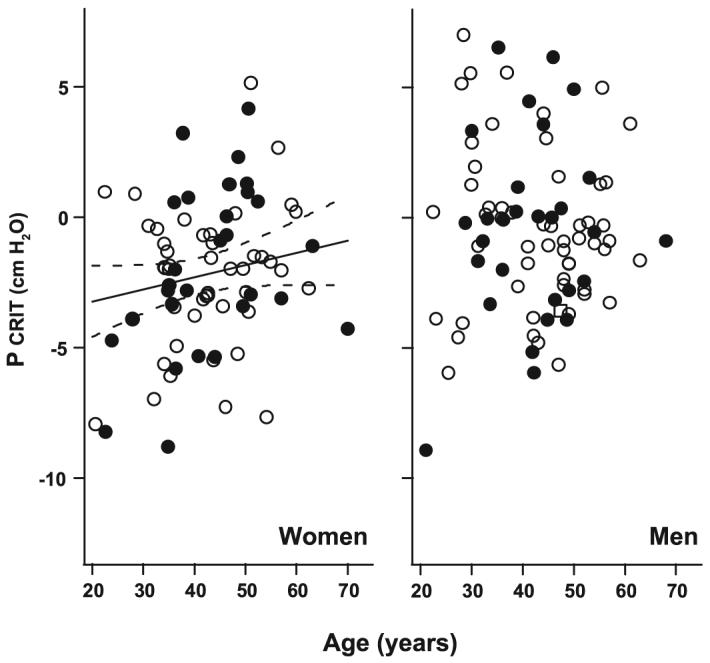
Passive Pcrit vs. age relationship in men and women. In the entire group, there was a significant correlation (solid line) between passive Pcrit and age (adjusted for BMI) in women, but not men (no line drawn). Note that the distribution of the subjects in the subset matched for RDI and BMI (closed symbols) did not differ from the distribution in the entire subject groups (open and closed symbols) for both men and women. Dashed lines represent 95% CIs.
In women, both BMI and age were independent predictors of passive Pcrit (Fig. 2, left, and Fig. 3, left). The age-adjusted passive Pcrit was 0.95 cmH2O higher per 10 kg/m2 increase in BMI (95% CI: 0.32-1.57 cmH2O per 10 kg/m2; P = 0.004). To determine the influence of menopause on BMI-related changes in passive Pcrit, we performed a subanalysis in women less than 45 yr old and found that the passive Pcrit rose by 2.06 cmH2O per 10 kg/m2 increase in BMI (95% CI: 1.35-2.78 cmH2O per 10 kg/m2; P < 0.0001). In contrast, there was no significant rise in passive Pcrit with BMI in women over 45 yr old.
Compared with men, women demonstrated a significant elevation in BMI-adjusted passive Pcrit with age of 0.92 cmH2O higher per decade (95% CI: 0.31-1.54 cmH2O per decade; P = 0.004). In contrast to BMI, age was not a significant determinant of passive Pcrit in the premenopausal subgroup (<45 yr old). Nevertheless, age became a significant determinant when the age range was extended to include all women less than 51 yr old and remained significant with the inclusion of women greater than 51 yr old, suggesting that perimenopausal changes may account for age-related elevations in passive Pcrit in women.
Sex Effect on Passive Pcrit in Matched Subsample
To assess the independent effect of sex on passive Pcrit, we accounted for differences in the distribution of BMI and sleep apnea severity between men and women (Table 1) by closely matching a subsample of men with women of comparable BMI and RDI (Table 2), a strategy that produced subgroups also matched for age (Table 2). Comparing matched male and female subgroups, we found that the age-adjusted passive Pcrit was 1.9 ± 0.9 cmH2O lower in the women compared with men (95% CI: 0.1-3.6 cmH2O, P = 0.02; Fig. 1, right). To minimize the influence of menopause on sex-related differences in passive Pcrit, we compared the passive Pcrit in men and women from the matched subsample who were less than 45 yr old. The passive Pcrit was 2.7 ± 1.0 cmH2O lower in women compared with men (95% CI: 0.7-4.7 cmH2O, P = 0.009).
Passive Pcrit and Sleep Apnea Severity
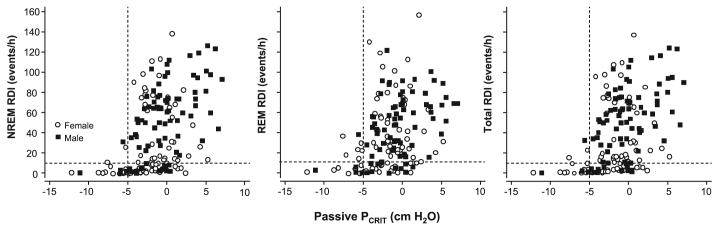
To determine whether upper airway mechanics mediate the relationship between known sleep apnea risk factors and sleep apnea severity, we examined the relationship between passive Pcrit and sleep apnea status and severity. Sleep apnea was largely absent in those whose passive Pcrit was less than -5 cmH2O, and increased markedly in severity when the passive Pcrit rose above this -5 cmH2O threshold in the entire sample stratified by NREM RDI (r = 0.46, 95% CI: 0.33-0.57, P < 0.001), REM RDI (r = 0.36, 95% CI: 0.21-0.49, P < 0.001), and total RDI (r = 0.46, 95% CI: 0.33-0.57, P < 0.001; see Fig. 4).
Fig. 4.
Passive Pcrit vs. RDI in all subjects. Sleep apnea prevalence (RDI > 10 events/h; see horizontal dashed line) and severity in non-rapid eye movement (NREM; left), rapid eye movement (REM; middle), and total sleep (right) increased with elevations in passive Pcrit. The presence of sleep apnea and sleep apnea severity increased markedly as passive Pcrit increased above -5 cmH2O (see vertical dashed line), although there was substantial variability (○, women; ■, men).
Using an RDI > 10 events/h to define the presence of sleep apnea, a 1 cmH2O increase in passive Pcrit was associated with an increased odds for sleep apnea in NREM (OR: 1.45; 95% CI: 1.26-1.69; P < 0.001), REM (OR: 1.41; 95% CI: 1.21-1.64, P < 0.001), and total sleep (OR: 1.43; 95% CI: 1.24-1.66; P < 0.001). The passive Pcrit had a predictive power of 0.73 (95% CI: 0.65-0.82) in predicting a total RDI > 10 events/h with similar results for NREM RDI and REM RDI. A passive Pcrit threshold of -5 cmH2O correctly classified sleep apnea disease status in 76.7% of subjects with a sensitivity of 96.5% and specificity of 30.6%.
DISCUSSION
Our study examined the modulation of passive Pcrit, a measurement of upper airway structural loads, by sex, age, and obesity. The major findings in the study were that passive Pcrit was markedly elevated in men compared with women independent of disease or obesity. We also found that obesity was associated with progressive elevations in passive Pcrit in both men and women and demonstrated that the increase in passive Pcrit with obesity was greater in men than women. In contrast to men, age was associated with elevations in passive Pcrit in the women, particularly in the perimenopausal years. Of note, a markedly negative passive Pcrit (less than -5 cmH2O) appears to protect against sleep apnea, whereas increases in the passive Pcrit above this threshold predict increases in sleep apnea prevalence and severity. Our findings suggest that passive Pcrit is differentially controlled by sex, age, and BMI, and that alterations in passive Pcrit partly mediate the relationship between these sleep apnea risk factors and OSA.
Sex Differences in Passive Pcrit
Men and women may differ in both the anatomic and neuromuscular control of upper airway patency during sleep (31, 39). In the present study, neuromuscular activity was minimized (60) by adopting methods for measuring the passive mechanical properties of the airway during sleep (49). Utilizing a similar approach, investigators previously noted an ∼3-cmH2O elevation in passive Pcrit in BMI-matched men compared with women; however, this difference did not remain after subjects were matched for sleep apnea severity (25). Our study overcame limitations in sample size and allowed us to model the effects of sex, BMI, and age on passive Pcrit. Given the ample sample size, we were able to deploy several distinct analytical approaches to unraveling the relationships between passive Pcrit and known sleep apnea risk factors. In separate approaches, we adjusted, stratified, and rigorously matched for specific factors in order to determine the impact and mediators of upper airway structural control by sex, age, and BMI. Multiple analytical approaches were utilized to demonstrate a robust ∼2- to 3-cmH2O elevation in Pcrit in men compared with women, independent of age, BMI, and sleep apnea severity, indicating increased mechanical instability of the pharynx. Sex differences in upper airway mechanical control may be related to differences in pharyngeal length and surrounding soft tissue structures (26, 27, 31, 43, 45) or to sex-related differences in fat distribution (see below).
Effect of Obesity and Age in Men vs. Women
Obesity was associated with elevations in passive Pcrit in both the men and women, reflecting its effect on upper airway structural properties and mechanical loads (6, 12, 14, 23, 28, 36, 37, 51, 60). Several structural differences can account for observed elevations in passive Pcrit with obesity. First, the pharynx is more elongated in men compared with women (31), leaving a greater region exposed to upper airway collapse. Second, increased fat deposits around the upper airway in obese compared with lean individuals may increase extraluminal tissue pressure and increase Pcrit (10, 26, 27, 33, 44, 46, 47, 56). Third, truncal fat and central adiposity (especially in men) may be associated with decreased lung volumes and diminish caudal traction on the upper airway, compromise upper airway patency (7, 17, 20), elevate Pcrit (23, 44, 45, 52, 56), and increase sleep apnea severity (18). Thus obesity imposes mechanical loads on the upper airway through its effect on lung volume, extraluminal tissue pressure, and/or pharyngeal size.
Although the effects of obesity were similar between men and women, age was associated with a higher passive Pcrit in the women but not in the men. Elevations in upper airway mechanical loads may be related to a redistribution of body fat from the gluteofemoral to intra-abdominal compartments with age, particularly as women pass through menopause (9). These alterations in body fat can account for the observed age-related elevations in passive Pcrit, which were only detected when women in the perimenopausal age range were included in our analysis. As fat redistributes from peripheral to central compartments with age (5), decreases in lung volume and/or increases in neck and peripharyngeal fat may account for observed increases in passive Pcrit. As women age, sleep apnea susceptibility may increase as the passive Pcrit rises or compensatory neuromuscular responses wane (37).
Passive Pcrit and Sleep Apnea Pathogenesis
A major finding was that the prevalence and severity of sleep apnea rose as the passive Pcrit increased above -5 cmH2O. These findings are consistent with prior work demonstrating a similar threshold for disease at this level (37), and a somewhat higher passive Pcrit threshold for REM-related sleep apnea (14). Our findings imply that the passive Pcrit mediates effects of sleep apnea risk factors on disease susceptibility. Nevertheless, some subjects with elevations in passive Pcrit did not have sleep apnea. This latter finding is consistent with the notion that while passive upper airway structural loads predispose to sleep apnea, the development of upper airway obstruction in subjects with elevations in passive Pcrit can elicit active neuromuscular responses that mitigate the obstruction and protect from sleep apnea (37, 51, 54).
Limitations
Our findings imply that sex, obesity, and aging increase the individual’s susceptibility to sleep apnea in part by elevating the passive Pcrit in men and women. These conclusions arise from cross-sectional analyses of associations in a cohort in which recruitment bias may exist. Nevertheless, our conclusions are strengthened by the large study group recruited for detailed physiological measurements, the wide range of age and weight, and the multiplicity of confirmatory analytic approaches afforded by our large sample size. In separate analyses, we minimized the impact of confounding and recruitment bias on our conclusions by adjusting for potential confounders in the entire sample population, stratifying by sex, and by rigorously matching on obesity, sleep apnea severity, and age between sex groups. Another limitation of the present study is that it did not account for measurements of neck size or body fat distribution (e.g., waist circumference) since these measurements were not available for the entire cohort. Future investigations will be necessary to identify anatomic and soft tissue compartments that influence mechanical loading of the upper airway. In addition, we did not have precise information regarding menopausal status in the women in this study sample. Nevertheless, we documented particular vulnerability in women in the perimenopausal years. Finally, we recognize that while the passive Pcrit represents a measure of upper airway structural properties, it may be partially influenced by neuromuscular tone since it is determined under conditions of neuromuscular hypotonia rather than atonia. Nevertheless, neuromuscular effects were not likely to confound our measurements of passive Pcrit, which were comparable to those previously reported under conditions of general anesthesia (12-14, 19) and neuromuscular blockade (22).
Implications
Our findings have major implications for our understanding of the mechanisms mediating OSA susceptibility in male, overweight, and aging populations. Epidemiological studies have demonstrated that age and obesity are associated with sleep apnea (4, 61, 62) and that changes in weight result in parallel changes in sleep apnea severity (34, 38). In prior work, investigators have attributed increases in upper airway collapsibility with weight gain (in obesity) (48) to alterations in mechanical loads or neural control (37). In particular, obesity has been associated with an ∼0.5-cmH2O·kg-1·m-2 decrease in upper airway critical pressures (48), which may have been due to improvements in the passive Pcrit and/or active responses to airway obstruction (21, 37). The present findings suggest that less than 50% of the overall Pcrit response to weight loss may be attributed to reductions in the passive Pcrit, which decreases by only ∼0.1-0.2 cmH2O per unit fall in BMI (kg/m2). The remaining ∼0.3- to 0.4-cmH2O reduction in Pcrit per unit fall in BMI (kg/m2) with weight loss may be attributed to concomitant improvements in upper airway neuromuscular control. Longitudinal studies will be required to establish the independent effects of age and weight on the passive and active control of upper airway patency during sleep.
ACKNOWLEDGMENTS
We acknowledge the staff of the Johns Hopkins Sleep Disorders Center and the Bariatric Surgery Program for assistance in this study.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-72126, HL-37379, HL-50381, and HL-77137; and by National Health and Medical Research Council of Australia Grant 353705. This publication was also made possible by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahmad D, Morgan WK. Obesity and lung function. Thorax. 2001;56:740–741. doi: 10.1136/thorax.56.9.740c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atlas Task Force EEG arousals: scoring rules and examples. A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:174–184. [PubMed] [Google Scholar]
- 3.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 4.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men. I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 5.Borkan GA, Hults DE, Gerzof SG, Robbins AH, Silbert CK. Age changes in body composition revealed by computed tomography. J Gerontol. 1983;38:673–677. doi: 10.1093/geronj/38.6.673. [DOI] [PubMed] [Google Scholar]
- 6.Boudewyns A, Punjabi N, Van de Heyning PH, De Backer WA, O’Donnell CP, Schneider H, Smith PL, Schwartz AR. Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest. 2000;118:1031–1041. doi: 10.1378/chest.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 7.Bradley TD, Brown IG, Grossman RF, Zamel N, Martinez D, Phillipson EA, Hoffstein V. Pharyngeal size in snorers, nonsnorers, and patients with obstructive sleep apnea. N Engl J Med. 1986;315:1327–1331. doi: 10.1056/NEJM198611203152105. [DOI] [PubMed] [Google Scholar]
- 8.Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prospective study of the determinants of age at menopause. Am J Epidemiol. 1997;145:124–133. doi: 10.1093/oxfordjournals.aje.a009083. [DOI] [PubMed] [Google Scholar]
- 9.Colombel A, Charbonnel B. Weight gain and cardiovascular risk factors in the post-menopausal women. Hum Reprod. 1997;12(Suppl 1):134–145. doi: 10.1093/humrep/12.suppl_1.134. [DOI] [PubMed] [Google Scholar]
- 10.Dancey DR, Hanly PJ, Soong C, Lee B, Shepard J, Jr, Hoffstein V. Gender differences in sleep apnea: the role of neck circumference. Chest. 2003;123:1544–1550. doi: 10.1378/chest.123.5.1544. [DOI] [PubMed] [Google Scholar]
- 11.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apneahypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 12.Eastwood PR, Platt PR, Shepherd K, Maddison K, Hillman DR. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology. 2005;103:470–477. doi: 10.1097/00000542-200509000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Eastwood PR, Szollosi I, Platt PR, Hillman DR. Collapsibility of the upper airway during anesthesia with isoflurane. Anesthesiology. 2002;97:786–793. doi: 10.1097/00000542-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Eastwood PR, Szollosi I, Platt PR, Hillman DR. Comparison of upper airway collapse during general anaesthesia and sleep. Lancet. 2002;359:1207–1209. doi: 10.1016/S0140-6736(02)08224-7. [DOI] [PubMed] [Google Scholar]
- 15.Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 16.Harik-Khan RI, Wise RA, Fleg JL. The effect of gender on the relationship between body fat distribution and lung function. J Clin Epidemiol. 2001;54:399–406. doi: 10.1016/s0895-4356(00)00318-8. [DOI] [PubMed] [Google Scholar]
- 17.Heinzer RC, Stanchina ML, Malhotra A, Fogel RB, Patel SR, Jordan AS, Schory K, White DP. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:114–117. doi: 10.1164/rccm.200404-552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinzer RC, Stanchina ML, Malhotra A, Jordan AS, Patel SR, Lo YL, Wellman A, Schory K, Dover L, White DP. Effect of increased lung volume on sleep disordered breathing in sleep apnoea patients. Thorax. 2006;61:435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillman DR, Platt PR, Eastwood PR. The upper airway during anaesthesia. Br J Anaesth. 2003;91:31–39. doi: 10.1093/bja/aeg126. [DOI] [PubMed] [Google Scholar]
- 20.Hoffstein V, Zamel N, Phillipson EA. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis. 1984;130:175–178. doi: 10.1164/arrd.1984.130.2.175. [DOI] [PubMed] [Google Scholar]
- 21.Horner RL. Contributions of passive mechanical loads and active neuromuscular compensation to upper airway collapsibility during sleep. J Appl Physiol. 2007;102:510–512. doi: 10.1152/japplphysiol.01213.2006. [DOI] [PubMed] [Google Scholar]
- 22.Isono S, Morrison DL, Launois SH, Feroah TR, Whitelaw WA, Remmers JE. Static mechanics of the velopharynx of patients with obstructive sleep apnea. J Appl Physiol. 1993;75:148–154. doi: 10.1152/jappl.1993.75.1.148. [DOI] [PubMed] [Google Scholar]
- 23.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of the pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–1326. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 24.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130:827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 25.Jordan AS, Wellman A, Edwards JK, Schory K, Dover L, MacDonald M, Patel SR, Fogel RB, Malhotra A, White DP. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol. 2005;99:2020–2027. doi: 10.1152/japplphysiol.00410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kairaitis K, Parikh R, Stavrinou R, Garlick S, Kirkness JP, Wheatley JR, Amis TC. Upper airway extraluminal tissue pressure fluctuations during breathing in rabbits. J Appl Physiol. 2003;95:1560–1566. doi: 10.1152/japplphysiol.00432.2003. [DOI] [PubMed] [Google Scholar]
- 27.Kairaitis K, Stavrinou R, Parikh R, Wheatley JR, Amis TC. Mandibular advancement decreases pressures in the tissues surrounding the upper airway in rabbits. J Appl Physiol. 2006;100:349–356. doi: 10.1152/japplphysiol.00560.2005. [DOI] [PubMed] [Google Scholar]
- 28.Kirkness JP, Madronio M, Stavrinou R, Wheatley JR, Amis TC. Relationship between surface tension of upper airway lining liquid and upper airway collapsibility during sleep in obstructive sleep apnea hypopnea syndrome. J Appl Physiol. 2003;95:1761–1766. doi: 10.1152/japplphysiol.00488.2003. [DOI] [PubMed] [Google Scholar]
- 29.Kirkness JP, Patil SP, Schneider H, Pichard LE, Maly JJ, McGinley BM, Lafan AM, Magnuson T, Schweitzer T, Lidor AO, Smith PL, Schwartz AR. Women are protected against obstructive sleep apnea through reduced upper airway mechanical loads and active neuromuscular responses (Abstract) Proc Am Thorac Soc. 2006;3:A314. [Google Scholar]
- 30.Kirkness JP, Patil SP, Schneider H, Smith PL, Schwartz AR. Obesity augments passive mechanical load on the upper airway in men but not women (Abstract) Sleep Biol Rhythms. 2006;4:A18. [Google Scholar]
- 31.Malhotra A, Huang Y, Fogel RB, Pillar G, Edwards JK, Kikinis R, Loring SH, White DP. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166:1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 32.Morabia A, Costanza MC, World Health Organization Collaborative Study of Neoplasia and Steroid Contraceptives International variability in ages at menarche, first livebirth, and menopause. Am J Epidemiol. 1998;148:1195–1205. doi: 10.1093/oxfordjournals.aje.a009609. [DOI] [PubMed] [Google Scholar]
- 33.Mortimore IL, Marshall I, Wraith PK, Sellar RJ, Douglas NJ. Neck and total body fat deposition in nonobese and obese patients with sleep apnea compared with that in control subjects. Am J Respir Crit Care Med. 1998;157:280–283. doi: 10.1164/ajrccm.157.1.9703018. [DOI] [PubMed] [Google Scholar]
- 34.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the sleep heart health study. Arch Intern Med. 2005;165:2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 35.Oliven A, Carmi N, Coleman R, Odeh M, Silbermann M. Age-related changes in upper airway muscles morphological and oxidative properties. Exp Gerontol. 2001;36:1673–1686. doi: 10.1016/s0531-5565(01)00127-9. [DOI] [PubMed] [Google Scholar]
- 36.Patil SP, Punjabi NM, Schneider H, O’Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med. 2004;170:86–93. doi: 10.1164/rccm.200309-1239OC. [DOI] [PubMed] [Google Scholar]
- 37.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102:547–556. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 38.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 39.Pillar G, Malhotra A, Fogel R, Beauregard J, Schnall R, White DP. Airway mechanics and ventilation in response to resistive loading during sleep: influence of gender. Am J Respir Crit Care Med. 2000;162:1627–1632. doi: 10.1164/ajrccm.162.5.2003131. [DOI] [PubMed] [Google Scholar]
- 40.Punjabi NM, O’Hearn DJ, Neubauer DN, Nieto FJ, Schwartz AR, Smith PL, Bandeen-Roche K. Modeling hypersomnolence in sleep-disordered breathing A novel approach using survival analysis. Am J Respir Crit Care Med. 1999;159:1703–1709. doi: 10.1164/ajrccm.159.6.9808095. [DOI] [PubMed] [Google Scholar]
- 41.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Brain Information Service/Brain Research Institute; Los Angeles, CA: 1968. [Google Scholar]
- 42.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149:722–726. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 43.Rowley JA, Permutt S, Willey S, Smith PL, Schwartz AR. Effect of tracheal and tongue displacement on upper airway airflow dynamics. J Appl Physiol. 1996;80:2171–2178. doi: 10.1152/jappl.1996.80.6.2171. [DOI] [PubMed] [Google Scholar]
- 44.Rowley JA, Sanders CS, Zahn BR, Badr MS. Gender differences in upper airway compliance during NREM sleep: role of neck circumference. J Appl Physiol. 2002;92:2535–2541. doi: 10.1152/japplphysiol.00553.2001. [DOI] [PubMed] [Google Scholar]
- 45.Rowley JA, Zhou X, Vergine I, Shkoukani MA, Badr MS. Influence of gender on upper airway mechanics: upper airway resistance and Pcrit. J Appl Physiol. 2001;91:2248–2254. doi: 10.1152/jappl.2001.91.5.2248. [DOI] [PubMed] [Google Scholar]
- 46.Schwab RJ, Pasirstein M, Kaplan L, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med. 2006;173:453–463. doi: 10.1164/rccm.200412-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Permutt S, Smith PL. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144:494–498. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz AR, O’Donnell CP, Baron J, Schubert N, Alam D, Samadi SD, Smith PL. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med. 1998;157:1051–1057. doi: 10.1164/ajrccm.157.4.9706067. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz AR, Schubert N, Rothman W, Godley F, Marsh B, Eisele D, Nadeau J, Permutt L, Gleadhill I, Smith PL. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1992;145:527–532. doi: 10.1164/ajrccm/145.3.527. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64:535–542. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 52.Series F, Cormier Y, Desmeules M. Influence of passive changes of lung volume on upper airways. J Appl Physiol. 1990;68:2159–2164. doi: 10.1152/jappl.1990.68.5.2159. [DOI] [PubMed] [Google Scholar]
- 53.Smith AH, Kark JD, Cassel JC, Spears GF. Analysis of prospective epidemiologic studies by minimum distance case-control matching. Am J Epidemiol. 1977;105:567–574. doi: 10.1093/oxfordjournals.aje.a112421. [DOI] [PubMed] [Google Scholar]
- 54.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol. 1988;64:789–795. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 55.Tagaito Y, Isono S, Nishino T. Upper airway reflexes during a combination of propofol and fentanyl anesthesia. Anesthesiology. 1998;88:1459–1466. doi: 10.1097/00000542-199806000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Thut DC, Schwartz AR, Roach D, Wise RA, Permutt S, Smith PL. Tracheal and neck position influence upper airway airflow dynamics by altering airway length. J Appl Physiol. 1993;75:2084–2090. doi: 10.1152/jappl.1993.75.5.2084. [DOI] [PubMed] [Google Scholar]
- 57.Ware JC, McBrayer RH, Scott JA. Influence of sex and age on duration and frequency of sleep apnea events. Sleep. 2000;23:165–170. [PubMed] [Google Scholar]
- 58.Wilhoit SC, Suratt PM. Obstructive sleep apnea in premenopausal women. A comparison with men and with postmenopausal women. Chest. 1987;91:654–658. doi: 10.1378/chest.91.5.654. [DOI] [PubMed] [Google Scholar]
- 59.Winter WC, Gampper T, Gay SB, Suratt PM. Lateral pharyngeal fat pad pressure during breathing in anesthetized pigs. J Appl Physiol. 1997;83:688–694. doi: 10.1152/jappl.1997.83.3.688. [DOI] [PubMed] [Google Scholar]
- 60.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–658. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 61.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 62.Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, Walsleben JA, Finn L, Enright P, Samet JM. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]