Abstract
Background
Plasma and serum levels of myeloperoxidase (MPO), a redox-active hemoprotein released by polymorphonuclear neutrophils (PMN) upon activation, is now recognized as a powerful prognostic determinant of myocardial infarction in patients suffering acute coronary syndromes. However, there is limited information on whether systemic MPO levels are also elevated and of discriminating value in patients with stable coronary artery disease (CAD) representing different ethnic groups.
Methods
Plasma levels of MPO and traditional CAD risk factors were quantified in African American and Caucasian patients (n=557) undergoing elective coronary angiography.
Results
MPO levels did not differ significantly between patients with or without CAD [421 pM (321, 533) vs. 412 pM (326, 500), p>0.05]. MPO levels were similar across ethnicity and gender, and correlated positively with CRP and fibrinogen levels (r=0.132, p=0.002 and r=0.106, p=0.011, respectively).
Conclusion
In conclusion, plasma MPO levels were not elevated in patients with stable CAD, suggesting that systemic release of MPO is not a characteristic feature of asymptomatic CAD.
Keywords: Cardiovascular diseases, Risk factors, Myeloperoxidase, Polymorphonuclear neutrophils
INTRODUCTION
Atherosclerosis in general and coronary artery disease (CAD) in particular is considered to have an inflammatory component [1, 2]. Clinically the presentation of CAD ranges from asymptomatic patients with stable CAD to patients with chest pain at rest experiencing acute coronary syndromes. Pathophysiologically, acute coronary disease is not only reflected by increased plaque vulnerability and elevated platelet activation, but also by recruitment and activation of leukocytes [3]. Notably, polymorphonuclear neutrophils (PMN), a cell type previously considered less relevant in coronary disease, were found to undergo site specific activation and degranulation in patients with unstable CAD and were localized to culprit lesions [4–6].
One of the principal enzymes released upon PMN activation is myeloperoxidase (MPO), a highly abundant redox-active hemoprotein [2, 7, 8]. MPO and its products display a diversity of pro-inflammatory and pro-atherogenic properties including catalytic consumption of endothelium-derived nitric oxide, LDL oxidation, modulation of metalloproteinase activities, and activation of PMN in a cytokine-like manner independent of the catalytic activity [7–10]. The significance of MPO in the development of CAD has been demonstrated in studies showing association of systemic MPO level and expression of MPO with the prevalence of CAD or with chronic heart failure [9, 11]. Interestingly, MPO serum and plasma levels are markedly elevated in patients with acute coronary disease, forming a firm mechanistic link between PMN activation, MPO release, and compromised vascular reactivity [12–16].
Given the diversity of the clinical presentations of patients with coronary disease we sought to evaluate whether systemic release of MPO is a characteristic feature in patients with stable CAD in the absence of acute events representing different ethnic groups. In the present study, we investigated MPO plasma levels in Caucasian and African American patients without acute coronary syndromes undergoing elective angiography who were participating in the Harlem-Bassett study undertaken to evaluate novel cardiovascular risk factors across ethnicity [17]. We have previously reported that other CAD risk factors, including metabolic syndrome components, differ across ethnicity among these high-risk patients [18]. In addition to characterizing MPO levels and their relation to disease, we explored the relationship between MPO levels and traditional coronary disease risk factors.
METHODS
Subjects
Subjects were recruited from a patient population scheduled for diagnostic coronary arteriography either at Harlem Hospital Center in New York City or at the Mary Imogene Bassett Hospital in Cooperstown, NY. A total of 648 consecutive subjects, 401 men and 247 women, ethnically self-identified as African Americans (n=232), Caucasians (n=344) or Other (n=72) were recruited from 1993–1997 as described in detail elsewhere [17, 19]. In the present study, results were available for 557 of the 576 African American and Caucasian subjects [211 Caucasian men (C/M), 120 Caucasian women (C/F), 127 African American men (AA/M), and 99 African American women (AA/F)]. Exclusion criteria were: age >70 years, recent (within 6 months) myocardial infarction or thrombolysis, a history of percutaneous transluminal coronary angioplasty, surgery during the previous six weeks, a known communicable disease such as hepatitis or AIDS, or current lipid-lowering medication. Information on diabetes mellitus, hypertension and smoking was obtained by a standardized questionnaire upon entry into the study. The study was approved by the Institutional Review Boards at Harlem Hospital, Bassett Healthcare, Columbia University College of Physicians and Surgeons, and the University of California, Davis, and informed consent was obtained from all participants.
Angiographic definition of CAD
Coronary angiograms were analyzed by 2 experienced investigators blinded to the patient’s identity, clinical diagnosis, and MPO levels [20]. A total of 15 pre-defined coronary artery segments were evaluated in each individual for degree of stenosis. Diagnosis of coronary artery disease was defined as a luminal narrowing of at least 50% of a vessel diameter in any of the analyzed 15 coronary artery segments. In patients defined as not having CAD, the majority (81%) had a maximum stenosis of less than 25%, whereas in 81% of the patients defined as having CAD, the luminal narrowing was at least 75%. A composite cardiovascular score (0–75) was calculated based on determination of presence of stenosis on a scale of 0–5 of the 15 predetermined coronary artery segments.
Analytical procedures
Plasma samples with ethylenediaminetetraacetic acid as an anticoagulant were drawn from every patient after an overnight fast and prior to the angiographic procedure. The blood samples were centrifuged for 20 min at 1300 g at 4°C, and the supernatant separated and aliquotted into storage vials. The vials were immediately frozen and stored at −80°C until further analyzed. All analytical procedures were performed by investigators blinded to the sample identity. Serum triglycerides, total and HDL cholesterol (HDL-C), LDL cholesterol (LDL-C), Lipoprotein (a) [Lp(a)], glucose, insulin, high sensitivity C-reactive protein (hs-CRP), fibrinogen, factor VII were measured by standard techniques as described previously [17].
Plasma MPO quantification
For determination of MPO in plasma samples an ELISA procedure was used. In brief, Maxisorb 96-well microtiter plates (Nalge Nunc International; Rochester, NY, USA) were coated with 100 μl purified monoclonal mouse anti-human MPO (Biomeda, Foster City, CA, USA) diluted 1:499 (final concentration 0.6 μg/ml) in carbonate buffer, pH 9.6 overnight at 4 °C. The plates were washed three times with washing buffer (0.01 M Tris buffer with 0.05% Tween, pH 7.4), blocked with 4% BSA in washing buffer for 2 hr at 25 °C, and again washed three times. Plasma samples diluted 1:4 in PBS containing 0.5% bovine serum albumin (BSA) were added to the wells (100 μl) and incubated for 2 hr at 25 °C. All samples were measured in duplicates. After washing 4 times, a purified polyclonal rabbit anti-human MPO antibody (Calbiochem, San Diego, CA, USA) diluted 1:399 in washing buffer containing 0.5% BSA was added to the wells (100 μl) and incubated for 2 hr at 25 °C, followed by washing 4 times. Bound rabbit IgG was detected using an alkaline phosphatase conjugated goat anti-rabbit IgG antibody (Zymed- Invitrogen, Carlsbad, CA, USA). Initially, serial dilutions of primary and both secondary Ab were carried out using washing buffer containing 0.5% BSA to determine saturation kinetics. Concentrations of both antibodies were carefully optimized to give linear signal in the range of expected values. Plasma dilution was selected based on the best ratio between a background signal and a signal of plasma spiked with 330 pM/L of MPO. The average recovery was over 20 %. After washing the plates four times, 4-nitrophenyl phosphate (Sigma-Aldrich Corp.; St. Louis, MO, USA) (1.5 g/l) in diethanolamine buffer (1 M) was added, the plates developed for 20 min and the absorbance read at 405 nm in PowerWavex UV-Vis plate reader (Bio-Tek Instruments, Winooski, VT, USA). Values were related to a set of purified human MPO (Calbiochem) standards to quantify the values in picomoles (pM) per liter. The linearity of the assay was in the range 70-2000 pM MPO in plasma. Average intra-assay variability was 10.4 % and the inter-assay CV was 11.9 %. The obtained over all average of MPO plasma level 416 pM (155 pM and 1064 pM minimum and maximum, respectively) was in good agreement with MPO levels in peripheral circulation reported in many other studies, including MPO levels observed by Vita et al. in control subjects and subjects with endothelial dysfunction 298 pM MPO (154.1 pM and 638.1 pM interquartile range) in serum [21], by Brennan et al. in patients presenting with chest pain 198 pM MPO (119 pM and 394 pM interquartile range) in plasma [13], by Hoy et al. in healthy individuals 5.4 – 141.6 μg/l MPO in serum [22], by Rudolph et al. in patients with impaired left ventricular function and controls 8.9 – 54 μg/l MPO in plasma [23], and by Baldus et al. in patients with and without CAD 7.66 – 13.10 μg/l MPO in plasma [14]. However, MPO levels reported herein were lower compared to levels reported by Baldus et al. in patients with acute coronary syndrome 287 μg/l (range 1.5 – 1112 μg/l) in plasma [12].
Statistical Analysis
Data are described as mean ± SD or as median and interquartile range as appropriate. Levels of MPO, triglycerides, insulin, hs-CRP, fibrinogen and HOMA were log transformed, and adiponectin levels were square root transformed to achieve normal distributions prior to statistical analysis. Comparisons of means between groups were made by Student’s t-test. Univariate relationships were described by Pearson’s correlation coefficients. Multiple logistic regression was used to assess the association of MPO with the various known and potential risk factors. Contingency table analysis was performed to assess differences in patients characteristics across MPO plasma level quartiles. All statistical analyses were done using SAS software (SAS Institute, Cary, NC). Statistical significance was set at P<0.05.
RESULTS
The demographic and clinical characteristics of the populations studied are summarized in Table 1. Patients with CAD were significantly older and had significantly higher levels of clinical and inflammatory markers associated with CAD risk such as the waist/hip ratio, glucose, HOMA, cholesterol, triglyceride, LDL-C, HDL-C, Factor VII, fibrinogen, and adiponectin. No significant difference was detected for body mass index (BMI) and insulin levels between those with and without CAD.
Table 1.
Demographic and clinical characteristics of subjects with and without CAD Results are expressed as means ± SD, or for non-normally distributed variables as median (interquartile range)
| no CAD (n=268) | CAD (n=289) | P | |
|---|---|---|---|
| Age (years) | 52.3 ± 10.3 | 58.8 ± 8.6 | <0.001 |
| Waist/hip ratio | 0.94 ± 0.08 | 0.96 ± 0.07 | 0.004 |
| BMI (kg/m2) | 29.3 ± 6.5 | 29.1 ± 5.8 | n.s |
| Insulin (mU/l) | 13.7 (8.8–22.9) | 15.2 (9.5–26.2) | n.s. |
| Glucose (mM/l) | 6.5 ± 2.6 | 7.4 ± 3.7 | 0.003 |
| HOMA | 0.62 ± 0.44 | 0.73 ± 0.46 | 0.011 |
| Cholesterol (mg/dl) | 190.0 ± 40.3 | 204.1 ± 42.6 | <0.0001 |
| Triglyceride (mg/dl) | 115.5 (85.3–165.0) | 143.0 (105.0–211.0) | <0.0001 |
| LDL-C (mg/dl) | 117.1 ± 34.6 | 129.3 ± 39.9 | <0.0001 |
| HDL-C (mg/dl) | 46.1 ± 15.9 | 41.9 ± 13.8 | 0.001 |
| Factor VII (%) | 100.2 ± 30.6 | 105.4 ±31.3 | <0.05 |
| Fibrinogen (mg/dl) | 324.5 (279.8–385.8) | 342.0 (291.0–408.8) | <0.05 |
| Adiponectin (μg/ml) | 9.6 (6.1–14.2) | 7.9 (5.0–11.7) | <0.0001 |
| hs-CRP (mg/l) | 3.1 (1.4–7.5) | 3.7 (1.8–11.5) | n.s |
MPO was detectable in plasma samples of all study subjects, and the frequency distribution was skewed. For all subjects, the median MPO level was 416 pM (323 pM upper and 512 pM lower quartile). There was no significant difference in the MPO plasma levels between subjects with and without stable CAD [421 pM (321 pM, 533 pM) vs. 412 pM (326 pM, 500 pM) in CAD vs controls, respectively p>0.05] (Fig. 1A). We then explored MPO levels across ethnicity and gender. As shown in Fig. 1B, there was no difference in plasma MPO levels between the four ethnicity/gender groups; African American and Caucasian men and women. Table 2 illustrates patients characteristics stratified according plasma MPO quartiles. Corresponding to other analysis, any significant difference was not observed across MPO quartiles (Table 2). Further, there was no significant relationship between MPO levels and CAD, expressed as cardiovascular score, in either Caucasians or African Americans. Thus, our results suggest that the plasma level of MPO did not identify patients with stable CAD and was also independent of gender and ethnicity.
Figure 1.
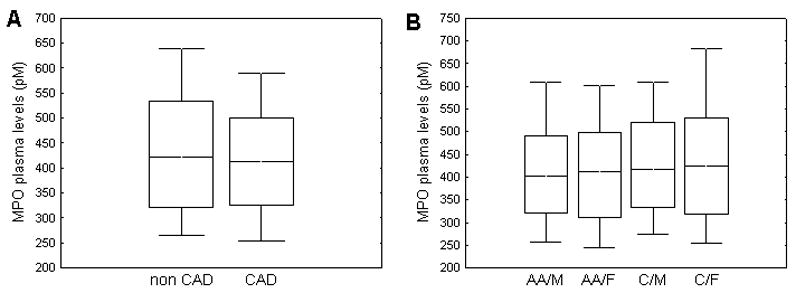
Comparison of MPO plasma levels between patients with and without CAD (A); Comparison of MPO plasma levels in four gender/ethnicity groups (AA/M - African American men, C/M - Caucasian men, AA/F - African American women, and C/F - Caucasian women) (B). The top, bottom, and line through the middle of the box correspond to the 75th percentile (top quartile), 25th percentile (bottom quartile), and 50th percentile (median) respectively. The whiskers on the bottom extend from the 10th percentile (bottom decile) and top 90th percentile (top decile).
Table 2.
Patient baseline characteristics across quartiles of MPO plasma levels Categorical data are presented as frequencies and percentages.
| MPO quartile (pM) | 155 – 323 | 324 – 416 | 417 – 511 | 512 – 1064 |
|---|---|---|---|---|
| African Americans | 76 (13.6) | 80 (14.4) | 83 (14.9) | 92 (16.5) |
| Caucasians | 63 (11.3) | 59 (10.6) | 56 (10.1) | 48 (8.6) |
| Male | 79 (14.2) | 96 (17.2) | 79 (14.2) | 84 (15.1) |
| Female | 60 (10.8) | 43 (7.7) | 60 (10.8) | 56 (10.1) |
| Cases with CAD | 71 (12.7) | 79 (14.2) | 75 (13.5) | 64 (11.5) |
Further, we considered whether the plasma MPO level was associated with other markers of stable CAD. Significant associations were found between MPO levels and the following markers in univariate analysis: factor VII (r=−0.251, p<0.0001); adiponectin (r=0.115, p=0.01); glucose (r=0.095, p=0.023); triglyceride (r=−0.186, p<0.0001); cholesterol (r=−0.081, p=0.05); hs-CRP (r=0.132, p=0.002); fibrinogen (r=0.106, p=0.011). There was no significant correlation between MPO plasma levels and age, BMI, waist/hip ratio, insulin, LDL-C, or HDL-C. Interestingly, Factor VII and adiponectin were significant predictors of MPO levels in a multiple regression model [Factor VII (r=−0.205, p<0.001) and adiponectin (r=0.119, p=0.021)]. In contrast, multiple regression analysis did not reveal levels of glucose, triglyceride, cholesterol, hs-CRP, and fibrinogen to be significant predictors of MPO plasma levels.
DISCUSSION
In the present study, we have aimed to determine whether MPO plasma levels identify patients with stable CAD. The principal finding of our study is that plasma MPO levels are not elevated in patients with stable CAD compared to non-CAD patients, and does not discriminate these cohorts of patients. CAD remains a heterogeneous disease with a wide range of clinical presentations and outcomes. Various systemic markers of inflammation have been investigated and linked to identify patients at risk of CAD and predict future cardiovascular events [1]. Recently, the importance of PMN degranulation of MPO in the coronary circulation is illustrated by the fact that systemic MPO plasma and serum are markedly elevated and have emerged as powerful predictors of adverse outcome in patients with acute CAD [12–16]. As opposed to these studies evaluating circulating MPO in symptomatic and unstable coronary disease we evaluated MPO levels in asymptomatic individuals: none of the patients, who underwent elective coronary angiography, were hospitalized because of symptomatic chest pain at rest, and none had evidence for ongoing myocardial ischemia or necrosis, respectively. Other studies showed that patients with stable CAD have significantly lower or no evidence of PMN recruitment and activation, as evidenced by unchanged CD11b expression and MPO content in PMN [4, 6]. Moreover, in patients with resolving unstable angina the decreased MPO content in PMN returned to levels similar to that in patients with chronic stable angina or subjects lacking coronary disease [6].
Whereas our data suggest that release of MPO is not a primary event in patients with stable CAD, it cannot be excluded that assessment of circulating MPO undervalues the extent of MPO sequestered into the vessel wall. There is now a large body of evidence revealing avid binding of MPO to heparan-sulfated glycosaminoglycans (GAGs) and subendothelial deposition of MPO in the vessel wall [8]. Heparinization with concomitant release of vessel-immobilized MPO revealed increased MPO burden in patients with CAD as compared to non diseased individuals, suggesting increased sequestration of leukocyte derived-MPO into the vessel wall [14]. This would also explain decreased MPO content in PMN in both acute and stable CAD patients compared to controls [5, 6, 9].
Despite MPO being equally distributed among patients with and without stable CAD, we observed weak but significant associations between plasma MPO levels and inflammatory markers known to indicate risk for CAD including CRP and fibrinogen. Similarly, Vita et al observed that serum MPO levels correlated with cardiovascular risk factors including hypertension, HDL cholesterol, C-reactive protein, serum triglycerides and smoking [21]. Recently, Meuwese et al. observed that elevated MPO plasma levels significantly predicted risk of future CAD in over all 3375 apparently healthy individuals [24]. However, the difference in medians of MPO plasma levels between controls and case subjects was around 10 %. Authors pointed out that relationship between MPO and CAD in these individuals was weaker than was reported in patients with acute CAD [24]. Lipid-lowering drugs statins downregulated MPO systemic levels in patients with acute coronary syndrome [25]. Interestingly, there are emerging data also linking MPO plasma levels with presence of heart failure [11, 16] and left ventricular dysfunction [23, 26]. However, no difference in MPO levels was observed between patients with and without CAD who had normal left ventricular function [23]. Furthermore, MPO plasma levels were neither related to intima media thickness, nor to progression of intima media thickness in 122 familiar hypercholesterolemia patients [27].
We acknowledge some of the limitations of this study. Subjects in our study were recruited from patients scheduled for coronary angiography and are likely more typical of a high-risk patient group than the healthy population at large. This may explain the relatively high levels of CRP and fibrinogen among our subjects. However, none of the patients had a history of acute coronary symptoms or surgical intervention within 6 months, arguing against any secondary increase in inflammatory parameters due to an acute CAD. Although the number of subjects representing each gender-ethnicity group was limited, the number of subjects, both Caucasians and African Americans was considerably higher than in some previous multi-ethnic studies [15]. Further, clinical and laboratory parameters were in agreement with differences generally observed between healthy African American and Caucasian populations from other studies [28, 29].
In conclusion, the present study reveals that plasma MPO levels do not identify patients with stable CAD. This is in contrast to determination of systemic MPO levels as emerging powerful and rapidly detectable markers of unstable CAD [12–16]. Our findings further support the concept that a robust release of MPO from activated PMN would unmask a state of acute inflammation in the coronary circulation preceding myocardial injury and this cannot be extended to stable cardiovascular disease using traditional blood drawing techniques. Further studies aiming to determine the pathophysiological role of MPO in acute coronary disease and addressing the pathophysiological heterogeneity of the different clinical presentations of coronary artery disease are needed.
Acknowledgments
This work was supported by a postdoctoral fellowship award from the Phillip Morris External Research Program (L.K.), grant GACR 524/06/1197 (L.K.), research plan AVOZ50040507 (L.K.), the University of California Davis Health System Research Award (J.P.E.), the Paul F. Gulyassy Endowed Professorship (J.P.E.), a grant from the Nora Eccles Treadwell Foundation (L.B. and J.P.E.), grant HL 62705 (L.B.) from NHLBI, and a grant BA 1870/3-3 from the Deutsche Forschungsgemeinschaft (S.B.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fichtlscherer S, Heeschen C, Zeiher AM. Inflammatory markers and coronary artery disease. Curr Opin Pharmacol. 2004;4:124–31. doi: 10.1016/j.coph.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–11. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 3.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. Jama. 1998;279:1477–82. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 4.Buffon A, Biasucci LM, Liuzzo G, et al. Widespread coronary inflammation in unstable angina. N Engl J Med. 2002;347:5–12. doi: 10.1056/NEJMoa012295. [DOI] [PubMed] [Google Scholar]
- 5.Leckie MJ, Gomma AH, Purcell IF, et al. Automated quantitation of peripheral blood neutrophil activation in patients with myocardial ischaemia. Int J Cardiol. 2004;95:307–13. doi: 10.1016/j.ijcard.2003.04.063. [DOI] [PubMed] [Google Scholar]
- 6.Biasucci LM, D'Onofrio G, Liuzzo G, et al. Intracellular neutrophil myeloperoxidase is reduced in unstable angina and acute myocardial infarction, but its reduction is not related to ischemia. J Am Coll Cardiol. 1996;27:611–6. doi: 10.1016/0735-1097(95)00524-2. [DOI] [PubMed] [Google Scholar]
- 7.Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med. 2000;28:1717–25. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 8.Eiserich JP, Baldus S, Brennan ML, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–4. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 9.Zhang R, Brennan ML, Fu X, et al. Association between myeloperoxidase levels and risk of coronary artery disease. Jama. 2001;286:2136–42. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 10.Sugiyama S, Okada Y, Sukhova GK, et al. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–91. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang WH, Brennan ML, Philip K, et al. Plasma myeloperoxidase levels in patients with chronic heart failure. Am J Cardiol. 2006;98:796–9. doi: 10.1016/j.amjcard.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Baldus S, Heeschen C, Meinertz T, et al. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–5. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 13.Brennan ML, Penn MS, Van Lente F, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 14.Baldus S, Rudolph V, Roiss M, et al. Heparins increase endothelial nitric oxide bioavailability by liberating vessel-immobilized myeloperoxidase. Circulation. 2006;113:1871–8. doi: 10.1161/CIRCULATIONAHA.105.590083. [DOI] [PubMed] [Google Scholar]
- 15.Cavusoglu E, Ruwende C, Eng C, et al. Usefulness of baseline plasma myeloperoxidase levels as an independent predictor of myocardial infarction at two years in patients presenting with acute coronary syndrome. Am J Cardiol. 2007;99:1364–8. doi: 10.1016/j.amjcard.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 16.Tang WH, Tong W, Troughton RW, et al. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. 2007;49:2364–70. doi: 10.1016/j.jacc.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 17.Jiang XC, Paultre F, Pearson TA, et al. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20:2614–8. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 18.Anuurad E, Chiem A, Pearson TA, Berglund L. Metabolic syndrome components in african-americans and European-american patients and its relation to coronary artery disease. Am J Cardiol. 2007;100:830–4. doi: 10.1016/j.amjcard.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Paultre F, Pearson TA, Weil HF, et al. High levels of Lp(a) with a small apo(a) isoform are associated with coronary artery disease in African American and white men. Arterioscler Thromb Vasc Biol. 2000;20:2619–24. doi: 10.1161/01.atv.20.12.2619. [DOI] [PubMed] [Google Scholar]
- 20.Miller M, Mead LA, Kwiterovich PO, Jr, Pearson TA. Dyslipidemias with desirable plasma total cholesterol levels and angiographically demonstrated coronary artery disease. Am J Cardiol. 1990;65:1–5. doi: 10.1016/0002-9149(90)90017-u. [DOI] [PubMed] [Google Scholar]
- 21.Vita JA, Brennan ML, Gokce N, et al. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation. 2004;110:1134–9. doi: 10.1161/01.CIR.0000140262.20831.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoy A, Tregouet D, Leininger-Muller B, et al. Serum myeloperoxidase concentration in a healthy population: biological variations, familial resemblance and new genetic polymorphisms. Eur J Hum Genet. 2001;9:780–6. doi: 10.1038/sj.ejhg.5200702. [DOI] [PubMed] [Google Scholar]
- 23.Rudolph V, Rudolph TK, Hennings JC, et al. Activation of polymorphonuclear neutrophils in patients with impaired left ventricular function. Free Radic Biol Med. 2007;43:1189–96. doi: 10.1016/j.freeradbiomed.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Meuwese MC, Stroes ES, Hazen SL, et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;50:159–65. doi: 10.1016/j.jacc.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 25.Zhou T, Zhou SH, Qi SS, et al. The effect of atorvastatin on serum myeloperoxidase and CRP levels in patients with acute coronary syndrome. Clin Chim Acta. 2006;368:168–72. doi: 10.1016/j.cca.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 26.Ng LL, Pathik B, Loke IW, et al. Myeloperoxidase and C-reactive protein augment the specificity of B-type natriuretic peptide in community screening for systolic heart failure. Am Heart J. 2006;152:94–101. doi: 10.1016/j.ahj.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Meuwese MC, Trip MD, van Wissen S, et al. Myeloperoxidase levels are not associated with carotid atherosclerosis progression in patients with familial hypercholesterolemia. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Paultre F, Tuck CH, Boden-Albala B, et al. Relation of Apo(a) size to carotid atherosclerosis in an elderly multiethnic population. Arterioscler Thromb Vasc Biol. 2002;22:141–6. doi: 10.1161/hq0102.101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez C, Pablos-Mendez A, Palmas W, et al. Comparison of modifiable determinants of lipids and lipoprotein levels among African-Americans, Hispanics, and Non-Hispanic Caucasians > or =65 years of age living in New York City. Am J Cardiol. 2002;89:178–83. doi: 10.1016/s0002-9149(01)02197-x. [DOI] [PubMed] [Google Scholar]


