Abstract
The present experiment examines the effects of NPY administered into the amygdala on ethanol drinking by alcohol-preferring P rats following long-term continuous ethanol access, with and without multiple periods of imposed ethanol abstinence. P rats had access to 15% (v/v) ethanol and water for 11 weeks followed by 2 weeks of ethanol abstinence, re-exposure to ethanol for 2 weeks, 2 more weeks of ethanol abstinence, and a final ethanol re-exposure. Immediately prior to the second ethanol re-exposure, 4 groups of rats received bilateral infusions NPY (0.25, 0.5, 1.0 μg) or artificial cerebrospinal fluid (aCSF) into the amygdala. Two additional groups were given uninterrupted ethanol access and were infused with a single NPY dose (1.0 μg) or aCSF. The highest NPY dose (1.0 μg) suppressed ethanol intake for 24 hrs in rats with a history of ethanol abstinence (i.e. deprivation) periods, but had no effect in rats with a history of continuous ethanol access. Water and food intakes were not altered. These results suggest that the amygdala mediates the suppressive effects of centrally administered NPY on ethanol drinking, and that NPY may block relapse-like drinking by opposing the anxiogenic effects of ethanol abstinence.
Keywords: Alcohol-preferring rats, neuropeptide Y, abstinence, deprivation, anxiety, amygdala, allostasis
Dysregulation of brain neuropeptide Y (NPY) systems involved in emotionality may mediate both the negative affective state that defines ethanol abstinence and the negative reinforcing effects of ethanol during relapse drinking (Valdez & Koob, 2004). NPY decreases anxiety-like behavior in rats in multiple behavioral assays (Heilig et al., 1989; 1992; Broqua et al., 1995; Britton et al., 1997; Sajdyk et al., 1999, 2008). The anxiolytic effects of NPY appear to be mediated by the central nucleus of the amygdala (CeA; Heilig et al., 1993), although a role has also been suggested for the basolateral nucleus of the amygdala (Sajdyk et al., 1999, 2008). Since there is a body of evidence suggesting that the CeA is involved in the regulation of both anxiety (Davis, 1992) and ethanol drinking (McBride, 2002), the focus of this investigation was to examine the effects of NPY infusions into the CeA on ethanol intake.
The alcohol-preferring (P) and high alcohol drinking (HAD1) lines of rats were selectively bred for high voluntary intake of ethanol solution with water and food concurrently available (Lumeng et al. 1995). When access to ethanol is limited to a brief (e.g., 2 hr) period each day, intracerebroventricular (ICV) administration of NPY decreases ethanol intake in P and HAD1 rats (Badia-Elder et al. 2001, 2003), but does not alter ethanol intake in selectively bred alcohol-nonpreferring (NP), low alcohol drinking (LAD1), or unselected Wistar rats (Badia-Elder et al., 2001, 2003; Katner et al., 2002a; Slawecki et al., 2000). However, ICV-administered NPY does effectively reduce limited-access ethanol intake in Wistar rats if they have a history of chronic intermittent exposure to ethanol vapor (Thorsell et al., 2005a, 2005b). With chronic continuous (24 hr/day) access to ethanol, ICV-administered NPY suppresses ethanol drinking for up to 24 hours in P rats, and the magnitude and duration of this effect is augmented following a period of imposed ethanol abstinence (Gilpin et al., 2003). Thus, the effectiveness of NPY in reducing ethanol drinking appears to depend on genetic background and on the duration and pattern of previous exposure to ethanol.
Several studies have examined the role of NPY activity in the CeA in ethanol drinking behavior. Infusion of NPY into the CeA does not affect limited-access ethanol intake in Wistar rats (Katner et al., 2002b), but suppresses ethanol drinking by Wistar rats that have been exposed to chronic intermittent ethanol vapor (Gilpin et al., 2008). Viral vector-induced increases in amygdalar NPY expression blunt excessive drinking associated with repeated cycles of ethanol liquid-diet access and periods of abstinence in Wistar rats (Thorsell et al., 2007). Injection into the CeA of a viral vector encoding prepro-NPY reduces continuous access-ethanol drinking by “anxious,” but not “nonanxious” Long Evans rats, divided based on behavior in an elevated plus maze (Primeaux et al. 2006). Finally, P rats with a brief history of ethanol self-administration consume less ethanol following NPY infusion into the CeA, and also following increases in NPY activity in the CeA produced via alterations in cAMP-responsive element-binding protein (CREB) function (Pandey et al. 2005). One contrary finding showed that infusion of a Y1 receptor antagonist into the CeA reduces lever-press responding maintained by ethanol in Wistar rats in 1-hr operant sessions (Schroeder et al., 2003), a finding that is seemingly inconsistent with the hypothesis that ICV NPY-induced suppression of ethanol intake is mediated by the CeA.
The purpose of the present study was to examine the effects of NPY in the CeA on ethanol intake in P rats using a procedure that includes chronic ethanol drinking punctuated by intermittent periods of imposed ethanol abstinence. This schedule models the cyclic pattern of chronic drinking, abstinence and relapse exhibited by human alcoholics (Finney & Moos, 1991; Spanagel & Hölter, 2000). It was predicted that infusion of NPY into the CeA would suppress ethanol drinking by P rats, and that this effect would be augmented following a period of imposed ethanol abstinence.
MATERIALS AND METHODS
Subjects
Subjects were experimentally naïve age-matched female P rats (n= 84) of the 55th generation of selective breeding obtained from the Indiana University School of Medicine Alcohol Research Center. Female P rats were used because they are more readily available than males. Female P rats generally consume more ethanol per day than males (Lumeng et al. 1995) but there appears to be no gender differences in the effects of ICV-administered NPY on ethanol drinking (Badia-Elder et al., 2001; Gilpin et al., 2003). Rats weighed 279.7 (±2.79) g at the end of the initial 11-week baseline drinking period. All rats were individually housed in plastic tub-style cages in a vivarium maintained on a 12:12 hr light/dark cycle (lights off at 1400 hrs). Food (Lab Diet 5001, PMI Nutrition International Inc., Brentwood, MO) and water were available ad libitum. The protocol for this study was approved by the IUPUI School of Science IACUC and was conducted in accordance with NIH guidelines (National Research Council, 1996).
Stereotaxic Surgery
Surgical implantation of cannulae was conducted using aseptic procedures as previously described (Badia-Elder et al., 2001). Rats were anesthetized via inhalation of isoflourane (IsoFlo, Abbott Laboratories, North Chicago, IL) and cannulae were implanted bilaterally and aimed at the CeA; the stereotaxic coordinates (AP-1.8, ML±4.2, DV-7.5) were taken from Paxinos and Watson (1998). Microinjection cannula components (Plastics One Inc., Roanoke, VA) included a guide cannula (26 gauge), an internal injection cannula (33 gauge), and a dummy cannula (33 gauge) that was placed in the guide cannula at all times except during infusions. The injection cannula extended 1.0 mm beyond the tip of the guide cannula when inserted. At the completion of all experimental manipulations, anatomical localization was confirmed after euthanization. A 1% solution of bromphenol blue dye in aCSF was injected for histological verification of cannulae patency and neuroanatomical placement.
Procedure
Rats were initially given 24-hour continuous access to 15% (v/v) ethanol and water for 11 weeks. The amount of fluid consumed was determined by weighing the drinking bottles at 1400 hr every day, just before the start of the dark cycle. The positions of the bottles were alternated daily (right/left) to control for side preferences. Based on average 24-hr ethanol drinking measures during the final 6 days of this initial drinking period, P rats were divided into two groups matched for ethanol intake (g ethanol/kg body weight [g/kg]), which would either be given uninterrupted continuous access to ethanol (CONT condition) for the duration of the experiment or be given access to ethanol with intermittent periods of imposed ethanol abstinence (ABST condition). Further, within the ABST condition, rats were subdivided into groups matched for ethanol intake (g/kg), which would later receive one of three doses of NPY (0.25, 0.5, 1.0 μg) or aCSF and, within the CONT condition, rats were subdivided into two groups matched for ethanol intake (g/kg), which would later receive either one dose of NPY (1.0 μg) or aCSF. Therefore, rats were divided into six groups: ABST-aCSF; ABST-0.25ug NPY; ABST-0.5ug NPY; ABST-1.0ug NPY; CONT-aCSF; and CONT-1.0ug NPY.
After the initial 11-weeks of ethanol drinking, rats in the ABST condition underwent a 2-week period with the ethanol removed and access to water and food only (abstinence period 1). Following this 2-week abstinence period, ethanol was returned (ethanol re-exposure 1) at 1400 hours (start of dark cycle) for 2 more weeks of continuous access to ethanol and water followed by another 2-week period of imposed ethanol abstinence (abstinence period 2). At the start of abstinence period 2, all rats underwent stereotaxic surgery. The rats in the CONT condition did not undergo any time without ethanol availability with the exception that ethanol was removed for 24 hours following surgery. All rats were weighed and monitored daily for one week following surgery. During the final five days of abstinence period 2, all rats underwent sham infusions during which they were handled as though being infused but no injector was inserted into the cannulae. On the final day of abstinence period 2, each rat received a single microinfusion of the appropriate NPY dose, and was immediately returned to the home cage and given free access to ethanol, water, and food.
Infusions
A Harvard 33 microinfusion syringe pump was used to administer drug infusions as previously described (Gilpin et al., 2004). Rats were microinfused with either aCSF [0.5 μl; Plasma-Lyte (Electrolyte) Solution, Baxter, Deerfield, IL] or one of three doses (0.25, 0.5, 1.0 μg; equivalent to 59, 118, 235 pmol) of NPY (Porcine; American Peptide Company, Sunnyvale, CA) dissolved in aCSF (0.5 μl). Fluid and food intakes were recorded at 2 hr and 24 hr following the infusion, and 24 hr fluid intakes were recorded for 13 post-infusion days. Following all experimental procedures, cannulae placements were histologically verified.
Data Analysis
Rats that lost headcaps, or had cannula that were determined to be not viable or inaccurately placed were eliminated from analyses of infusion data (n=45). Therefore, 39 rats were included in analyses of pre-infusion and infusion day data and they were distributed across treatment groups as follows: ABST-aCSF (n=5); ABST-0.25ug NPY (n=6); ABST-0.5ug NPY (n=8); ABST-1.0ug NPY (n=6); CONT-aCSF (n=5); and CONT-1.0ug NPY (n=9).
To determine changes in fluid consumption by ABST rats on the day of re-exposure to ethanol following abstinence period 1, ethanol intake (ml and g/kg) and water intake (ml) were analyzed using two-way repeated-measures analyses of variance (RM ANOVA). Ethanol history (ABST vs CONT) was the between-subjects factor and day (pre-abstinence baseline vs re-exposure) was the within-subjects factor. For infusion day data, 2 hr and 24 hr measures of the same dependent variables plus 2-hr and 24-hr food intake (g) were analyzed using one-way (NPY dose) and two-way (NPY dose x ethanol History) ANOVAs as appropriate. All post-hoc analyses were conducted using Student-Newman-Keuls method for multiple comparison tests. In all cases, significance was determined at p<0.05.
RESULTS
Pre-surgery ethanol drinking
Re-exposure to Ethanol Following Abstinence
Table 1 shows 24-hr ethanol intake (ml, g/kg) and water intake (ml) by rats in the ABST and CONT groups on the days preceding and following abstinence period 1. A two-way RM ANOVA on ethanol intake (ml) revealed significant main effects of ethanol history, F(1,37)=4.54, p=0.04, and day, F(1,37)=20.06, p<0.001, and a significant ethanol history x day interaction effect, F(1,37)=9.62, p=0.004. Post-hoc analyses revealed that ABST rats exhibited higher 24-hr ethanol intake (ml) on re-exposure day relative to baseline (p<0.001), and relative to CONT rats (p=0.003). A two-way RM ANOVA on ethanol intake (g/kg) yielded the same pattern of results. A two-way RM ANOVA on water intake (ml) yielded significant main effects of ethanol history, F(1,37)=6.40, p=0.016, and day, F(1,37)=18.38, p<0.001, and a significant history x day interaction, F(1,37)=11.39, p=0.002. Post-hoc analyses revealed that ABST rats exhibited lower 24-hr water intake on re-exposure day relative to baseline (p<0.001), and relative to CONT rats (p<0.001). Thus, rats in the ABST group showed an alcohol deprivation effect on re-exposure to ethanol following abstinence period 1.
Table 1.
Fluid intake is shown for the days preceding and following abstinence period 1. Data are mean ±SEM 24-hr intake of 15% (v/v) ethanol solution (ml, g/kg) and water intake (ml) by rats in the ABST and CONT groups. The baseline was calculated by averaging each rat’s drinking over the final 6 days of the initial 11-week baseline drinking period, and re-exposure data were the average for the day that ethanol was returned to rats in the ABST group.
| ABST rats (n=54) | Ethanol intake (ml) | Ethanol intake (g/kg) | Water intake (ml) |
|---|---|---|---|
| Baseline | 15.80 ± 1.02 | 6.82 ± 0.54 | 23.93 ± 1.03 |
| Re-exposure | 20.68 ± 1.08 *# | 8.71 ± 0.47 *# | 17.22 ± 0.83 *# |
| CONT rats (n=30) | Ethanol intake (ml) | Ethanol intake (g/kg) | Water intake (ml) |
|
| |||
| Baseline | 14.72 ± 1.04 | 6.37 ± 0.56 | 24.54 ± 1.47 |
| Re-exposure | 15.68 ± 1.05 | 6.14 ± 0.66 | 23.74 ± 1.63 |
p<0.05 significant difference between baseline and re-exposure.
p<0.05 significant difference between ABST and CONT rats.
Post-NPY infusion fluid & food intake
Two sets of analyses were performed on infusion day fluid intake data. First, to establish an NPY dose-response, data for rats from only the ABST group were analyzed with one-way ANOVAs with NPY dose (0.0, 0.25, 0.50, 1.0 μg) as the between-subjects factor. Second, to determine the effects of ethanol abstinence on sensitivity to NPY, data from rats in the ABST and CONT groups, infused with either 1.0 μg or aCSF, were analyzed with two-way ANOVAs with NPY dose (0.0, 1.0 μg) and drinking history (ABST vs. CONT) as the between-subjects factors.
2-hr post-NPY infusion fluid & food intake
ABST dose-response
One-way ANOVAs for data from only rats in the ABST group yielded no effects of NPY dose on intake of ethanol intake (ml or g/kg), water intake (ml) or food intake (g).
ABST vs. CONT rats
Two-way ANOVAs for 2-hr data from rats in the ABST and CONT groups infused with either 1.0 μg or aCSF yielded no main effects of NPY dose or ethanol history nor an NPY dose x ethanol history interaction effect on intake of ethanol (ml or g/kg), water (ml) or food intake (g).
24-hr post-NPY infusion fluid & food intake
Figure 1 shows ethanol and water intake (ml; Fig. 1A) and food intake (g; Fig. 1B) by rats in the ABST group and CONT groups during the 24 hrs following infusion of NPY. Table 2 shows ethanol intake (ml and g/kg), water intake (ml), ethanol preference ratios, and food consumption (g) by rats in the ABST and CONT groups during the 24 hrs following infusion of all NPY doses.
Figure 1.
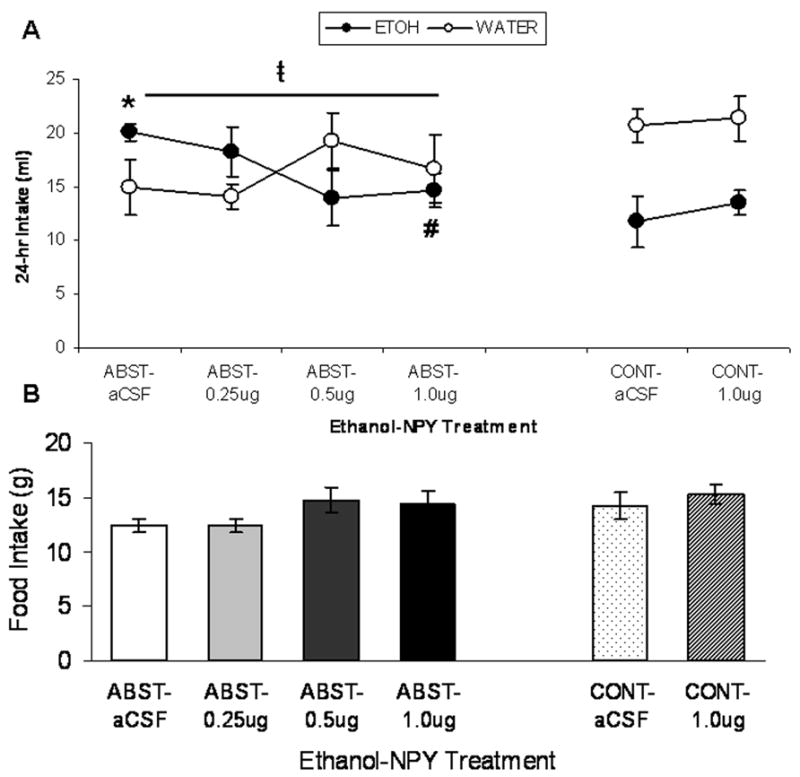
(A) Mean ± SEM 24-hr consumption (ml) of 15% (v/v) ethanol (closed circles) and water (open circles) by P rats in the ABST group (infused in CeA with 0.0, 0.25, 0.5, or 1.0 μg NPY) and CONT group (infused in CeA with 0.0 or 1.0 μg NPY). ABST rats were infused with NPY immediately prior to re-exposure to ethanol following a two-week period of ethanol abstinence. (B) Mean ± SEM intake of concurrently available food (g) by the same groups of rats. * p<0.05 significant difference between ABST and CONT groups that were infused with aCSF; # p<0.05 significant difference from aCSF group with identical drinking history; t̵p<0.05 significant linear trend of dose on ethanol intake (ml).
Table 2.
Mean (±SEM) ethanol intake (ml and g/kg), water intake (ml), ethanol preference (E:T), and food consumption (g) measured 24 hr following aCSF or NPY infusions into the CeA of P rats. Rats in the ABST group received infusions directly prior to re-exposure to ethanol availability following a 2-week period of ethanol abstinence. Rats in the CONT group had uninterrupted access to ethanol and were infused in parallel with rats in the ABST group. For ethanol intake, ANOVA indicated significant effects of group and the interaction of group with NPY dose.
| ABST groups | ||||||
|---|---|---|---|---|---|---|
| NPY dose (μg) | n | Ethanol intake (ml) | Ethanol Intake (g/kg) | Water intake (ml) | Ethanol Preference | Food intake (g) |
| 0.0 (aCSF) | 5 | 20.06 ± 0.82 * | 8.03 ± 0.41 * | 14.92 ± 2.55 | 0.58 ± 0.05 | 12.36 ± 0.61 |
| 0.25 | 5 | 18.22 ± 2.31 | 7.79 ± 1.06 | 14.13 ± 1.14 | 0.56 ± 0.05 | 12.42 ± 0.61 |
| 0.5 | 7 | 13.91 ± 2.55 | 5.67 ± 1.01 | 19.26 ± 2.65 | 0.42 ± 0.07 | 14.71 ± 1.13 |
| 1.0 | 6 | 14.63 ± 1.60 # | 6.23 ± 0.77 | 16.68 ± 3.19 | 0.48 ± 0.07 | 14.28 ± 1.29 |
| CONT groups | ||||||
| NPY dose (μg) | n | Ethanol intake (ml) | Ethanol Intake (g/kg) | Water intake (ml) | Ethanol Preference | Food intake (g) |
|
| ||||||
| 0.0 (aCSF) | 5 | 11.78 ± 2.37 | 4.69 ± 0.89 | 20.64 ± 1.58 | 0.36 ± 0.06 | 14.22 ± 1.21 |
| 1.0 | 7 | 13.48 ± 1.14 | 5.62 ± 0.59 | 21.37 ± 2.07 | 0.40 ± 0.05 | 15.28 ± 0.89 |
p<0.05 significant difference between ABST and CONT groups that were infused with aCSF.
p<0.05 significant difference from aCSF group with identical drinking history.
ABST dose-response
One-way ANOVAs for ethanol and water consumption (ml), ethanol intake (g/kg), ethanol preference, and food intake (g) from only rats in the ABST group yielded no significant effects. However, linear trend analysis yielded a significant downward linear trend of NPY dose on ethanol intake (ml) by these rats, F(1,21)=4.70, p=0.042.
ABST vs. CONT rats
A two-way (NPY dose x ethanol history) ANOVA of ethanol intake (ml, Figure 1) revealed a significant effect of ethanol history, F(1,21)=11.16, p=0.003, indicating that, overall, ABST rats drank more ethanol solution than CONT rats. There was a significant ethanol history x NPY dose interaction F(1,21)=6.26, p=0.02. Post-hoc analyses revealed that infusion of 1.0 μg NPY into the CeA significantly suppressed ethanol intake (ml) in ABST rats (p=0.016) but not CONT rats (p=0.385), relative to respective aCSF controls. Post hoc analyses also indicated that ABST rats that received aCSF infusions had higher ethanol intake than CONT rats that received aCSF infusions (p=0.001), but this difference was abolished by 1.0 μg NPY (p>0.05). A separate two-way ANOVA of ethanol intake (g/kg, Table 2) showed the same pattern of results. A two-way (NPY dose x ethanol history) ANOVA of ethanol preference (Table 2) revealed a significant effect of ethanol history, F(1,21)=8.34, p=0.009, indicating that, overall, ABST rats exhibited higher ethanol preference than CONT rats. A two-way (NPY dose x ethanol history) ANOVA of water intake (ml, Figure 1) revealed a significant effect of ethanol history, F(1,21)=4.99, p=0.036, indicating that, overall, ABST rats drank less water than CONT rats. Analysis of food intake (g, Figure 1) yielded no significant effects.
DISCUSSION
The most important finding presented here is that NPY infused into the amygdala suppresses ethanol intake in ethanol-abstinent P rats, but the same NPY dose does not alter ethanol intake in long-term drinking P rats that have not undergone periods of imposed ethanol abstinence. That is, infusion of NPY into the CeA blocked the elevated alcohol drinking observed in P rats that endured multiple periods of abstinence (i.e. deprivation). This suppressive effect on ethanol drinking occurred in the absence of effects on any other intake measure (i.e., intake of water and food). Although the cannulae were aimed at the CeA, and placement of the cannulae tips were histologically verified, with the procedures used in the present investigation it cannot be ruled out definitively that the NPY infusions may have spread to other amygdalar subcompartments such as the BLA to produce observed effects. Therefore, to be conservative, the findings of this study are interpreted to be the results of NPY acting in the amygdala, rather than solely in the CeA.
The results presented here are in agreement with past findings that amygdalar NPY suppresses ethanol intake in “vulnerable” subpopulations of rats. NPY infused into the CeA suppresses ethanol drinking by Wistar rats that have been exposed to chronic intermittent ethanol vapor, but not in controls exposed to ambient air (Gilpin et al., 2008). Also, increases in amygdalar NPY expression suppress excessive drinking associated with repeated cycles of ethanol liquid-diet access and periods of abstinence in Wistar rats (Thorsell et al., 2007). Injection into the CeA of a viral vector encoding prepro-NPY selectively reduces ethanol drinking by Long Evans rats determined to be “anxious” based on behavior in an elevated plus maze (Primeaux et al. 2006). Finally, Pandey and coworkers (2005) showed that infusion of NPY (100 pmol) into the CeA reduced ethanol drinking in P rats with continuous access to ethanol solution. However, in contrast to that finding, NPY-induced reductions in ethanol intake in the present investigation were only observed in rats following a period of ethanol abstinence. This discrepancy may be accounted for by differences in ethanol exposure protocols. In the study by Pandey et al. (2005), P rats had only seven days access to 9% (v/v) ethanol and approximately 5–6 g ethanol/kg/day. In contrast, P rats in the present investigation drank 15% (v/v) ethanol solution for 11 weeks and consumed approximately 8 g ethanol/kg/day at the end of this baseline period. Regardless, the sum of these results suggests that the ability of NPY to suppress ethanol drinking in rats is mediated by the CeA.
The efficacy of NPY in altering behavior appears to be determined by the type of ethanol exposure history. In the present investigation, NPY reduced ethanol drinking only following a period of imposed ethanol abstinence. This finding parallels studies in which ICV-administered NPY produced enhanced suppression of ethanol drinking (Gilpin et al., 2003, 2005) and larger increases in feeding (Gilpin et al., 2005) in P rats with a history of chronic ethanol drinking punctuated by periods of ethanol abstinence relative to long-term drinking P rats with continuous ethanol exposure. Results from studies in genetically heterogeneous animals are also in agreement with these findings. Increases in NPY activity more effectively suppress ethanol consumption in Wistar rats following chronic intermittent exposure to ethanol vapor or ethanol liquid-diet (Rimondini et al., 2005; Thorsell et al., 2005a, 2007; Gilpin et al., 2008). Also, ICV administration of BIIE0246, a Y2 autoreceptor-selective antagonist, produced greater sedative effects following chronic exposure to high doses of ethanol and periods of ethanol abstinence (Rimondini et al., 2005). It should be mentioned that P rats with continuous access to ethanol had more overall days of ethanol access than rats that underwent periods of abstinence, and the possibility can not be excluded that this difference contributed to the differential NPY effects.
The present investigation also underscores the dissociation between the suppressive effects of NPY on ethanol drinking and the orexigenic effects of NPY. Infusion of NPY directly into the paraventricular nucleus of the hypothalamus (PVN) appears to increase ethanol drinking and produces a robust increase in food intake (Gilpin et al., 2004; Kelley et al., 2001; Stanley et al., 1985). Alternatively, in the present study, infusions of NPY into the amygdala suppressed ethanol drinking in ethanol-abstinent P rats, but had no effect on feeding. These results suggest a role for the amygdala, but not the PVN, in mediating the suppressive effects of NPY on ethanol drinking. Consistent with this notion, lower NPY tissue concentrations in the amygdala are correlated with selection for alcohol preference in both P and HAD1 selectively bred lines of rats (Ehlers et a., 1998; Hwang et al., 1999). In spite of the mutual exclusivity of these pathways and the behaviors they mediate, it is intriguing that the sensitized effects of NPY during ethanol abstinence extend to multiple behaviors. More specifically, the orexigenic effects of NPY are mediated by the PVN (Stanley et al., 1985), the sedative effects by the posterior hypothalamic nucleus (Naveilhan et al., 2001), and the CeA mediates the suppressive effects of NPY on anxiety-like behavior (Heilig et al., 1993) and possibly ethanol drinking (Pandey et al., 2005); the effects of NPY on most, if not all, of these behaviors is augmented following periods of ethanol abstinence (Gilpin et al., 2005; Rimondini et al., 2005). Therefore, chronic ethanol exposure followed by ethanol abstinence may produce a global dysregulation of NPY systems.
Voluntary consumption by P rats of amounts of ethanol similar to those in the present study produce pharmacologically significant blood ethanol levels, ranging from 50 to 200 mg% (Li et al., 1979; Lumeng & Li, 1986; Murphy et al., 1986), and these levels of consumption by P rats produce tolerance to the effects of ethanol (Gatto et al., 1987; Lumeng & Li, 1986) and perhaps even dependence (Kampov-Polevoy et al., 2000; Waller et al., 1982). Ethanol-dependent animals exhibit a long-lasting negative affective state defined partly by elevated anxiety-like behavior (Valdez et al., 2002; Koob and LeMoal, 1997) that may be attributable to decreased NPY levels in the amygdala during ethanol abstinence (Roy & Pandey, 2002). Therefore, the negative reinforcing effects produced by ethanol when consumed following abstinence (i.e. relapse drinking) may be mediated by NPY brain systems involved in regulating anxiety-like behavior (Valdez & Koob, 2004), and the enhanced ability of NPY to suppress ethanol drinking during abstinence may occur via opposition of the high-anxiety state produced by the absence of ethanol itself. Because multiple amygdalar subcompartments (i.e. CeA and BLA) have been implicated in the anxiolytic effects of NPY (Heilig et al., 1993; Sajdyk et al., 1999, 2008), it is not surprising that the amygdala is also implicated in the suppressive effects of the peptide on ethanol drinking. It is also logical that exogenously administered NPY more effectively suppresses ethanol drinking in abstinent relative to non-abstinent rats.
It is not clear why there were no effects of NPY on ethanol drinking two hours following infusion, but it is possible that this measurement period was too short to allow for separation of treatment groups (i.e. floor effect; all groups consumed <2 g/kg ethanol during first two hours post-NPY infusion). Regardless, it is interesting that a single infusion of NPY produced an effect that lasted 24 hours following infusion. The duration of this effect suggests that ethanol abstinence may produce increases in function of downstream NPY targets (e.g., Y receptor upregulation or intracellular signaling cascades), although this question has not been directly examined. Future studies should attempt to track changes in NPY neuronal function more specifically within the CeA during ethanol abstinence and examine the receptor pharmacology of this effect.
Acknowledgments
This investigation was supported by National Institutes of Alcohol Abuse and Alcoholism grants, AA12857, AA07611, and AA10722.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badia-Elder NE, Stewart RB, Powrozek TA, Murphy JM, Li T-K. Effects of Neuropeptide Y on sucrose and ethanol intake and on the elevated plus maze test of anxiety in high alcohol drinking (HAD) and low alcohol drinking (LAD) rats. Alcohol Clin Exp Res. 2003;27:894–899. doi: 10.1097/01.ALC.0000071929.17974.DA. [DOI] [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, Li T-K. Effect of neuropeptide Y (NPY) on oral ethanol intake in Wistar, alcohol-preferring (P), and –nonpreferring (NP) rats. Alcohol Clin Exp Res. 2001;25:386–390. [PubMed] [Google Scholar]
- Britton KT, Southerland S, Van Uden E, Kirby D, Rivier J, Koob G. Anxiolytic activity of NPY receptor agonists in the conflict test. Psychopharmacol. 1997;132:6–13. doi: 10.1007/s002130050313. [DOI] [PubMed] [Google Scholar]
- Broqua P, Wettstein JG, Rocher MN, Gauthier-Martin B, Junien JL. Behavioral effects of neuropeptide receptor agonists in the elevated plus-maze and fear-potentiated startle procedure. Behav Pharmacol. 1995;6:215–222. [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Li T-K, Lumeng L, Hwang BH, Somes C, Jiminez P, Mathe AA. Neuropeptide Y levels of ethanol-naïve alcohol-preferring and -nonpreferring rats and in Wistar rats after ethanol exposure. Alcohol Clin Exp Res. 1998;22:1778–1782. [PubMed] [Google Scholar]
- Finney JW, Moos RH. The long-term course of treated alcoholism: I Mortality, relapse and remission rates and comparisons with community controls. J Stud Alcohol. 1991;52:44–54. doi: 10.15288/jsa.1991.52.44. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, Murphy JM, Waller MB, McBride WJ, Lumeng L, Li TK. Chronic ethanol tolerance through free-choice drinking in the P line of alcohol-preferring rats. Pharmacol Biochem Behav. 1987;28:111–115. doi: 10.1016/0091-3057(87)90021-9. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol Biochem Behav. doi: 10.1016/j.pbb.2008.04.006. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Badia-Elder NE. Neuropeptide Y in the paraventricular nucleus of the hypothalamus increases ethanol intake in high- and low-alcohol-drinking rats. Alcohol Clin Exp Res. 2004;28:1492–1498. doi: 10.1097/01.alc.0000141813.27875.d5. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Badia-Elder NE. Sensitized effects of neuropeptide Y on multiple ingestive behaviors in P rats following ethanol abstinence. Pharmacol Biochem Behav. 2005;81:740–749. doi: 10.1016/j.pbb.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Li T-K, Badia-Elder NE. Neuropeptide Y reduces oral ethanol intake in alcohol-preferring (P) rats following a period of imposed ethanol abstinence. Alcohol Clin Exp Res. 2003;27:787–794. doi: 10.1097/01.ALC.0000065723.93234.1D. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacol. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Koob GF, Britton KT. Anxiolytic-like effect of neuropeptide Y (NPY), but not other peptides in an operant conflict test. Regulat Pept. 1992;41:61–69. doi: 10.1016/0167-0115(92)90514-u. [DOI] [PubMed] [Google Scholar]
- Heilig M, Söderpalm B, Engel JA, Widerlöv E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacol. 1989;98:524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- Hwang BH, Zhang J-K, Ehlers CL, Lumeng L, Li T-K. Innate differences of neuropeptide Y (NPY) in hypothalamic nuclei and central nucleus of the amygdala between selectively bred rats with high and low alcohol preference. Alcohol Clin Exp Res. 1999;23:1023–1030. [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol Clin Exp Res. 2000;24:278–284. [PubMed] [Google Scholar]
- Katner SN, Slawecki CJ, Ehlers CL. Neuropeptide Y administration into the third ventricle does not increase sucrose or ethanol self-administration but does affect the cortical EEG and increases food intake. Psychopharmacol. 2002a;160:146–154. doi: 10.1007/s00213-001-0950-9. [DOI] [PubMed] [Google Scholar]
- Katner SN, Slawecki CJ, Ehlers CL. Neuropeptide Y administration into the amygdala does not affect ethanol consumption. Alcohol. 2002b;28:29–38. doi: 10.1016/s0741-8329(02)00235-5. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Nannini MA, Bratt AM, Hodge CW. Neuropeptide-Y in the paraventricular nucleus increases ethanol self-administration. Peptides. 2001;22:515–522. doi: 10.1016/s0196-9781(01)00361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Waller MB, Hawkins DT. Progress toward a voluntary oral-consumption model of alcoholism. Drug Alcohol Dep. 1979;4:45–60. doi: 10.1016/0376-8716(79)90040-1. [DOI] [PubMed] [Google Scholar]
- Lumeng L, Li TK. The development of metabolic tolerance in the alcohol-preferring P rats: comparison of forced and free-choice drinking of ethanol. Pharmacol Biochem Behav. 1986;25:1013–1020. doi: 10.1016/0091-3057(86)90079-1. [DOI] [PubMed] [Google Scholar]
- Lumeng L, Murphy JM, McBride WJ, Li T-K. Genetic influences on alcohol preference in animals. In: Begleiter H, Kissin B, editors. The Genetics of Alcoholism. New York: Oxford University Press; 1995. pp. 165–201. [Google Scholar]
- McBride WJ. Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav. 2002;71:509–515. doi: 10.1016/s0091-3057(01)00680-3. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li T-K. Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol. 1986;3:331–336. doi: 10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington D.C: National Research Council; 1996. [Google Scholar]
- Naveilhan P, Canals JM, Valjakka A, Vartiainen J, Arenas E, Ernfors P. Neuropeptide Y alters sedation through a hypothalamic Y1-mediated mechanism. Eur J Neurosci. 2001;13:2241–2246. doi: 10.1046/j.0953-816x.2001.01601.x. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Primeaux SD, Wilson SP, Bray GA, York DA, Wilson MA. Overexpression of neuropeptide Y in the central nucleus of the amygdala decreases ethanol self administration in “anxious” rats. Alcohol Clin Exp Res. 2006;30:791–801. doi: 10.1111/j.1530-0277.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Thorsell A, Heilig M. Suppression of ethanol self-administration by the neuropeptide Y (NPY) Y2 receptor antagonist BIIE0246: evidence for sensitization in rats with a history of dependence. Neurosci Letters. 2005;375:129–133. doi: 10.1016/j.neulet.2004.10.084. [DOI] [PubMed] [Google Scholar]
- Roy A, Pandey SC. The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcohol Clin Exp Res. 2002;26:796–803. [PubMed] [Google Scholar]
- Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, Gehlert DR, Urban JH, Shekhar A. Neuropeptide Y in the Amygdala Induces Long-Term Resilience to Stress-Induced Reductions in Social Responses But Not Hypothalamic–Adrenal–Pituitary Axis Activity or Hyperthermia. J Neurosci. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999;368:143–7. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Olive F, Koenig H, Hodge CW. Intra-amygdala infusion of the NPY Y1 receptor antagonist BIBP 3226 attenuates operant ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1884–1891. doi: 10.1097/01.ALC.0000098875.95923.69. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Walpole T, Ehlers CL. Increases in sucrose consumption, but not ethanol consumption, following ICV NPY administration. Pharmacol Biochem Behav. 2000;66:591–594. doi: 10.1016/s0091-3057(00)00215-x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Hölter SM. Pharmacological validation of a new animal model of alcoholism. J Neural Trans. 2000;107:669–680. doi: 10.1007/s007020070068. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Chin AS, Leibowitz SF. Feeding and drinking elicited by central injection of neuropeptide Y: evidence for a hypothalamic site(s) of action. Brain Research Bulletin. 1985;14:521–524. doi: 10.1016/0361-9230(85)90100-5. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Repunte-Canonigo V, O’Dell LE, Chen SA, King AR, Lekic D, Koob GF, Sanna PP. Viral vector-induced amygdala NPY overexpression reverses increased alcohol intake caused by repeated deprivations in Wistar rats. Brain. 2007;130:1330–7. doi: 10.1093/brain/awm033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y on appetitive and consummatory behaviors associated with alcohol drinking in Wistar rats with a history of ethanol exposure. Alcohol Clin Exp Res. 2005a;29:584–590. doi: 10.1097/01.alc.0000160084.13148.02. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y and corticotropin-releasing factor on ethanol intake in Wistar rats: interaction with chronic ethanol exposure. Behav Brain Res. 2005b;161:133–140. doi: 10.1016/j.bbr.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Koob GF. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol Biochem Behav. 2004;79:671–689. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Lumeng L, Li T-K. Induction of dependence on ethanol by free-choice drinking in alcohol-preferring rats. Pharmacol Biochem Behav. 1982;16:501–507. doi: 10.1016/0091-3057(82)90459-2. [DOI] [PubMed] [Google Scholar]


