Abstract
Decreases in serotonergic activity in the central nucleus of the amygdala reduce responses to stressors, suggesting an important role for serotonin in this region of the amygdala in stress reactivity. However, it is not known whether exposure to stressors actually increases serotonin release in the central nucleus of the amygdala. The current experiment tested the hypothesis that restraint stress increases extracellular serotonin within the central nucleus of the amygdala and adjacent medial amygdala using in vivo microdialysis in awake male rats during the dark phase of the light-dark cycle. Serotonin release in the central nucleus increased immediately in response to restraint stress. In contrast, there was no change in serotonin release within the adjacent medial amygdala during or following restraint. Since corticotropin-releasing factor is an important mediator of both responses to stressors and serotonergic activity, subsequent experiments tested the hypothesis that central nucleus serotonergic response to restraint stress is mediated by central corticotropin-releasing factor receptors. Administration of the corticotropin-releasing factor type 1 and 2 receptor antagonist d-Phe-CRF (icv, 10 μg/5 μl) prior to restraint stress suppressed restraint-induced serotonin release in the central nucleus. The results suggest that restraint stress rapidly and selectively increases serotonin release in the central nucleus of the amygdala by the activation of central corticotropin-releasing factor receptors. Furthermore, the results imply that corticotropin-releasing factor mediated serotonergic activity in central nucleus of the amygdala may be an important component of a stress response.
Keywords: d-Phe-CRF, medial amygdala, microdialysis, rat
1. Introduction
The amygdala is comprised of a group of nuclei important for the processing of emotional information [25, 37]. Hyperactivity of the amygdala, as observed in individuals suffering from anxiety and depression [17,41], is thought to contribute to the negative cognitive and affective symptoms characterizing such disorders [46]. The central nucleus of the amygdala (CeA) receives highly processed information via the lateral and basolateral amygdala from wide range of cortical and subcortical structures and, in turn, projects to the hypothalamus and brainstem [1, 38]. Experimental evidence in rodents suggests both activational and integrative roles for the CeA in responses to stressors. For example, stimulation of the CeA activates the hypothalamic - pituitary - adrenal (HPA) axis [19], while bilateral lesions of the CeA inhibit the activation of the HPA axis in response to stressful stimuli, including restraint [6-8, 29, 45, although see 11, 15].
The CeA is also thought to be involved in mediating the expression of fear or anxiety behaviors exhibited in response to stressors. For example, lesion studies in rats suggest that the CeA plays a critical role in fear learning by associating predictive stimuli with the affective aspects of the stimuli [5, 9]. Furthermore, fear-potentiated startle is blocked by lesions or pharmacological inactivation of the CeA [13]. Although a role for the CeA in anxiety behavior is not apparent with lesion methodology [13], application of glucocorticoids to the rat CeA increases anxiety-like behavior in the elevated plus maze [32, 43].
Central serotonin (5-HT) is well documented to be involved in responses to stressors, and alterations of 5-HT levels are associated with fear and anxiety behaviors [10, 23, 31]. The dorsal raphe nucleus (dRN) provides 5-HT innervation to the CeA [36]. Increased 5-HT release in the CeA has been positively correlated with the expression of pharmacologically-induced freezing behavior in rats [23]. Furthermore, 5-HT receptor stimulation in the CeA is sufficient and necessary for stress-induced activation of the HPA axis [18, 20]. Thus, 5-HT release and activity within the CeA appears to be important for the behavioral and endocrine activational role attributed to the CeA. However, it is not known whether 5-HT release in the CeA is actually activated by stressful or fearful stimuli.
The localization of corticotropin-releasing factor (CRF) cell bodies in CeA and its function as a major extrahypothalamic site of expression of CRF also suggests a pivotal role of CeA in stress responses [3]. Corticotropin-releasing factor, acting as a neurotransmitter, is involved in initiating stress responses, partially by modulating serotonergic systems arising from the dRN [3, 27]. The CeA is one of the major sources of CRF innervation to the dRN [24], and CRF type 1 and type 2 (CRF1/2) receptors are present in the dRN [14, 42]. The release of CRF from the CeA has been detected during stress [30] and CRF administered to the dRN concurrently increases freezing behavior and 5-HT release within the CeA [23]. Therefore, we hypothesized that 5-HT release in the CeA will increase in response to a stressor (restraint stress) via central CRF receptor activation.
2. Materials and Methods
Animals
Adult male Sprague Dawley rats (300 – 350 g) were purchased from the University of South Dakota Animal Resource Center. Rats were housed in groups of four and maintained at 22 °C, on a reverse 12h light/12 h dark cycle (lights off at 0:900 h) with free access to food and water. The following procedures were approved by the Institutional Animal Care and Use Committee of the University of South Dakota, and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
Surgery
Rats were anaesthetized with xylazine/ketamine mixture (80 mg ketamine/10 mg xylazine, ip.; Med-Vet, Libertyville, IL) and placed within a small mammal stereotaxic frame (Stoelting, Wood Dale, IL). A stainless-steel guide cannula (20 gauge; Plastics One, Roanoke, VA) for later insertion of the microdialysis probe, was stereotaxically implanted 1 mm above the CeA (AP: -2.1 mm from bregma; ML: 4.0 mm from midline; DV: -6.3 mm from dura; [34]). In experiment 2, a second guide cannula was implanted on the contralateral side for intracerebroventricular (icv) injection within the lateral ventricle at the following coordinates: AP: -1.0 mm from bregma; ML: 1.5 mm from midline; DV: -3.0 mm from dura [34]. Cannulae were fixed to the skull with glass ionomer cement (GC Corp., Alsip, IL), and permanently secured with a coating of cranioplastic cement (Plastics One) over the surface of the exposed skull with dental screws in the skull serving as anchor points. Rats were housed individually and allowed to recover for 3 days before undergoing further experimental procedures [23].
Microdialysis, Restraint Stress and Drug Infusions
During the light phase of the light-dark cycle, rats were lightly anaesthetized with ketamine/xylazine mix to allow insertion of the microdialysis probe. A laboratory-made concentric microdialysis probe (2.4 mm exposed membrane length, 5000 MW cut-off, typical recovery 20%; [23]) was inserted into the CeA to a depth of 8.8 mm from dura. Artificial cerebrospinal fluid (aCSF) was perfused through the probe at a rate of 0.4 μl/min via PE 20 tubing connected to a 1 ml syringe using a microinfusion pump (CMA, North Chelmsford, MA). Rats were then left overnight in a 10 gallon glass testing chamber with sawdust substrate, and allowed free access to food and water.
Experiments began twelve hours following probe implantation, during the dark phase of the photoperiod, with the testing room dimly illuminated by red light for the entire duration of the experiment. Dialysates were collected at 20 min intervals and extracellular 5-HT levels were measured by HPLC with electrochemical detection as previously described [23]. Mobile phase (150 mg EDTA, 432 mg sodium octanesulfonate, 4.8 g NaH2PO4, 200 μl triethylamine and 122 ml acetonitrile per liter, pH 5.45) was pumped through a UniJet 3 μm C18 microbore column (Bioanalytical Systems, West Lafayette, IN) under nitrogen gas pressure (2000 psi). Dialysates were injected onto the chromatographic system using a rheodyne injector via a 5 μl loop (Bioanalytical Systems). The collection rate of 0.4 μl/min resulted in approximately 8 μl of dialysate to insure that the loop was overfilled during each sample period. Following separation by the column, 5-HT was detected by a glassy carbon electrode (Bioanalytical Systems) maintained at +0.5 V with respect to an Ag/AgCl2 reference electrode with a LC-4C potentiostat (Bioanalytical Systems). The voltage output was recorded by CSW32 v1.4 Chromatography Station for Windows (DataApex, Prague, Czech Republic). Elution time for 5-HT was 11-13 min, and individual 5-HT peaks were identified by comparison to a 5-HT standard (7.9 pg/5 μl).
After at least 3 consecutive stable 5-HT baseline samples (less than 10% variation) were collected, the rats in experiment 1 were directly placed in a restraining tube (6 cm id, 27 cm long), constructed from PVC piping with a narrow channel cut in the top to allow passage of the lines attached to the microdialysis probe. These tubes provided sufficient room for movement associated with respiration, but did not allow the rat to move in any direction. Rats remained in restraining tubes for 40 min (equivalent of 2 microdialysis sampling periods), before being released back into the testing chamber.
In experiment 2, a 5 μl Hamilton syringe with a 28 gauge needle (Fisher Scientific, Tustin, CA) was used to inject either vehicle (5 μl aCSF) or 10 μg d-Phe-CRF in 5 μl [44] icv over a two min period without restraining the animal [23]. This was achieved by lifting the lid of the testing chamber, and inserting the needle/syringe from above (without handling the rat) into the guide cannula to a depth of 3.4 mm below the skull, as indicted by a stopper on the needle. The syringe was gently and slowly depressed for 2 min, and left in situ for a further minute to allow diffusion from the needle into the lateral ventricle before the experimenter removed the needle. The dose (10 μg icv) of d-Phe-CRF used has been shown to reverse the behavioral effects of restraint stress in ethanol-withdrawn rats [44]. Ten min following icv injection, rats were placed into the restraining tube as described for experiment 1. For both experiments 1 and 2, dialysates were collected until 5-HT returned to baseline levels for 3 consecutive samples following treatment. In the case of rats where no alterations in 5-HT levels were observed, 4-6 post-stress samples were collected.
Histology
Following experimentation, rats were killed (0.5 ml Fatal Plus, ip.; Vortech, Dearborn, MI), and brains removed and fixed in 10% formalin. Brains were sectioned at 60 μm on a cryostat (-14 °C), and examined under a light microscope by two investigators blind to treatment, to determine placements of probes and cannulae.
Statistics
Extracellular 5-HT levels in the three baseline dialysis samples were averaged, and post-treatment 5-HT levels were calculated as a percentage change from mean baseline levels for each rat. For experiment 1, the effects of restraint stress on CeA 5-HT levels were determined by a one-way ANOVA with one repeated measure (time), followed by Dunnett's posthoc test, where the sample immediately preceding the initiation of stress served as the control. For experiment 2, CeA 5-HT levels were compared between rats injected icv with vehicle (aCSF) or d-Phe-CRF using a two-way ANOVA with one repeated measure (time). A significant effect of time was further analyzed by one-way ANOVAs with one repeated measure followed by Dunnett's posthoc tests, as described above. A significant effect of icv treatment was further analyzed using Tukey's posthoc test for multiple comparisons at each time point. Five vehicle-treated rats from experiment 2 had probe placements within the medial amygdala (MeA) adjacent to the CeA. The MeA has also been implicated in restraint-induced activation of the hypothalamus [14]. Therefore, the regional specificity of restraint stress on amygdala 5-HT was assessed by determining the effects of restraint on MeA 5-HT levels, using a one-way ANOVA with one repeated measure followed by Dunnett's posthoc test, as described for experiment 1. Analyses were performed using SigmaStat v.2.03, with the alpha level set at 0.05.
3. Results
Histology and Baseline 5-HT Levels
The locations of probe placements for experiments 1 and 2 are illustrated in Fig. 1. The probe placements were similarly distributed in the CeA of vehicle (n = 5) and d-Phe-CRF (n = 6) treated rats. Baseline 5-HT levels in the CeA were 0.32 +/- 0.11 pg/ μl (uncorrected for probe recovery), with a 2:1 signal to noise ratio of 0.06 +/- 0.02 pg/ μl. Probe membranes located outside of the CeA were present in the MeA in sufficient numbers to allow evaluation of restraint effects on MeA 5-HT (n = 5; Fig 1). Baseline 5-HT levels in the MeA were 0.24 +/- 0.06 pg/ μl (uncorrected for probe recovery), with a 2:1 signal to noise ratio of 0.06 +/- 0.01 pg/ μl.
Figure 1.
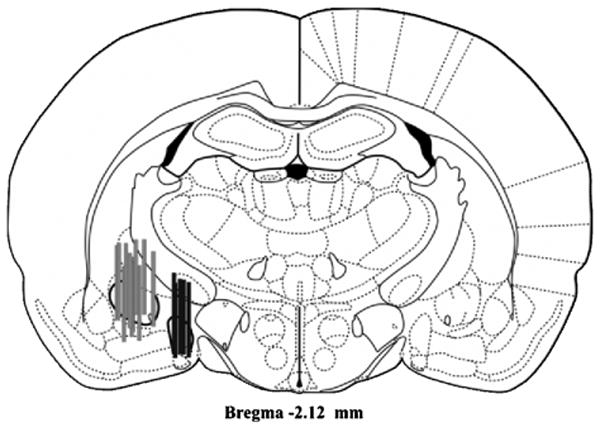
Representative coronal section of the rat brain showing placement of the active surface of microdialysis probes in the central nucleus of the amygdala (grey bars; n = 16) and the medial amygdala (black bars; n = 5). Figures adapted from [34].
Experiment 1: Effects of Restraint Stress on CeA 5-HT Levels
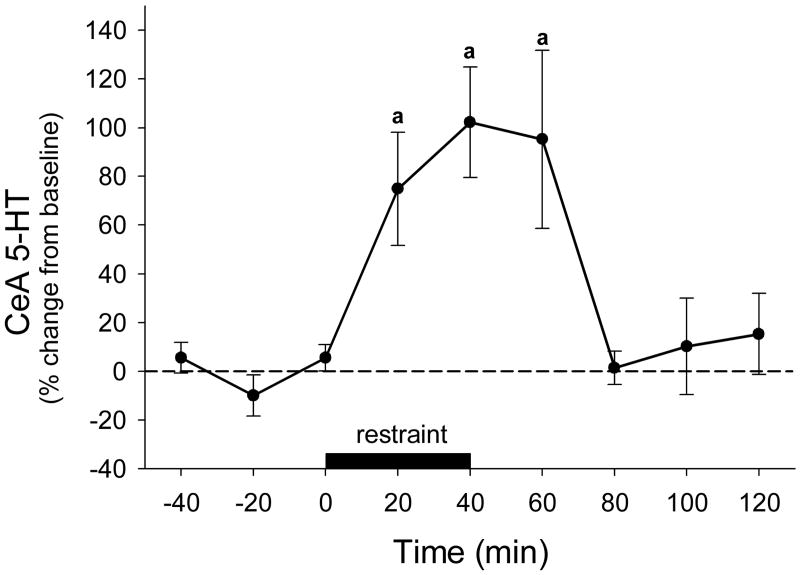
Rats subjected to restraint stress for 40 minutes showed a rapid increase in CeA 5-HT levels (F8,28 = 7.198, P<0.001). Levels of 5-HT were significantly elevated over pre-stress values during the restraint period (20-40 min) and in the dialysate sample following restraint (60 min) (Dunnet's P<0.05; Fig 2).
Figure 2.
Effects of restraint stress on serotonin (5-HT) levels in the central nucleus of the amygdala (CeA; n = 5), data points represent mean +/- SEM. a = significantly different from pre-stress levels, P<0.05.
Experiment 2: Effects of CRF1/2 Receptor Antagonism on Restraint-Induced CeA 5-HT
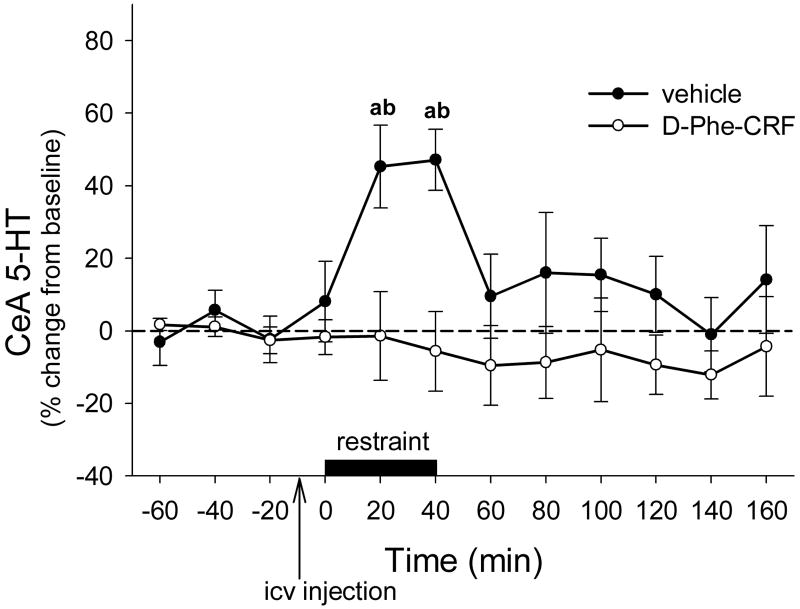
Intracebroventricular administration of the CRF receptor antagonist d-Phe-CRF blocked the increase in CeA 5-HT levels during restraint stress (Fig 3). There were significant effects of drug treatment (F1,9 = 5.837, P<0.05), time (F11,88 = 2.692, P<0.01) and a treatment × time interaction (F11,88 = 2.786, P<0.01). An effect of time was apparent in vehicle-treated rats (one-way ANOVA F11,47 = 4.182, P<0.001), but not in d-Phe-CRF -treated rats (one-way ANOVA F11,47 = 0.608, P=0.821). Increased levels of 5-HT were detected in the CeA of vehicle-treated rats during the restraint period (20-40 min) compared to pre-stress baseline values (Dunnet's P<0.05) and compared to 5-HT values obtained from restrained rats pre-treated with d-Phe-CRF (Tukey's P<0.001).
Figure 3.
Effects of vehicle (artificial cerebrospinal fluid; n = 5) or d-Phe-CRF (n = 6) icv pretreatment on restraint-induced levels of 5-HT in the CeA, data points represent mean +/- SEM. a = significantly different from pre-stress levels, b = significantly different from d-Phe-CRF pretreated rats; P<0.05.
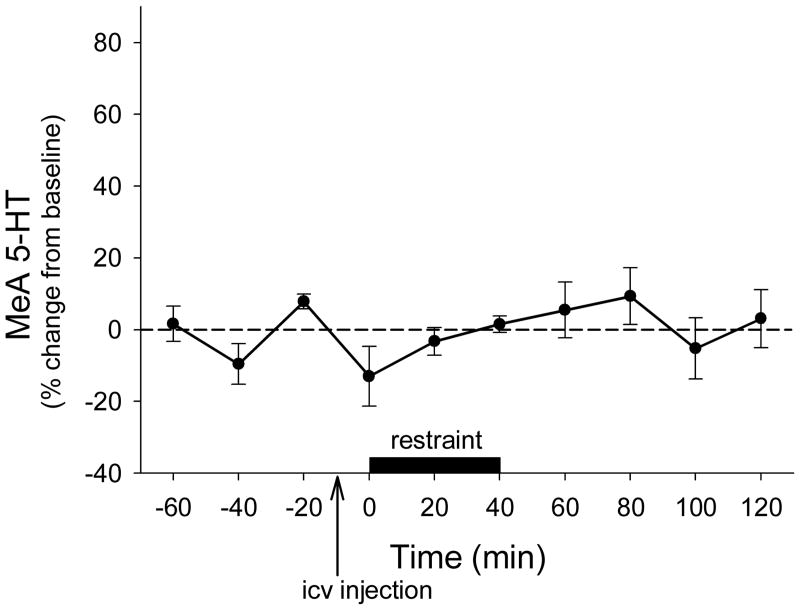
In rats that received icv vehicle treatment and had probe placements within the MeA, restraint stress did not have a significant effect on 5-HT levels (F9,36 = 0.949, P=0.497; Fig 4).
Figure 4.
Effects of restraint stress on 5-HT levels in the medial amygdala (MeA) in vehicle-treated rats (n = 5), data points represent mean +/- SEM.
4. Discussion
This study demonstrates for the first time that restraint stress increases extracellular levels of 5-HT within the CeA. Central nucleus 5-HT levels were elevated during the 40 min of restraint and 5-HT levels remained elevated in the first dialysate collected after the removal of restraint in rats that did not receive icv injections. In contrast to the CeA, 5-HT levels in the MeA were not affected by restraint stress. Interestingly, a comparison of Figures 2 and 3 suggest that the magnitude and duration of the CeA 5-HT responses elicited by restraint stress were less in rats that received an icv injection of vehicle (Figure 3) compared to those that did not receive an icv injection at all (Figure 2). It is possible that the icv injection procedure represented a mild stressor for the rats, which resulted in blunting of the CeA 5-HT response to 40 min of restraint stress. The possibility that a mild stressor immediately prior to a second stressor could blunt the CeA 5-HT responses to this second more severe stressor should be examined in the future.
Previous studies suggest that 5-HT neurotransmission in the CeA is important for the expression of endocrine and behavioral responses to stressors, although it is not clear how 5-HT receptor activation in this region translates to behavioral and endocrine output. Agonist –induced stimulation of 5-HT1A receptors in the CeA activates the HPA axis [20] while depletion of 5-HT in CeA or application of 5-HT2 receptor antagonists in the CeA eliminate the excitatory effects of the CeA on the HPA axis [18]. Furthermore, increased 5-HT levels in the CeA are associated with the onset of pharmacologically-induced fear behavior [23]. These previous studies all involved pharmacological manipulation of 5-HT systems, thus our current study adds to this by demonstrating that restraint stress rapidly increases 5-HT release within the CeA. Therefore, exposure to an acute stressor increases 5-HT release in the CeA, an amygdala region important for adaptive behavioral and endocrine stress responses [18, 20, 22].
Similar to the CeA, lesions of the adjacent MeA have been shown to abolish restraint-stress activation of the hypothalamus [15]. However, the current results suggest that the activational role of the MeA on HPA axis activity is independent of 5-HT activity, since restraint did not alter 5-HT levels over the time course evaluated. Our result is consistent with the finding that 5-HT turnover in rats following 60 min of restraint does not increase in the MeA [7]. Taken together, these results suggest that 5-HT neurotransmission in the CeA, but not in the MeA, increases in response to acute restraint stress. Serotonergic neurotransmission within the CeA in response to stressors may play a role in stress-exacerbated anxiety and depression. It has been suggested that increased serotonergic tone is an important mediator of amygdala hyper-reactivity observed in emotional disorders [21]. Therefore, it would be important to determine the effects of chronic stress on 5-HT levels in the CeA in future studies.
Administration of the CRF1/2 receptor antagonist d-Phe-CRF (icv) prior to the onset of restraint stress inhibited the stress-induced increase in extracellular 5-HT in the CeA. The finding that central CRF receptor mechanisms mediate stress-induced CeA 5-HT release supports a growing body of literature demonstrating central CRF receptor-mediated regulation of stress responses, anxiety, anorexia and 5-HT release in a variety of the limbic brain regions [for example, 4, 16, 23, 26, 28, 39, 40, 42]. However, this is the first report of CRF-mediation of stress-induced 5-HT release in the amygdala. The potential significance of CRF-mediation of amygdala 5-HT release is illustrated by several reports of CRF hyper-secretion in depressed populations [as reviewed by 33]. Since CRF receptor activation is responsible for stress-induced 5-HT release in the CeA as shown by the current study, and increased 5-HT tone in the amygdala is thought to contribute to amygdala hyper-reactivity in depression [21], it is possible that altered central CRF-mediation of amygdala 5-HT release could contribute to the symptoms of stress-induced depression.
It is likely that central CRF receptor antagonism abolished restraint-induced increases in CeA 5-HT release by blocking CRF receptors located in the serotonergic cell body region, the dRN. Restraint stress increases the release of CRF, and CRF infused directly into the dRN produces an increase in CeA 5-HT release similar to that observed during restraint stress [23; 30]. Our preliminary studies show that electrical stimulation of the CeA CRF cell-body region, using parameters designed to mimic firing rates observed during restraint stress, results in increased forebrain extracellular 5-HT levels [22]. Importantly, this effect is blocked by pre-treatment of the dRN with d-Phe-CRF [22], again suggesting that stress activates CRF neurons in the CeA, resulting in increased CRF release and CRF receptor activation in the dRN. These observations, taken with the current results, provide a hypothesis for the presence of a reciprocal stress-activated circuit in which restraint-induced release of CRF by the CeA acts on CRF receptors in the dRN to increase 5-HT release, with the resulting activation of 5-HT receptors in the CeA subsequently leading to the facilitation of endocrine and behavioral responses to stressors [23].
While not tested in the current study, CRF type 2 (CRF2) receptors may play an important role in stress-induced activation of 5-HT release in the CeA. Activation of CRF2 receptors in the dRN appear to increase firing rates of 5-HT neurons, probably by disinhibition of 5-HT neuronal activity by CRF2-mediated inhibition of non-serotonergic (presumably GABAergic) neurons within the dRN [35]. Likewise, CRF2 receptors in the dRN are required for CRF-induced release of 5-HT within another forebrain limbic site, the nucleus accumbens, whereas CRF type 1 receptor activation results in decreased accumbal 5-HT release [28]. Furthermore, CRF2 receptor activation in the dRN by urocortin II produces increased 5-HT release in the basolateral amygdala [2]. Finally, chronic activation of CRF2 receptors in the dRN result in altered serotonergic gene expression and corresponding behavioral changes that resemble those induced by chronic stress of rats, such as decreased novel object and open field exploration [12]. Subsequent work should determine whether CRF2 receptors in the dRN are required for stress-induced increases in CeA 5-HT release, as would be predicted by results from previous studies.
Conclusions
In summary, this study directly demonstrates that acute restraint stress increases 5-HT release in the CeA and that the elevation of CeA 5-HT during restraint stress is under the regulation of CRF receptor activity. These results imply that CRF-mediated effects on 5-HT activity in CeA may be an important component of the circuitry involved in responses to stressors.
Acknowledgments
This work was supported by grants NIH COBRE P20 RR15567 which is designated a Center of Biomedical Research Excellence, and NIDA R01 DA019921. We thank Dr. Michael Watt for helpful suggestions regarding the design of these experiments.
Footnotes
Conflict of Interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akmaev IG, Kalmullina LB, Sharipova LA. The central nucleus of the amygdala body of the brain: Cytoarchitectonics, neuronal organization, connections. Neurosci Behavioral Physiol. 2004;34:603–610. doi: 10.1023/b:neab.0000028292.14402.ad. [DOI] [PubMed] [Google Scholar]
- 2.Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 3.Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav. 2005;48:1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Bakshi VP, Newman SM, Smith-Roe S, Jochman KA, Kalin NH. Stimulation of lateral septum CRF2 receptors promotes anorexia and stress-like behaviors: functional homology to CRF1 receptors in basolateral amygdala. J Neurosci. 2007;27:10568–10577. doi: 10.1523/JNEUROSCI.3044-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. TINS. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Beaulieu S, Di Paolo T, Barden N. Implications of the serotonergic system in the decreased ACTH response to stress after lesion of the amygdaloid central nucleus. Prog Neuro-Psychopharmacol & Biol Psychiat. 1985;9:665–669. doi: 10.1016/0278-5846(85)90037-5. [DOI] [PubMed] [Google Scholar]
- 7.Beaulieu S, Di Paolo T, Barden N. Control of ACTH secretion by the central nucleus of the amygdala: Implications of the serotonergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinol. 1986;44:247–254. doi: 10.1159/000124652. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu S, Di Paolo T, Cote J, Barden N. Participation of the central amygdaloid nucleus in the responses of adrenocorticotropin secretion to immobilization stress: Opposing roles of the noradrenergic and dopaminergic systems. Neuroendocrinol. 1987;45:37–46. doi: 10.1159/000124701. [DOI] [PubMed] [Google Scholar]
- 9.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 10.Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 11.Carter RN, Pinnock SB, Herbert J. Does the amygdala modulate adaptation to repeated stress? Neurosci. 2004;126:9–19. doi: 10.1016/j.neuroscience.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Clark MS, McDevitt RA, Hoplight BJ, Neumaier JF. Chronic low dose ovine corticotropin releasing factor or urocortin II into the rostral dorsal raphe alters exploratory behavior and serotonergic gene expression in specific subregions of the dorsal raphe. Neurosci. 2007;146:1888–1905. doi: 10.1016/j.neuroscience.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- 14.Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5-HT1A, alpha 1b adrenergic, CRH-R1 and CRH-R2 receptor mRNA in serotonergic, gama-aminobutyric acidergic and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- 16.de Groot L, Penalva RG, Flachskamm C, Reul JM, Linthorst AC. Differential monoaminergic, neuroendocrine and behavioural responses after central administration of corticotropin-releasing factor type 1 and type 2 agonists. J Neurochem. 2005;94:45–56. doi: 10.1111/j.1471-4159.2005.03164.x. [DOI] [PubMed] [Google Scholar]
- 17.Drivets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann NY Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 18.Feldman S, Newman ME, Gur E, Weidenfeld J. Role of serotonin in the amygdala in hypothalamo-pituitary-adrenocortical responses. Neuroreport. 1998;9:2007–2009. doi: 10.1097/00001756-199806220-00017. [DOI] [PubMed] [Google Scholar]
- 19.Feldman S, Weidenfeld J. The excitatory effects of the amygdala on hypothalamo-pituitary-adrenocortical responses are mediated by hypothalamic norepinephrine, serotonin, and CRF-41. Brain Res Bull. 1998;45:389–393. doi: 10.1016/s0361-9230(97)00384-5. [DOI] [PubMed] [Google Scholar]
- 20.Feldman S, Newman ME, Weidenfeld J. Effects of adrenergic and serotonergic agonists in the amygdala on the hypothalamo-pituitary-adrenocortical axis. Brain Res Bull. 2000;52:531–536. doi: 10.1016/s0361-9230(00)00292-6. [DOI] [PubMed] [Google Scholar]
- 21.Fisher PM, Meltzer CC, Ziolko SK, Price JC, Moses-Kolko EL, Berga SL, Hariri AR. Capacity for 5-HT-1A-mediated autoregulation predicts amygdala reactivity. Nature Neuroscience. 2006;9:1362–1363. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- 22.Forster GL, Feng N, Watt MJ, Summers CH, Renner KJ. Electrical stimulation of the central nucleus of the amygdala evokes delayed increases in medial prefrontal cortex serotonin: Mediation by corticotropin-releasing factor receptors in the dorsal raphe nucleus. Soc Neurosci Abst. 2004:760.9. [Google Scholar]
- 23.Forster GL, Feng N, Watt MJ, Mouw NJ, Korzan WJ, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neurosci. 2006;141:1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Gray T. Amygdaloid CRF pathways. Ann NY Acad Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- 25.Harmer CJ, MacKay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59:816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Linthorst AC, Penalva RG, Flachskamm C, Holsboer F, Reul JM. Forced swim stress activates rat hippocampal serotonergic neurotransmission involving a corticotropin-releasing hormone receptor-dependent mechanism. Eur J Neurosci. 2002;16:2441–2452. doi: 10.1046/j.1460-9568.2002.02400.x. [DOI] [PubMed] [Google Scholar]
- 27.Lowry CA, Moore FL. Regulation of behavioral responses by corticotropin-releasing factor. Gen Comp Endocrinol. 2006;146:19–27. doi: 10.1016/j.ygcen.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphé differentially affect serotonin release in the nucleus accumbens. Eur J Pharmacol. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcilhac, Siaud P. Regulation of the adrenocorticotrophin response to stress by the central nucleus of the amygdala in rats depends upon the nature of the stressor. Exp Physiol. 1996;81:1035–1038. doi: 10.1113/expphysiol.1996.sp003987. [DOI] [PubMed] [Google Scholar]
- 30.Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci. 1998;18:4758–4766. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millan MJ. The neurobiology and control of anxious states. Prog Neurobio. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 32.Myers DA, Gibson M, Schulkin J, Greenwood Van-Meerveld B. Corticosterone implants to the amygdale and type 1 CRH receptor regulation: Effects on behavior and colonic sensitivity. Behav Brain Res. 2005;161:39–44. doi: 10.1016/j.bbr.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien D, Skelton KH, Owens MJ, Nemeroff CB. Are CRF receptor antagonists potential antidepressants? Human Psychopharmacol. 2001;16:81–87. doi: 10.1002/hup.187. [DOI] [PubMed] [Google Scholar]
- 34.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd. Academic Press; New York: 1997. [Google Scholar]
- 35.Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrov T, Krukoff TL, Jhamandas JH. Chemically defined collateral projections from the pons to the central nucleus of the amygdala and hypothalamic paraventricular nucleus in the rat. Cell Tissue Res. 1994;277:289–295. doi: 10.1007/BF00327776. [DOI] [PubMed] [Google Scholar]
- 37.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 38.Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. TINS. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 39.Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin releasing factor on brain serotonergic activity. Neuropsychopharm. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- 40.Price ML, Kirby LG, Valentino RJ, Lucki I. Evidence for corticotropin-releasing factor regulation of serotonin in the lateral septum during acute swim stress: adaptations produced by repeated swimming. Psychopharmacol. 2002;162:406–414. doi: 10.1007/s00213-002-1114-2. [DOI] [PubMed] [Google Scholar]
- 41.Rauch SC, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann NY Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 42.Roche M, Commons K, Peoples A, Valentino R. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci. 2003;23:970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- 44.Valdez G, Zorrilla E, Roberts A, Koob G. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol withdrawal. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- 45.Van de Kar LD, Piechowski RA, Rittenhouse PA, Gray TS. Amygdaloid lesions: Differential effects on conditioned stress and immobilization-induced increases in corticosterone and renin secretion. Neuroendocrinol. 1991;54:89–95. doi: 10.1159/000125856. [DOI] [PubMed] [Google Scholar]
- 46.Whalen PJ, Shin LM, Somerville LH, McLean AA, Kim H. Functional neuroimaging studies of the amygdala in depression. Sem Clin Neuropsychiat. 2002;7:234–242. doi: 10.1053/scnp.2002.35219. [DOI] [PubMed] [Google Scholar]