Summary
The HIV-1 accessory protein Vpu counteracts a cellular factor that restricts the release of virions from infected cells. Here we show that the interferon-induced cellular protein BST-2/HM1.24/CD317 fulfills criteria as this factor. BST-2 is down-regulated from the cell surface by Vpu, and it is expressed constitutively in a cell-type specific manner that correlates with the virology of Vpu. Exogenous expression of BST-2 potently inhibits the release of HIV-1 virions, and suppression of its constitutive expression relieves the requirement for Vpu. Efficient down-regulation of BST-2 requires both the transmembrane, ion-channel domain and conserved serines in the cytoplasmic domain of Vpu. Endogenous BST-2 co-localizes with the HIV-1 structural protein Gag in endosomes and at the plasma membrane, suggesting that BST-2 traps virions within and on infected cells. The unusual structure of BST-2, which includes a transmembrane domain as well as a lumenal GPI-anchor, may allow it to retain nascent enveloped virions on cellular membranes, providing a novel mechanism of viral restriction counteracted by a specific viral accessory protein.
Introduction
Viral “accessory” proteins are so-named due to their relative dispensability for replication in simple in vitro culture systems, an observation often explained by their roles in evasion of innate and adaptive immunity in the infected host (Sheehy et al., 2002; Collins et al., 1998). In certain examples, specific in vitro culture systems either do or do not reveal the phenotype of such genes, because the cell lines used either do or do not express specific inhibitory cellular factors that these genes counteract (Sheehy et al., 2002). The HIV-1 accessory gene vpu encodes a small transmembrane protein known to enhance the release of infectious progeny virions from infected cells, but only in certain cell types (Klimkait et al., 1990; Sakai et al., 1995). Heterokaryons formed by the fusion of cells that support the phenotype of vpu with cells that do not are supportive of the Vpu-effect, suggesting that Vpu counteracts an inhibitor of virion-release (Varthakavi et al., 2003). Cells that do not support the effect of Vpu can be induced to do so by treatment with type I interferons, suggesting that the inhibitor is a component of the interferon-mediated innate immune response to viral infection (Neil et al., 2007).
The inefficient release of virions in the absence of Vpu is associated with the accumulation of nascent virions along the plasma membrane and within clathrin-coated endosomes (Klimkait et al., 1990; Van Damme and Guatelli, 2007). Virions trapped on the plasma membrane can be released by treatment with proteases, suggesting that the inhibitor that Vpu overcomes is a cell-surface-associated protein (Neil et al., 2006). We were intrigued by the proteomic analysis of Bartee and colleagues, who revealed down-regulation of the interferon-inducible protein BST-2/CD317/HM1.24 from the plasma membrane by the Kaposi’s sarcoma associated herpes virus (KSHV) protein K5, an immunomodulatory viral ubiquitin ligase; BST-2 was also noted to be modulated by HIV-1 Vpu (Bartee et al., 2006). Based on these data, we hypothesized that BST-2 is the inhibitor of virion-release that is counteracted by Vpu. This hypothesis has been supported by the recent findings of Neil and colleagues, who refer to BST-2/CD317 as “tetherin” based on its ability to inhibit the release of HIV virions from cells (Neil et al., 2008). The data herein corroborate the role of BST-2/CD317 as the elusive restriction factor targeted by Vpu and further suggest that down-regulation of BST-2 from the cell surface is the mechanism by which Vpu counteracts this cellular antiviral defense.
Results
HIV-1 Vpu down-regulates BST-2 from the cell surface
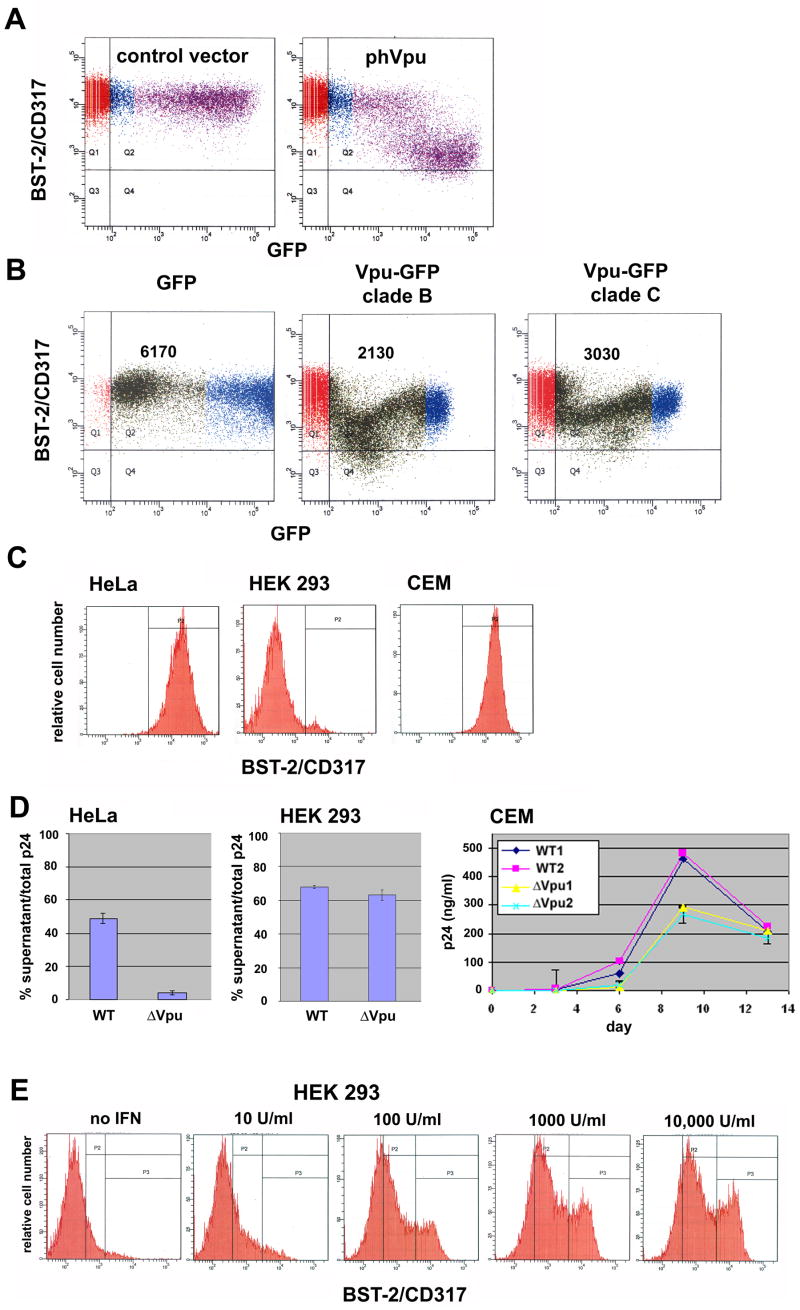
We determined using flow cytometry that BST-2 is constitutively expressed on the surface of HeLa cells and that it is down-regulated by Vpu as expressed via transient transfection (Figure 1A). The extent of down-regulation of BST-2 in highly expressing cells was approximately 10-fold. Down-regulation of BST-2 was also observed using Vpu-GFP fusion proteins in which the Vpu sequence was obtained from a laboratory-adapted subtype B virus (HXB2) as well as from a clinical isolate of subtype C from Botswana (Figure 1B).
Figure 1. BST-2/CD317 is down-regulated from the cell surface by HIV-1 Vpu; it is expressed constitutively in a cell-type-specific manner that correlates with the virology of Vpu, and its expression is induced by interferon-α.
A) HeLa cells were transfected to express transiently either no viral protein (“control vector”) or a codon-optimized version of HIV-1NL4-3 Vpu (“phVpu”) along with GFP encoded on a separate plasmid, then stained the next day for surface BST-2/CD317 and analyzed by two-color flow cytometry. B) HeLa cells were transfected to express transiently GFP fusion proteins containing N-terminal Vpu of clade BHXB-2 or clade C, then stained the next day for surface BST-2/CD317 and analyzed by two-color flow cytometry. Numbers within each panel are the mean fluorescence intensities of cell surface BST-2 for the low- to mid-GFP positive cells (green). Note that the GFP moiety appeared to interfere with the activity of Vpu at high levels of expression. C) Cell surface expression of BST-2 in HeLa cells (clone P4.R5), HEK 293 cells, and CEM T lymphoblastoid cells measured using flow cytometry. D) Virologic effects of Vpu in the different host cells. Left two panels: percent p24 capsid secretion measured as the fraction of the total p24 capsid antigen produced by cultures of cells transfected with full length viral genomes that was secreted into the media. Right panel: growth curve of wild-type (“WT”) versus vpu-negative (“ΔVpu”) virus during spreading infection in CEM T cells. “1 and “2” indicate independently infected cultures. Error bars are the standard deviation. The difference between the concentrations of p24 antigen in the media of cultures infected with wild-type or ΔVpu at nine days after inoculation was significant (p=0.001 by t-test). E) Induction of BST-2 expression at the surface of HEK 293 cells by treatment with IFN-α. Cells were treated overnight with the indicated concentrations of interferon then analyzed by flow cytometry. In panels A-C, horizontal and vertical lines indicate gates set using either GFP-negative cells or a primary antibody isotype control for BST-2; colors are arbitrary and distinguish GFP-negative, -low positive, and -high positive cells. In panel E, the leftmost vertical line is the gate set using an antibody isotype control.
Expression of BST-2 at the cell surface correlates with the virology of Vpu
BST-2 was expressed robustly on CEM T cells, a CD4-positive leukemic cell line, as well as on HeLa cells, but was nearly undetectable on the surface of HEK 293 cells (Figure 1C). This pattern of expression matched the ability of these cells to support the effect of Vpu on virion-release (Figure 1D). Treatment of HEK-293 cells with interferon-α induced the expression of BST-2 (Figure 1E), consistent with the ability of type-I interferons to confer Vpu-dependent virion-release to these cells (Neil et al., 2007).
BST-2 inhibits the release of virions from cells
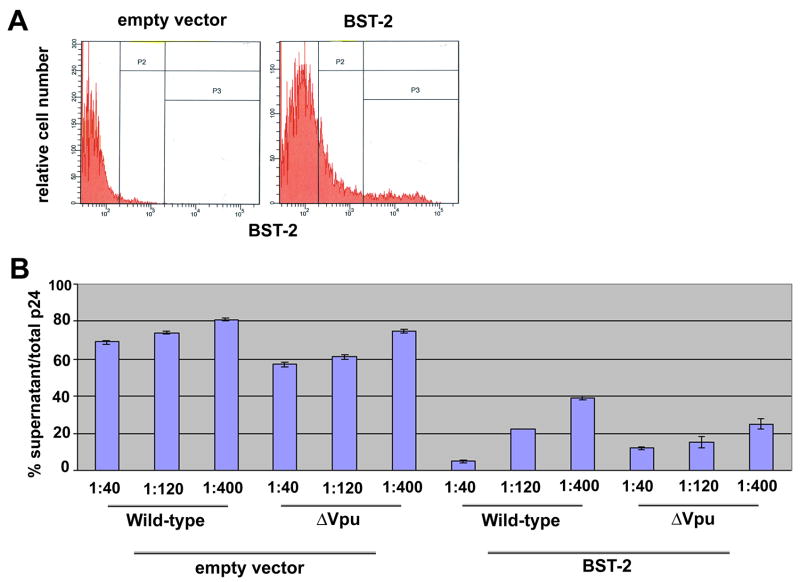
To determine whether BST-2 is an inhibitor of virion-release, we expressed the protein in HEK 293 cells by transient transfection together with full-length HIV-1 proviral DNA (Figure 2). BST-2 was a strikingly potent inhibitor of virion-release. Relatively selective inhibition of virus lacking a vpu gene occurred only when very low amounts of plasmid expressing BST-2 were used: when the weight-ratio of co-transfected plasmids expressing BST-2 and the HIV genome was 1:400, Vpu enhanced the efficiency of virion-release by approximately two-fold (Figure 2B). This effect of Vpu was reproducible and not increased by further reduction in BST-2 expression (data not shown). In contrast, at higher concentrations, the release of both wild-type as well as vpu-negative viruses was markedly inhibited, suggesting that the ability of Vpu to counteract the inhibitory action of BST-2 is readily saturable.
Figure 2. Exogenous expression of BST-2 in HEK 293 cells inhibits virion-release.
A) HEK 293 cells were transfected to express either no protein (“empty vector”) or BST-2 (“BST-2”) and analyzed by flow cytometry for surface BST-2; 1.6 μg of DNA was used in each transfection. The leftmost vertical line is the gate set using an antibody isotype control. B) HEK 293 cells were transfected to express transiently the wild-type and vpu-negative HIV-1 genomes together with either no BST-2 (“empty vector”) or BST-2. Ratios are the relative amounts of plasmids by weight (empty vector or BST-2 vector: proviral (HIV-1) vector; total DNA was 1.6 μg in each transfection. The following day, the fraction of the total p24 capsid antigen produced that was secreted into the media was measured.
BST-2 is necessary for the maximal effect of Vpu on virion release
To determine whether BST-2 is necessary for the effect of Vpu, we inhibited the expression of the protein in HeLa cells by RNA interference (Figure 3). Plasmid vectors encoding short, hairpin RNAs (shRNAs) effectively inhibited the expression of BST-2 at the surface of HeLa cells (Figure 3A). Expression of these shRNAs reduced the phenotype of the vpu gene: the release of virions from cells expressing vpu-negative virus was increased much more than the release of virions from cells expressing the wild-type (Figure 3B). Expression of an “off-target” shRNA had no effect (data not shown). The incomplete rescue of virion-release from cells expressing vpu-negative virus likely reflects the high potency of BST-2 as shown above, although alternatively it could indicate that BST-2 is not the sole protein mediating inhibition of virion-release in these cells. Interestingly, the small but consistent increase in the release of virions from cells expressing wild-type virus by these shRNAs suggests that the activity of BST-2 is not fully negated by Vpu during viral replication.
Figure 3. Reduction of constitutive BST-2-expression in HeLa cells decreases the virologic phenotype of vpu.
A) HeLa cells were transfected to express transiently either no short-hairpin (sh) RNA (“empty vector”) or various shRNAs targeting different sequences in BST-2 mRNA (“TI-1”, “TI-3”, or “TI–4”), along with GFP encoded on a separate plasmid, then stained three days later for surface BST-2/CD317 and analyzed by two-color flow cytometry. Horizontal and vertical lines indicate gates set using either GFP-negative cells or a primary antibody isotype control for BST-2; colors are arbitrary and distinguish GFP-negative, -low positive, and -high positive cells. Total DNA in each transfection was 1.6 μg, 1.4 of which was the shRNA expression vector. B) HeLa cells were transfected to express either no shRNA or shRNAs TI-1, -3, -4, or an equimolar mixture of TI-1, -3, and –4, along with wild-type or vpu-negative full length viral DNA at a weight ratio of 2:1:: shRNA-vector: proviral plasmid. A CXCR4 receptor blocker (AMD3100) was used to limit viral production to the initially transfected cells, and the fraction of the total p24 capsid antigen produced that was secreted into the media was measured two days later.
Vpu down-regulates BST-2 when expressed in the context of the complete viral genome: role of the Vpu transmembrane domain and conserved serines in the cytoplasmic domain
To determine the requirements in Vpu for the down-regulation of BST-2 and to characterize this effect in the context of the complete viral genome, we expressed proviral mutants of HIV-1 in HeLa cells (Figure 4A). Expression of the complete viral genome down-regulated BST-2 from the cell surface. This effect required the vpu gene. To examine the roles of Vpu domains, we tested the proviral mutant VpuRD, which encodes a Vpu transmembrane domain (TMD) of scrambled sequence, and the mutant Vpu2/6, which encodes alanine substitutions of serine residues 52 and 56 in the Vpu cytoplasmic domain. Both the VpuRD and Vpu2/6 mutants were impaired in their abilities to down-regulate BST-2. These domains of Vpu have been associated with distinct functional attributes: the TMD with ion channel activity and serines 52 and 56 with the linkage of Vpu to β-TrCp and a Skp1-ubiquitin ligase, through which it triggers the degradation of CD4, the primary viral receptor (Schubert et al., 1996a; Schubert et al., 1996b; Margottin et al., 1998). However, both domains contribute to the enhancement of virion-release by Vpu [Figure 4B and (Schubert et al., 1996b; Schubert and Strebel, 1994)]. To test the hypothesis that Vpu down-regulates BST-2 via the proteasome, we examined whether the peptide aldehyde proteasome inhibitor MG-132 blocked this activity (Figure 4C). Only a modest effect of MG-132 was observed, consistent with an alternative mechanism that is independent of proteasomal activity.
Figure 4. Vpu-mediated down-regulation of BST-2 in the context of the complete HIV-1 genome requires the Vpu transmembrane domain and conserved serines in the cytoplasmic domain but is not prevented by inhibition of the proteasome.
A) HeLa cells were transfected to express complete HIV-1 genomes, either wild-type (“WT”), vpu-negative (“ΔVpu”), a mutant encoding a Vpu protein whose transmembrane domain is scrambled (“VpuRD”), or a mutant in which serines 52 and 56 in the Vpu cytoplasmic domain are replaced by alanines (“Vpu2/6”), in each case along with GFP encoded on a separate plasmid. The cells were stained the next day for surface BST-2 and analyzed by two-color flow cytometry. B) HeLa cells were transfected with the indicated proviral genomes, and the fraction of the total p24 capsid antigen produced that was secreted into the media was measured one day later. C) HeLa cells were transfected to express transiently either no viral protein (“mock”) or a codon-optimized version of HIV-1NL4-3 Vpu along with GFP encoded on a separate plasmid. The next day, cells were either treated for five hours with 25 μM MG-132 (an inhibitor of the proteasome) or left untreated, then stained for surface BST-2 and analyzed by two-color flow cytometry. In panels A and C, horizontal and vertical lines indicate gates set using either GFP-negative cells or a primary antibody isotype control for BST-2; colors are arbitrary and distinguish GFP-negative, -low positive, and -high positive cells.
Vpu and endogenous BST-2 co-localize within the cytoplasm of cells expressing the viral genome
To determine whether Vpu might affect BST-2 within the endosomal system, we examined the subcellular distributions of these proteins in HeLa cells expressing Vpu from the complete HIV-1 genome. Native, endogenous BST-2 co-localized with Vpu within cytoplasmic structures visualized by immunofluorescence microscopy (Figure 5). The non-functional Vpu expressed by the VpuRD mutant co-localized slightly less extensively with BST-2. These data suggest that co-residence of Vpu and BST-2 within the endosomal system may be functionally important.
Figure 5. BST-2 co-localizes with Vpu.
HeLa cells were transfected to express transiently either the wild-type or VpuRD viral genomes. The next day, the cells were stained by indirect immunofluorescence using antibodies to BST-2 and Vpu. Images were obtained using a spinning disc confocal microscope and processed using a deconvolution algorithm; single planes are shown. Insets show the boxed regions indicated in the “merge”panels. Vpu: green; BST-2: red. Scale bars are 10 microns.
BST-2 co-localizes with the HIV structural protein Gag both in endosomes and along the plasma membrane
The accumulation of virions along the plasma membrane and within endosomes of cells expressing vpu-negative virus led to the hypothesis that a specific cellular factor “tethers” nascent virions to cellular membranes (Neil et al., 2006). If BST-2 is this factor, then it should co-localize microscopically with virion structural proteins along the plasma membrane and in endosomes. To test this, we first expressed complete viral genomes in HeLa cells and co-stained the cells for both p55/p17 Gag and endogenous, native BST-2 (Figure 6A). As described previously, the accumulation of Gag in endosomal structures and along the plasma membrane was more prominent in the absence of vpu (Klimkait et al., 1990; Neil et al., 2006; Van Damme and Guatelli, 2007). Nevertheless, in cells expressing either wild-type or vpu-negative virus, BST-2 and HIV-1 Gag co-localized both along the plasma membrane and in endosomes, consistent with the hypothesis that BST-2 traps virions on and within virus producing cells. Similar results were obtained using HEK 293 cells expressing exogenous BST-2 (Figure 6B).
Figure 6. BST-2 co-localizes with HIV-1 Gag (p17/p55) both along the plasma membrane and in endosomes.
A) HeLa cells were transfected to express transiently either the wild-type or vpu-negative viral genome, along with GFP encoded on a separate plasmid. The next day, the cells were stained by indirect immunofluorescence using antibodies to BST-2 and HIV-1 Gag (p17/p55). Images were obtained using a spinning disc confocal microscope and processed using a deconvolution algorithm; single planes are shown. Insets show the boxed regions indicated in the “merge”panels. GFP: green; BST-2: red; p17/p55 Gag: blue. B) HEK 293 cells were transfected to express transiently either the wild-type or vpu-negative viral genome along with BST-2 encoded on a separate plasmid; the ratio of BST-2-expression plasmid: proviral plasmid was 1:4 by weight. The next day, the cells were stained by indirect immunofluorescence using antibodies to BST-2 and HIV-1 Gag (p17/p55); images were obtained as described above. p17/p55 Gag: green; BST-2: red. Scale bars in all panels are 10 microns.
BST-2 traps viral particles on the cell surface
To determine whether BST-2 is able to retain nascent virions at the cell surface, we used scanning electron microscopy (SEM) to visualize virus-like particles (VLPs) on the surface of HEK 293 and HT1080 cells; neither of these cell types support the effect of Vpu on virion-release, and neither express BST-2 [Figure 1 and (Neil et al., 2008)]. The VLPs were expressed from a proviral genome lacking vpu and well as vpr, vif, env, and nef genes. The density of VLPs on the surface of HEK 293 or HT1080 cells was typically low (Figure 7a and b; e and f), but when the cells were transfected to express BST-2, we observed surface domains with remarkably high densities of VLPs (Figure 7c and d; g and h). These data suggest that BST-2 acts at least in part by trapping virions at the cell surface.
Figure 7. BST-2 retains virus-like particles (VLPs) at the cell surface.
HEK 293 cells (a–d) or HT1080 cells (e–h) were transfected to express transiently a provirus lacking intact vpu, vpr, vif, env and nef genes, along with either a vector expressing no BST-2 (a and b; e and f) or BST-2 (c and d; g and h). The proviral plasmid also encoded the fluorescent protein TdTomato, enabling assessment of the efficiency with which individual cells were transfected by fluorescence microscopy (insets), before visualization by SEM. Scales bars are 2 microns.
Discussion
We have shown that the HIV-1 Vpu protein down-regulates the interferon-induced cellular protein BST-2/HM1.24/CD317 from cell surfaces. BST-2 is a potent inhibitor of HIV-1 virion-release, and it appears both sufficient to confer vpu-dependent virion release to HEK 293 cells and necessary for a robust vpu-phenotype in HeLa cells.
BST-2 has an unusual topology that may render it uniquely suited to tethering nascent enveloped virions to cellular membranes: it is a type II transmembrane protein whose lumenal domain is predicted to be modified by linkage to glycosylphosphatidylinositol (GPI) (Kupzig et al., 2003). This structure could enable BST-2 to function as a bifunctional lipid-binding agent that directly links cellular and viral membranes. BST-2 associates with cholesterol-enriched lipid rafts, presumably via its GPI anchor, and it has been proposed to cross-link rafts by dimerization (Kupzig et al., 2003). HIV-1, as well as several other enveloped viruses including influenza A and Ebola virus, have cholesterol-enriched lipid membranes due to budding from raft-enriched domains (Aloia et al., 1993; Scheiffele et al., 1999; Nguyen and Hildreth, 2000; Panchal et al., 2003). Consequently, it is tempting to speculate that BST-2 may inhibit the release of a number of enveloped viruses, especially those that bud from cholesterol-enriched rafts. This hypothesis is consistent with the ability of Vpu to enhance the release of murine leukemia virus and Ebola virus-like-particles (Neil et al., 2007; Gottlinger et al., 1993), as well as with the observation that down-regulation of BST-2 is a function of proteins encoded by proteins of both HIV-1 (Vpu) and KSHV (K5), viruses that have little in common except a lipid envelope (Bartee et al., 2006).
Although BST-2 is a ubiquitous protein following interferon stimulation, it is expressed constitutively on B cells, dendritic cells, and activated T cells (Vidal-Laliena et al., 2005; Blasius et al., 2006). Until now, its function has been essentially unknown. Activated T cells are a major host cell for productive HIV-1 replication in vivo, where the inhibitory effect of BST-2 on virion-release could play a role in restricting viral replication, consistent with the significant effect of Vpu on replication and pathogenesis in animal models (Hout et al., 2005). In addition, BST-2 could play a role in capturing enveloped viruses via their lipid domains for uptake into the professional antigen presenting cells noted above.
How does Vpu counteract the inhibitory effect of BST-2? Vpu could decrease the expression of BST-2 by targeting it for degradation, similar to the mechanism of its effect on CD4 (Schubert et al., 1998). This hypothesis is consistent with the role of serines 52 and 56 in the cytoplasmic domain of Vpu in the down-regulation of BST-2 observed here (Margottin et al., 1998). However, data herein suggest that inhibition of the proteasome does not efficiently reverse the Vpu-mediated down-regulation of BST-2 from the cell surface, a result consistent with the observation that the effect of Vpu on virion-release persists in the presence of such inhibitors (Schubert et al., 2000). Notably, the transmembrane domain of Vpu and its ion channel activity have been correlated more closely with the virion-release phenotype than have the above serine residues (Schubert et al., 1996a). Nevertheless, we find as reported previously that serines 52 and 56 as well as the transmembrane domain contribute to optimal virion-release (Schubert et al., 1996b; Schubert and Strebel, 1994).
Conceivably, the ion channel activity of Vpu could affect the intracellular trafficking of BST-2, which, although readily detectable by flow cytometry at the surface of cells such as HeLa, CEM, and interferon-treated HEK 293, is observed to a large extent within the endosomal system by immunofluorescence microscopy. Vpu is itself found predominantly within the Golgi, trans-Golgi network (TGN), and the endosomal system, and its ability to distort the TGN and delay transport through secretory membranes could relate directly to an effect on BST-2 (Van Damme and Guatelli, 2007; Varthakavi et al., 2006; Vincent and Abdul Jabbar, 1995). Indeed, an orthologue of BST-2 reportedly plays a key role in the maintenance of Golgi structure (Li et al., 2007). Interestingly, the requirement of functional recycling endosomes for the enhancement of virion-release suggests that Vpu may act on BST-2 within the endosomal system (Varthakavi et al., 2006). These hypotheses are supported by the apparent co-localization of BST-2 and Vpu within endosomal structures.
Although we detected remarkable modulation of cell-surface BST-2 by Vpu, whether the surface-level of BST-2 is a direct, quantitative correlate of the efficiency of virion-release remains an open question. For example, the inhibition of virion-release induced by exogenous expression of BST-2 in HEK 293 cells appears to occur at surface levels below that detected on HeLa cells. This observation, together with the ease with which virion-release is inhibited by the exogenous expression of BST-2 in HEK 293 cells even when Vpu is present, suggests that differentially expressed but as yet unidentified cellular factors may modulate the ability of BST-2 to restrict virion-release and/or the effectiveness of Vpu as a viral countermeasure.
While this paper was in preparation, similar conclusions regarding the role of BST-2 as a restriction factor counteracted by Vpu were reported by Neil and colleagues, although these investigators did not address the potential effect of Vpu on the expression of BST-2 at the cell surface as highlighted herein (Neil et al., 2008). Nevertheless, comparison of these two data sets reveals interesting differences in the apparent efficacy of Vpu as an antagonist of BST-2. In the experiments herein, the exogenous expression of BST-2 in HEK 293 cells consistently inhibited the release of wild-type virions, whereas Neil and colleagues reported little to no effect of BST-2 expression on the wild-type. Similarly, in the experiments herein, Vpu did not appear to counteract fully the effect of BST-2 in HeLa cells, because the suppression of BST-2 expression in these cells increased the efficiency of virion-release even in the case of wild-type virus. In contrast, Neil and colleagues reported no increase in the release of wild-type virions when the expression of BST-2 was suppressed in HeLa cells. The reasons for these differences are not clear, but they could be due to the different methods used to quantify “virion-release.” Here, we measured the fractional secretion of p24 capsid antigen, the structural component of the virion-core; this measurement may be complicated by the presence of secreted p24 antigen that is not virion-associated. Neil and colleagues measured secreted infectivity in a virologic assay; this measurement may be complicated by intrinsic differences in the particle/infectivity ratio of wild-type and vpu-negative virions. While these issues remain to be fully resolved, the magnitude of the effects of Vpu on virion-release reported here is consistent with previously reported measurements (Schubert et al., 1996b; Schubert and Strebel, 1994).
As noted above, the data herein, together with those of Neil and colleagues, raise the intriguing possibility that BST-2 is a broadly acting inhibitor of virion-release (Neil et al., 2008). Vpu has been categorized as a virally encoded ion channel or “viroporin”, along with several viral gene products including the M2 protein of influenza A virus, the 6K protein of Sindbis virus, and the p7 protein of hepatitis C virus (Gonzalez and Carrasco, 2003). Like Vpu, these proteins affect virion-release and/or protein transport along secretory membranes. Given the potential for BST-2 to act as a broad inhibitor of enveloped viruses and the role of the Vpu transmembrane domain in the modulation of BST-2, we wonder whether other viroporins may down-regulate BST-2 as a countermeasure to the innate immune response of the host to viral infection.
Experimental Procedures
Plasmids, antibodies, and reagents
The proviral plasmid pNL4-3 was obtained from the National Institutes of Health (NIH) AIDS Research & Reference Reagent Program (Adachi et al., 1986). The pNL4-3 mutants ΔVpu (containing an out-of-frame deletion in the 5′ region of the vpu gene that preserves the start codon and does not affect env), VpuRD (encoding a Vpu protein in which the sequence of the transmembrane domain is randomized) and Vpu2/6 (encoding a Vpu protein in which serines 52 and 56 in the cytoplasmic domain are substituted with alanines), as well as the pcDNA3.1-based plasmid expressing codon-optimized Vpu were provided by Klaus Strebel (Schubert et al., 1996a; Nguyen et al., 2004). The plasmid expressing GFP, pCG-GFP, was provided by Jacek Skowronski (Greenberg et al., 1997). Plasmids expressing the fusion proteins of GFP with Vpu from clade B or C HIV-1 were described previously (Pacyniak et al., 2005). The plasmid expressing BST-2 was obtained from OriGene (Rockville, MD); the coding sequence of BST-2 was confirmed. The corresponding empty vector, pCMV6-XL5, was also obtained from OriGene. Plasmids expressing 29mer shRNAs targeting three distinct regions of the BST-2 mRNA (pRS: TI357701, -3, and –4; “TI-1, TI-3, and TI-4”), the empty vector pRS, and an off-target control vector encoding an inactive shRNA targeted to GFP, were obtained from OriGene. The pNL4-3 derived “HIV-tomato” plasmid does not express Vpr, Vif, Vpu, or Env; it encodes GFP in the nef position and the protein TdTomato downstream of a CMV promoter located outside the proviral sequence. The murine monoclonal antibody to BST-2/HM1.24/CD317 was provided by Chugai Pharmaceutical Co., Kanagawa, Japan. For flow cytometry, an IgG2a antibody isotype control and a goat, anti-mouse IgG antibody conjugated to allophycocyanin (APC) was obtained from BioLegend (San Diego, CA). Rabbit antisera to HIV-1 Vpu and p17/p55 were obtained from the NIH AIDS Research & Reference Reagent Program. Secondary antibodies for immunofluorescence were obtained from Jackson ImmunoResearch (West Grove, PA). The peptide-aldehyde proteasome inhibitor MG-132 was obtained from Calbiochem, and the CXCR4 co-receptor antagonist AMD3100 was obtained from Sigma-Aldrich.
Cells and transfections
HEK 293T cells were obtained from Ned Landau and were maintained in EMEM plus 10% fetal bovine serum (FBS) and L-glutamine. HeLa P4.R5 cells were also obtained from Ned Landau and were maintained in DMEM plus 10% FBS, penicillin/streptomycin, and puromycin; these cells stably express CD4 and CCR5 and are a derivative of clone P4 (Clavel and Charneau, 1994). CEM cells were obtained from Douglas Richman and maintained in RPMI 1640 plus 10% FBS and penicillin/streptomycin. HT1080 cells were obtained from David Pintel. Cells were transfected using Lipofectamine2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions, or with Fugene for electron microscopy.
Infections
For viral infections, CEM T cells were exposed for five hours to cell free virions produced from HeLa P4.R5 cells transiently transfected with proviral plasmids. The cells were washed and the cultures were split at days 3, 6, and 9 to maintain a nominal cell density between 4×105 and 2×106 cells/ml. The concentrations of p24 antigen in the cell-free supernatants of the cultures were measured by ELISA (Perkin-Elmer).
Flow cytometry
Cells were stained before fixation in phosphate buffered saline (PBS) including sodium azide and FBS at 4 C using an indirect method to detect BST-2: the HM1.24 murine monoclonal antibody was followed by a goat anti-mouse IgG conjugated to APC. Gates for BST-2 were set using an antibody isotype control (IgG2a) as the primary antibody, and gates for GFP were set using non-GFP expressing cells. After staining, the cells were fixed in formaldehyde and analyzed by flow cytometry.
Virion-release assays
A p24 antigen capture ELISA (Perkin-Elmer) was used to determine the concentration of viral capsid protein in culture supernatants that were first clarified by centrifugation at 400g as well as the concentration of capsid protein in detergent lysates (0.5% Triton-X-100 in PBS) of the adherent cells. The percentage of p24 capsid secreted into the culture media was determined as the concentration of p24 antigen in the supernatants divided by the concentration of p24 antigen in the total culture (supernatant plus cells) ×100. Assays were run in duplicate.
Immunofluorescence microscopy
Cells were stained for HIV-1 Gag and BST-2 using the antibodies above after fixation in 3% formaldehyde and permeabilization using 0.1% NP40, as previously described (Van Damme and Guatelli, 2007). Images were obtained using a spinning disc confocal fluorescence microscope fitted with a 100x objective (Olympus). For each field, a Z-series of images was collected, and the data were processed using a deconvolution algorithm (Slidebook software, Imaging Innovations, Inc). Composite multi-color images of single optical sections were assembled using Adobe Photoshop software.
Scanning electron microscopy (SEM)
SEM analyses were performed as described previously (Larson et al., 2005). HEK 293T cells or HT1080 cells were plated onto glass coverslips containing a gold finder grid for cell identification, then transfected using Fugene (Roche) with “HIV-tomato”: BST-2 or “HIV-tomato”: empty vector (pCMV6-XL5) at a plasmid weight ratio of 50:1. Nineteen hours after transfection, cells were fixed in 4% paraformaldehyde and imaged by fluorescence microscopy to identify the transfected cells and assess relative transfection levels. The cells were then fixed in 2.5% glutaraldehyde, dehydrated, critical point dried, coated with a thin layer of platinum, and imaged at the University of Missouri Electron Microscopy core facility on a Hitachi S4700 Cold-cathode SEM.
Acknowledgments
We thank Klaus Strebel for providing the ΔVpu, VpuRD, and Vpu2/6 proviral plasmid mutants of HIV-1NL4-3 as well as the pcDNA3.1-based plasmid expressing codon-optimized Vpu; Jacek Skowronski for pCG-GFP, and Chugai Pharmaceuticals for the antibody to HM1.24/BST-2. Antibody to HIV-1 p17/p55 was originated by Paul Spearman, and antibody to Vpu was originated by Klaus Strebel; both were obtained via the National Institutes of Health AIDS Research & Reference Reagent Program. We thank Sherri Rostami, Deya Collier, and Nancy Keating for the p24 ELISAs; Neal Sekiya, Peggy O’Keefe and Judy Nordberg for the flow cytometry; and Richard Kornbluth for review of the manuscript. This work was supported by the following grants from the National Institutes of Health: AI038201 and AI076040 to J.G.; AI51981 to E.S., AI073098 to M.J; and AI36214, the UCSD Center for AIDS Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. Production of acquired immunodeficiency syndrome-associated retrovirus in human and non-human cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia RC, Tian H, Jensen FC. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci USA. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartee E, McCormack A, Fruh K. Quantitative membrane proteomics reveals new cellular targets of viral immune modulators. PLoS Pathog. 2006;2:e107. doi: 10.1371/journal.ppat.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- Clavel F, Charneau P. Fusion from without directed by human immunodeficiency virus particles. J Virol. 1994;68:1179–1185. doi: 10.1128/jvi.68.2.1179-1185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–400. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- Gonzalez ME, Carrasco L. Viroporins. FEBS Lett. 2003;552:28–34. doi: 10.1016/s0014-5793(03)00780-4. [DOI] [PubMed] [Google Scholar]
- Gottlinger HG, Dorfman T, Cohen EA, Haseltine WA. Vpu protein of human immunodeficiency virus type 1 enhances the release of capsids produced by gag gene constructs of widely divergent retroviruses. Proc Natl Acad Sci USA. 1993;90:7381–7385. doi: 10.1073/pnas.90.15.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Bronson S, Lock M, Neumann M, Pavlakis GN, Skowronski J. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 1997;16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hout DR, Gomez ML, Pacyniak E, Gomez LM, Inbody SH, Mulcahy ER, Culley N, Pinson DM, Powers MF, Wong SW, Stephens EB. Scrambling of the amino acids within the transmembrane domain of Vpu results in a simian-human immunodeficiency virus (SHIVTM) that is less pathogenic for pigtailed macaques. Virology. 2005;339:56–69. doi: 10.1016/j.virol.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Klimkait T, Strebel K, Hoggan MD, Martin MA, Orenstein JM. The human immunodeficiency virus type 1-specific protein vpu is required for efficient virus maturation and release. J Virol. 1990;64(2):621–629. doi: 10.1128/jvi.64.2.621-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupzig S, Korolchuk V, Rollason R, Sugden A, Wilde A, Banting G. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 2003;4:694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- Larson DR, Johnson MC, Webb WW, Vogt VM. Visualization of retrovirus budding with correlated light and electron microscopy. Proc Natl Acad Sci USA. 2005;102:15453–15458. doi: 10.1073/pnas.0504812102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kaloyanova D, van EM, Eerland R, van der GG, Oorschot V, Klumperman J, Lottspeich F, Starkuviene V, Wieland FT, Helms JB. Involvement of a Golgi-resident GPI-anchored protein in maintenance of the Golgi structure. Mol Biol Cell. 2007;18:1261–1271. doi: 10.1091/mbc.E06-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margottin F, Bour SP, Durand H, Selig L, Benichou S, Richard V, Thomas D, Strebel K, Benarous R. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell. 1998;1:565–574. doi: 10.1016/s1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- Neil SJ, Eastman SW, Jouvenet N, Bieniasz PD. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2006;2:e39. doi: 10.1371/journal.ppat.0020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJ, Sandrin V, Sundquist WI, Bieniasz PD. An interferon-alpha-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe. 2007;2:193–203. doi: 10.1016/j.chom.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- Nguyen DH, Hildreth JEK. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KL, llano M, Akari H, Miyagi E, Poeschla EM, Strebel K, Bour S. Codon optimization of the HIV-1 vpu and vif genes stabilizes their mRNA and allows for highly efficient Rev-independent expression. Virology. 2004;319:163–175. doi: 10.1016/j.virol.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Pacyniak E, Gomez ML, Gomez LM, Mulcahy ER, Jackson M, Hout DR, Wisdom BJ, Stephens EB. Identification of a region within the cytoplasmic domain of the subtype B Vpu protein of human immunodeficiency virus type 1 (HIV-1) that is responsible for retention in the golgi complex and its absence in the Vpu protein from a subtype C HIV-1. AIDS Res Hum Retroviruses. 2005;21:379–394. doi: 10.1089/aid.2005.21.379. [DOI] [PubMed] [Google Scholar]
- Panchal RG, Ruthel G, Kenny TA, Kallstrom GH, Lane D, Badie SS, Li L, Bavari S, Aman MJ. In vivo oligomerization and raft localization of Ebola virus protein VP40 during vesicular budding. Proc Natl Acad Sci US A. 2003;100:15936–15941. doi: 10.1073/pnas.2533915100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Tokunaga K, Kawamura M, Adachi A. Function of human immunodeficiency virus type 1 Vpu protein in various cell types. J Gen Virol. 1995;76(Pt 11):2717–2722. doi: 10.1099/0022-1317-76-11-2717. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Rietveld A, Wilk T, Simons K. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J Biol Chem. 1999;274:2038–2044. doi: 10.1074/jbc.274.4.2038. [DOI] [PubMed] [Google Scholar]
- Schubert U, Anton LC, Bacik I, Cox JH, Bour S, Bennink JR, Orlowski M, Strebel K, Yewdell JW. CD4 glycoprotein degradation induced by human immunodeficiency virus type 1 Vpu protein requires the function of proteasomes and the ubiquitin-conjugating pathway. J Virol. 1998;72:2280–2288. doi: 10.1128/jvi.72.3.2280-2288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert U, Bour S, Ferrer-Montiel AV, Montal M, Maldarell F, Strebel K. The two biological activities of human immunodeficiency virus type 1 Vpu protein involve two separable structural domains. J Virol. 1996a;70:809–819. doi: 10.1128/jvi.70.2.809-819.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert U, Ferrer-Montiel AV, Oblatt-Montal M, Henklein P, Strebel K, Montal M. Identification of an ion channel activity of the Vpu transmembrane domain and its involvement in the regulation of virus release from HIV-1-infected cells. FEBS Lett. 1996b;398:12–18. doi: 10.1016/s0014-5793(96)01146-5. [DOI] [PubMed] [Google Scholar]
- Schubert U, Ott DE, Chertova EN, Welker R, Tessmer U, Princiotta MF, Bennink JR, Krausslich HG, Yewdell JW. Proteasome inhibition interferes with gag polyprotein processing, release, and maturation of HIV-1 and HIV-2. Proc Natl Acad Sci USA. 2000;97:13057–13062. doi: 10.1073/pnas.97.24.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert U, Strebel K. Differential activities of the human immunodeficiency virus type 1-encoded Vpu protein are regulated by phosphorylation and occur in different cellular compartments. J Virol. 1994;68:2260–2271. doi: 10.1128/jvi.68.4.2260-2271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- Van Damme N, Guatelli J. HIV-1 Vpu inhibits accumulation of the envelope glycoprotein within clathrin-coated, Gag-containing endosomes. Cell Microbiol. 2007 doi: 10.1111/j.1462-5822.2007.01101.x. epub doi: 10.1111. [DOI] [PubMed] [Google Scholar]
- Varthakavi V, Smith RM, Bour SP, Strebel K, Spearman P. Viral protein U counteracts a human host cell restriction that inhibits HIV-1 particle production. Proc Natl Acad Sci USA. 2003;100:15154–15159. doi: 10.1073/pnas.2433165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varthakavi V, Smith RM, Martin KL, Derdowski A, Lapierre LA, Goldenring JR, Spearman P. The pericentriolar recycling endosome plays a key role in Vpu-mediated enhancement of HIV-1 particle release. Traffic. 2006;7:298–307. doi: 10.1111/j.1600-0854.2005.00380.x. [DOI] [PubMed] [Google Scholar]
- Vidal-Laliena M, Romero X, March S, Requena V, Petriz J, Engel P. Characterization of antibodies submitted to the B cell section of the 8th Human Leukocyte Differentiation Antigens Workshop by flow cytometry and immunohistochemistry. Cell Immunol. 2005;236:6–16. doi: 10.1016/j.cellimm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Vincent MJ, Abdul JM. The human immunodeficiency virus type 1 Vpu protein: a potential regulator of proteolysis and protein transport in the mammalian secretory pathway. Virology. 1995;213:639–649. doi: 10.1006/viro.1995.0035. [DOI] [PubMed] [Google Scholar]