Abstract
Axonal projections from the dorsal nucleus of the lateral lemniscus (DNLL) distribute contralaterally in a pattern of banded layers in the central nucleus of the inferior colliculus (IC). The banded pattern of DNLL projections is already in the IC by onset of hearing in postnatal rat pups. Previously, it was shown that unilateral cochlear ablation in neonatal rat pups disrupted the banded pattern in IC for the projections of the DNLL contralateral to the ablation but not those of the DNLL ipsilateral to the ablation. In the present study, bilateral cochlear ablation or sham surgery was performed at postnatal day 9 (P9) after which rat pups were sacrificed at P12 and the brains removed to study axonal projections of the DNLL. A lipophilic carbocyanine dye, DiI, was placed in the dorsal tegmental commissure of Probst to label decussating DNLL axons that end in the central nucleus of the contralateral IC. The distribution of labeled fibers across the central nucleus of the IC was analyzed in digital images by comparing the pattern of labeling with a sine model of periodic distribution of banded layers. In the control group, labeled axons formed a regular pattern of dense banded layers in IC. In the bilateral cochlear ablation group, labeled axons in the IC were distributed diffusely and there was little or no regular pattern of dense bands of axonal labeling. The influence of the cochlea on developing auditory circuits possibly mediated by activity-dependent mechanisms is discussed.
Keywords: auditory, refinement, plasticity, pattern formation, carbocyanine dye, DiI
It is clear that early sensory experience or, more specifically, evoked activity plays a critical role in the functional refinement of synaptic circuits. However, the earliest evoked responses recorded from the central nucleus of the inferior colliculus (IC) suggest that complex neural circuits in IC develop before hearing onset (Aitkin and Moore, 1975; Moore and Irvine, 1980; 1981; Ehret and Romand, 1992). In the auditory brainstem, as well as in other sensory systems, spontaneous activity has been shown to influence early development of neural circuits not withstanding other activity-independent influences (for general review see Sur and Rubenstein, 2005; Friauf and Lohmann, 1999). The cochlea is one source and perhaps the major source of spontaneous activity in developing auditory pathways (Walsh and McGee, 1987; Kotak and Sanes, 1995; Lippe, 1995).
We have chosen to study development of laminar circuits in the IC. Both structural (e.g., Oliver and Shneiderman, 1989) and functional (e.g., Semple and Aitkin, 1979; Wenstrup et al., 1986; Schreiner and Langner, 1988; Brückner and Rübsamen, 1995) evidence indicates that specific inputs are segregated in afferent compartments in IC layers. Afferent projections that distribute in banded layers and either overlap or are segregated from other projections are illustrative of organization in IC (e.g., Shneiderman and Henkel, 1987; Shneiderman et al., 1988; Oliver et al., 1997). One of these inputs, the dorsal nucleus of the lateral lemniscus (DNLL), gives rise to a pattern of afferent bands in IC that is well-refined by hearing onset (Gabriele et al., 2000a) and other banded projections to the IC have been demonstrated prior to hearing in other species (Brunso-Bechtold and Henkel, 2005; Henkel et al., 2005). Axonal projections from the DNLL to the central nucleus of the IC are initially sparse at birth in rat pups, proliferate diffusely throughout the first postnatal week, and then begin to segregate into a pattern of dense banded layers (Gabriele et al., 2000a).
In previous studies, unilateral cochlear ablation in neonatal rat pups disrupted the development of afferent projections from the DNLL to the central nucleus of the IC (Gabriele et al., 2000b; Franklin et al., 2006). Somewhat unexpectedly in those studies crossed projections of the deprived DNLL to IC on the side of the ablation were affected but not those of the mirror image projections. Hebbian activity-dependent competition seems unlikely given the stability of undeprived inputs and loss of banding of deprived DNLL inputs to IC. The mechanisms that mediate this effect, however, remain to be elucidated.
In order to examine further the influence of the cochlea on developing neural circuits in the IC, cochlear ablation was performed bilaterally in postnatal day 9 (P9) rat pups. Any influence of the cochlea on developing DNLL projections would be symmetric on each side in such cases in contrast to unilateral ablation cases in previous studies (Gabriele et al., 2000b; Franklin et al., 2006). If spontaneous activity-dependent competition is the organizing mechanism for band development, then bilateral ablation as opposed to unilateral ablation should not perturb the pattern of DNLL bands. Alternatively, if spontaneous activity apart from competition acts as a permissive factor for band development, then bilateral ablation should disrupt the banded pattern of crossed DNLL projections to both ICs.
Axonal projections from DNLL to the central nucleus of the IC were labeled with a lipophilic carbocyanine dye, DiI, and the pattern of labeling in the ablated group was compared with surgical controls. The results show that in spite of symmetric influence of bilateral cochlear ablation on the volume of the cochlear nuclei in neonatal rat pups the banded pattern of DNLL projections to IC layers is disrupted.
Preliminary results have been published previously in abstract form (Franklin et al., 2006).
EXPERIMENTAL PROCEDURES
In this study, a carbocyanine dye, DiI, was used to label axonal projections from the DNLL to the central nucleus of the IC in P12 rat pups that had been operated to remove the cochlea bilaterally three days earlier at P9. A total of 25 experimental and 5 control animals were used for the study. The experimental design is illustrated in Figure 1. All animal care and experimental procedures were in compliance with the Guide for the Care and Use of Laboratory Animals in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC). All efforts were made to minimize the suffering and the number of animals used in this study.
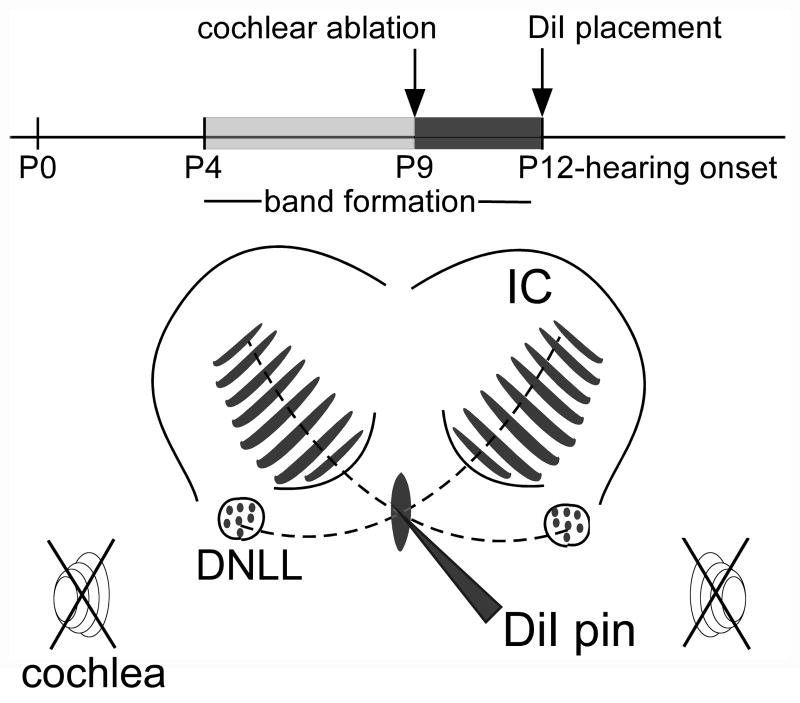
Figure 1.
Schematic illustration of the experimental design. A time line shows the relationship of time points for bilateral cochlear ablation (indicated by crossed out spiral symbols) and DiI labeling of DNLL projections in the IC to the time course of development of afferent bands in the central nucleus of the IC (CNIC) and hearing onset. The line drawing of the dorsal part of the midbrain indicates where DiI pins were positioned to label DNLL fibers as they cross the midline in the dorsal tegmental commissure (of Probst) and distribute in a pattern of afferent bands (shaded regions) in the contralateral CNIC. Other abbreviations: DCIC, dorsal cortex of the IC; ECIC external cortex of the IC.
Cochlear ablation
In P9 rat pups, both the left and right cochleas were ablated by aspiration as previously described (Gabriele et al., 2000b; Franklin et al., 2006). Briefly, P9 rat pups in the experimental group were anesthetized by hypothermia and a skin incision was made ventral to the pinna. The external auditory canal was exposed and followed to the tympanic membrane, taking care to avoid injury to the root of the facial motor nerve. The tympanic membrane then was pierced and the middle ear cavity cleared of mesenchyme. After removing the primordia of the ossicles, a syringe needle was inserted through the round window and sterile water was flushed through the perilymphatic spaces of the cochlea to destroy hair cells. Next the bone between the oval and round windows was pinched and the cochlear contents were aspirated. In age-matched control animals, the skin was incised but the middle and inner ears were not opened. The wound was closed with cyanoacrylate glue (Vetbond Tissue Adhesive, 3M, St. Paul, MN, or equivalent) and the rat placed on a warming pad to recover. After recovery, ablated and control pups were returned to the litter and reared to P12.
Labeling DNLL commissural axons
On P12, rat pups were given an overdose of ketamine and xylazine and perfused through the heart with 4% paraformaldehyde. The brains were removed and postfixed in 4% paraformaldehyde overnight. The temporal bones were inspected to verify cochlear ablations. Brains were blocked in the coronal plane, embedded in a 2% gelatin-egg yolk mixture, and immersed overnight in 4% paraformaldehyde. Using a Vibratome (St. Louis, MO), sections were cut from the rostral face of the embedded block until commissural fibers ventral to the periaqueductal gray matter were visualized at the superior colliculus-inferior colliculus junction. At this site, the block was impaled on the midline with a glass pin coated with DiI (1,1′-dioctodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Invitrogen-Molecular Probes, Eugene, OR) and any stray flecks of dye were rinsed off the block. The tissue block was then returned to 4% paraformaldehyde and incubated in the dark at 37°C for 6 weeks to allow diffusion of the dye to axonal branches and endings in the IC.
Histology
After 6 weeks incubation, the brains were cut in the coronal plane on a Vibratome. Serial, 75 μm thick sections through the IC and cochlear nucleus (CN) were collected in 0.1 M phosphate buffer, mounted onto +/− charged slides, and coverslipped using a permanent aqueous mounting media (Gel/Mount, Biomeda, Foster City, CA, Catalogue #M01). The tissue was examined with an epifluorescent photomicroscope (Olympus BX2, Olympus America, Inc., Melville, NY) equipped with a Cy5 filter set (Chroma Technologies, Brattleboro, VT) to visualize DiI. Images of all sections containing the IC, DNLL, or CN were collected within 24 hrs using a Spot RT Slider digital camera (1600×1200 pixel resolution) and software (Diagnostic Instruments, Sterling Heights, MI).
Analysis of CN and IC volume
To obtain volume measurements from each case, the borders of the left and right CN and IC in serially spaced, Nissl stained sections were traced and the nuclear volume calculated with a computer controlled microscope mapping system (Neurolucida software, MBF Bioscience, Williston, VT). Volumes in control and cochlear ablation groups were compared with a Student-t test (Prism, GraphPad Software, Inc., San Diego, CA).
Analysis of DiI labeling
Images of DiI-labeled DNLL projections to the central nucleus of the IC were processed and analyzed in a manner similar to that described previously for analysis of DNLL bands (Gabriele et al., 2000a; Henkel et al., 2005; Franklin et al., 2006). Briefly, to assess the relative density of labeled fibers and the pattern of distribution in the IC on each side in ablated and control groups, 8 bit gray level images were captured at 4× magnification (1600×1200 pixels, 0.542 pixels/micron) and examined using ImageJ software (NIH, Bethesda, MD; http://rsb.info.nih.gov/ij/). The densest area of labeling was located consistently in the middle third of the rostral-caudal axis of the central nucleus of the IC. In each case, the labeling pattern in three sections from this region of dense labeling was analyzed. For analysis, a line intersecting the midpoint of the axonal labeling was selected and rotated so that it was approximately orthogonal to the layers. A line plot profile of gray level (density) changes along this line was generated with ImageJ software. The high frequency noise in the gray level values of the images was smoothed digitally with a 15×15 Gaussian filter before analysis.
Nonlinear regression was used to fit the line density profile representing the labeling pattern to a sine curve modeling a periodic pattern of density changes across IC layers (Prism, GraphPad Software, Inc., San Diego, CA). Results for samples from three sections were averaged for each case. Four measures were obtained: baseline –representing overall density of the projection, standard deviation – representing density of the bands, frequency – from which the thickness or spacing of the bands was derived, and R2-goodness of fit value – representing the degree of periodicity in the pattern of band labeling (see TABLE I). To test for symmetry of labeling in the left and right IC as well as for effect of bilateral cochlear ablation, the mean values for the four variables in the labeling pattern in each IC were compared using a MANOVA design (Statistica, StatSoft, Inc., Tulsa, OK). A Tukey post test was performed to examine any effect of interaction of the variables. Data are expressed as mean ± standard deviation.
TABLE I.
Data from line density analysis and curve fitting of labeling pattern comparing the parameters of the best fit sine curves in control and ablation groups. Mean and standard deviation are given for control (N=5) and experimental group with bilateral cochlear ablation (N=8).
| Left Control | Right Control | Left Experimental | Right Experimental | |
|---|---|---|---|---|
| BaseLine | 65.9±6.6 | 77.0±6.7 | 79.4±3.6 | 80.8±3.9 |
| Amplitude | 14.5±2.8 | 15.5±2.9 | 6.3±1.2** | 5.3±0.7** |
| Period (μm) | 158±12 | 142±11 | 110±10** | 114±9** |
| R2 | 0.49±0.05 | 0.49±0.07 | 0.43±0.04 | 0.41±0.05 |
RESULTS
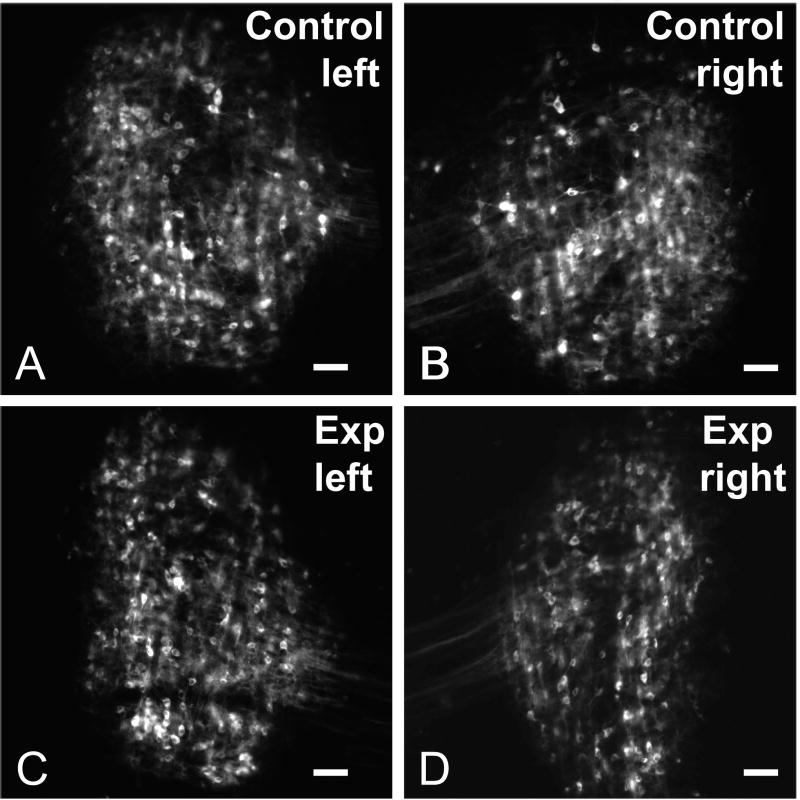
In order to label crossed axonal projections of the DNLL, DiI labeled pins were placed in the dorsal tegmental commissure (of Probst) in P12 rat pups that had been operated on at P9 to remove the cochlea bilaterally (Figure 1). Note that at the time of cochlear ablation, normal development of DNLL projections to IC has been shown previously to have progressed to the stage where axons segregate into banded layers in the central nucleus of the IC (Gabriele et al., 2000a). Crossed DNLL projections to IC in rat pups were compared with projections in age-matched, sham-operated controls. In both the control and bilateral cochlear ablation group, retrogradely labeled cells were labeled throughout the dorsal-to-ventral and medial-to-lateral expanse of the DNLL and were distributed symmetrically on the left and right sides (Figure 2).
Figure 2.
Paired images illustrating retrogradely labeled neurons in the DNLL for the control group (A, B) and experimental (Exp) group (C, D) cases after DiI-pins were placed in the dorsal tegmental commissure (see Experimental Design in Figure 1) to label decussating fibers. Note the symmetric labeling in DNLL on each side in both groups. Scale bars equal 50 μm.
Analysis of symmetry of CN volume for case selection
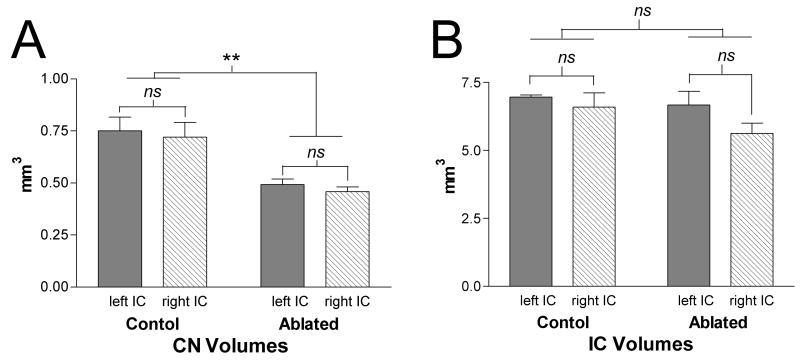
Only 8 of 25 experimental cases with evidence of symmetrical bilateral cochlear ablation based on postmortem visual inspection of the temporal bones and confirmed by symmetric reduction of the left and right CN volumes by at least 25% (consistent with Franklin et al., 2006) were used for analyses in the present study. As illustrated in Figure 3A, CN volume in these cases (N=8) was 0.48±0.07 mm3 compared to 0.74±0.16 mm3 in control cases (N=5). The right and left CN volumes were not significantly different indicating symmetrical effects of ablation but both were significantly reduced compared to controls (p<0.05, Student T-test, GraphPad Prism, GraphPad Software, Inc., Sand Diego, CA).
Figure 3.
Bar graphs illustrating the mean percent difference in volumes for CN (A) and IC (B) in the P9 cochlear ablation group compared to controls. Whiskers indicate standard error of the mean (N=8). There is significant reduction in volume of about 35% for both the left and right CN compared to controls. IC volumes in the ablated group were unchanged from the control condition. No differences were observed in these cases between reduction of the left and right CN volumes consistent with symmetrical cochlear ablation on each side.
Although the sensitive period for deafferentation induced cell loss in CN (Trune, 1982; Nordeen et al., 1983; Moore and Kowalchuk, 1988; Hashisaki and Rubel, 1989; Hardie and Shepherd, 1999; Gabriele et al., 2000a) ends abruptly prior to P9 in rats (Moore et al., 1998; see also Tierney et al., 1997 -gerbil), shrinkage in the absence of significant cell loss has been attributed to neuron atrophy and other presynaptic and postsynaptic changes in the CN (Moore et al., 1998).
Stability of IC volume in bilateral cochlear ablation cases
To affirm that changes in the pattern of afferent projections, such as the dimension of spacing or thickness of bands, were not the result of changes in the size of IC after cochlear ablations, the volumes of IC in control and experimental animals were compared. There was no significant difference between volumes in the two groups as indicated in Figure 3B.
Qualitative description of distribution of DiI labeled axons
DiI pins placed in the dorsal tegmental commissure of Probst labeled axons decussating from the DNLL on each side and entering the contralateral IC in both the control and experimental rat pups. Labeled axons entered IC ventrolaterally and dispersed along layers in the central nucleus. The labeled fibers typically were distributed throughout the rostral-caudal (longitudinal) axis of the central nucleus of theIC as well as across its ventromedial-dorsolateral (cochleotopic) axis. Terminations of at least some of these labeled fibers extended into the deepest layers of the dorsal cortex of the IC. A lateral area in the deep part of the external nucleus of the IC was also occupied by labeled axons; however, only changes in distribution of axonal labeling in the central nucleus of IC are described in this account.
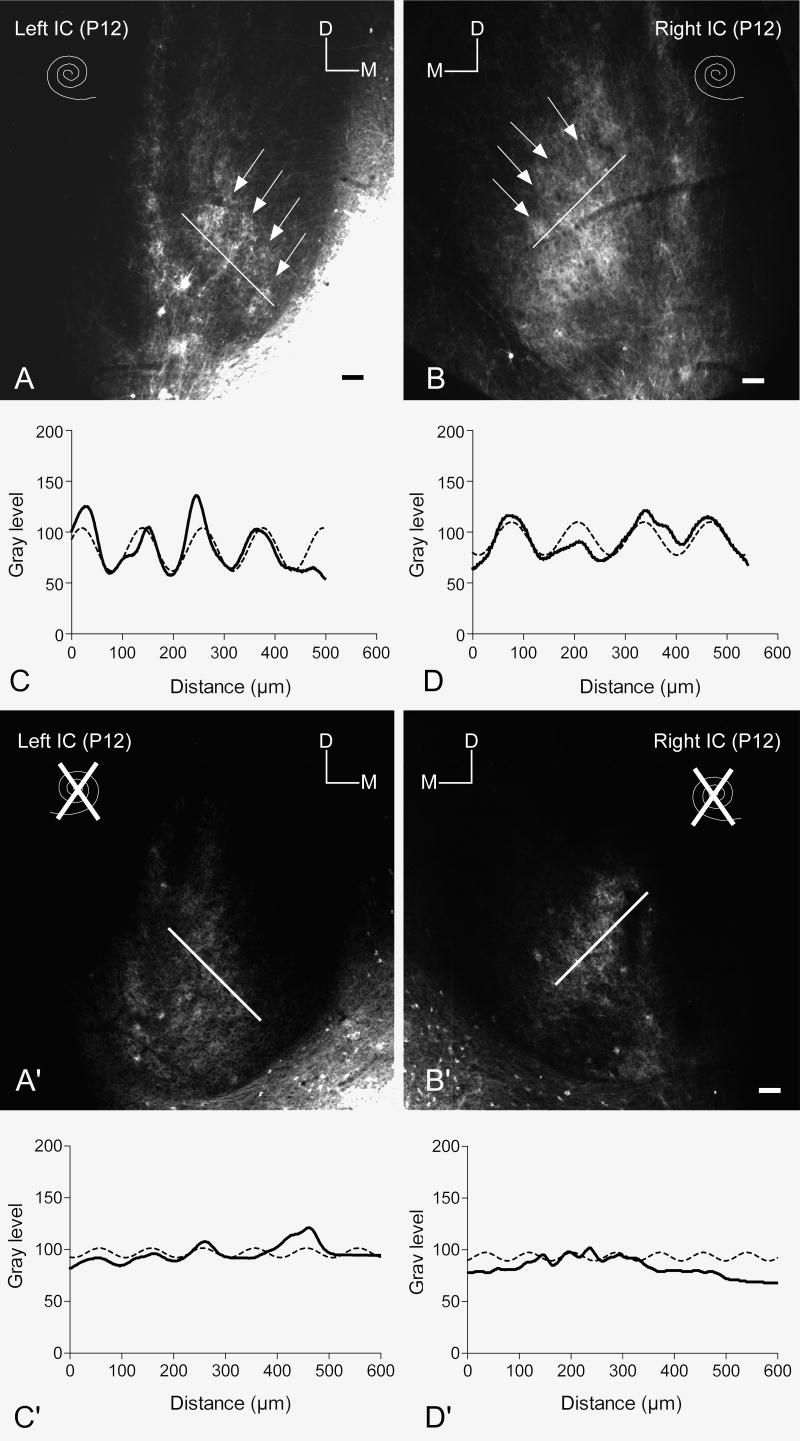
In both control and experimental cases, the density of labeled DNLL projections appeared to be symmetrical in the left and right IC (see Figure 4). However, there were marked differences in the pattern of distribution of labeled fibers in the central nucleus of the IC in the bilateral cochlear ablation cases compared to that in control cases. In the controls, labeled axons and endings were distributed along layers of the central nucleus of IC in densely labeled bands alternating with sparsely labeled or unlabeled areas (arrows in Figure 4A, B) as described previously in P12 rat pups (Gabriele et al., 2000a; Franklin et al., 2006). This banded pattern of distribution was most evident in the middle extent of the rostral-caudal target of DNLL fibers in the IC. In contrast to the banded pattern of labeling in IC in control cases, labeled DNLL axons appeared more diffusely distributed in bilateral ablation cases. The pattern of distribution for a typical case is shown in Figure 4A′ and B′. The labeled DNLL projections were quite dense as in the controls and symmetrically distributed in the left and right IC. Labeled axons in these cases also coursed mostly parallel to the plane of layers in the central nucleus of IC but lacked the obvious bands and patches of denser terminal regions that characterized the distribution in control cases.
Figure 4.
Comparison of the pattern of labeled DNLL projections to the contralateral IC in control and bilateral ablation cases
A, B. Paired images illustrating the symmetrical density and pattern of DiI labeled DNLL axons and endings distributed in the IC on each side in a P12 rat pup from the control group (intact condition of the cochleas indicated by the spiral symbols). Bright fluorescent labeling is segregated along bands in the central nucleus at the arrows. Markers indicate dorsal (D) and medial (M) orientation of the images. The bright region in the lower right corner of image A is spread the DiI pin. Scale bars equal 100 μm.
C, D. Line graphs illustrating regularly spaced changes in gray level along the white lines in images A and B from the left and right IC, respectively. Peaks in the line density plot (bold line) indicate the position of bright bands (arrows) in the corresponding images. Note the ‘fit’ of the best fit sine curves (dashed lines) to the density data.
A′, B′. Paired images illustrating the density and pattern of DiI labeled DNLL axons and endings distributed in the IC on each side in a P12 rat pup from the ablation group (condition of the cochleas indicated by the spiral symbols). Bright fluorescent labeling is segregated is distributed more uniformly in the central nucleus of IC compared to the distribution in the control group shown (A and B). Markers indicate dorsal (D) and medial (M) orientation of the images. Scale bars equal 100 μm.
C′, D′. Line graphs illustrating changes in gray level along the white lines in images A′ and B′ from the left and right IC, respectively. Peaks in the line density plot (bold line) are not at all as prominent as in the control group (A and B). The amplitude of the best fit sine curves (dashed lines) also is small compared to the controls.
Analysis of changes in labeling pattern after bilateral cochlear ablation
Densitometry was used to analyze the change in spatial distribution of axonal labeling in IC after bilateral cochlear ablation (see EXPERIMENTAL PROCEDURES). In general, line density profiles representing the spatial pattern of labeling in the left and right IC were similar (compare Figure 4C with 4D and 4C′ with 4D′) whereas an obvious difference was seen between the control and experimental group (compare Figure 4C, D with Figure 4C′, D′).
Curve-fitting with a nonlinear regression algorithm determined the sine function that best fit the profile of the line plot of labeling density for each IC (dashed line graphs in Figures 4C, D, C′, D′). Four parameters from the best fit sine curves for the data were compared in each group: baseline or overall density, amplitude or band density, frequency from which band spacing was derived, and R2 or periodicity factor. For the controls, the mean best fit curve modeled the evenly spaced dense bands observed in the labeled DNLL projection with peaks in the curve corresponding to dense labeling and troughs corresponding to the sparsely labeled regions. As shown in TABLE I, the four parameters of best fit curves were similar for the left and right IC in the control group and also the left and right IC in the ablation group and MANOVA showed no statistical effect of side for either group (F(5,20)= 0.683, p= 0.642) for these measures.
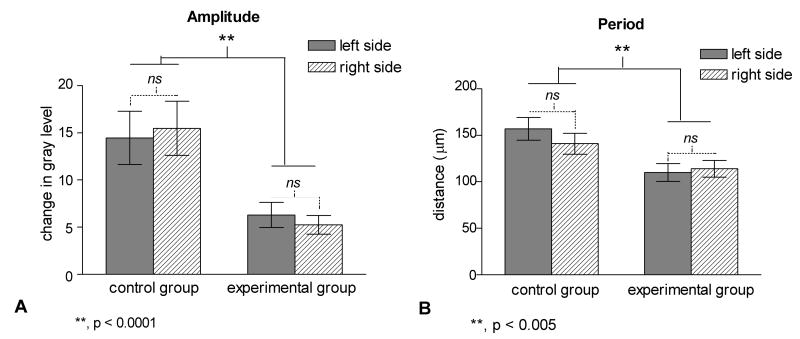
In contrast to the similarity shown by densitometric analyses in distribution of labeling between sides, there was a highly significant effect of condition (control versus ablation) on the pattern of distribution of DNLL fibers in IC (MANOVA F(3,22)=12.02, p<0.0001) (Figure 5). A Tukey posthoc test revealed a significant interaction of both band spacing (p<0.005) and band density (p< 0.001) on differences between conditions. The amplitude of the best fit sine curves indicating the gray level change within and between bands was 14.5±2.8 for the left IC and 15.5±2.9 for the right IC in controls but only 6.3±1.2 and 5.3±0.7, respectively, for the experimental group (TABLE I). Note that in the ablation case shown in Figure 4(C′, D′), the band density measured as the maximum gray level difference between the peaks and valleys of the line graphs was less than half of that shown for the example of a control case (Figure 4C, D). Likewise, the period of the best fit sine curves corresponding to the center-to-center distance between bands in IC was 158±12 μm for the left IC and 142±11 μm for the right IC for the control group compared to 110±10 and 114±9, respectively for the ablation group (TABLE I).
Figure 5.
Bar graphs comparing data from the control and ablation groups for the mean amplitude (A) and period (B) of fluctuation of gray level across the cochleotopic axis in the region of labeled DNLL projections of the left (solid bar) and right IC (hatched bar). Note that condition has a significant effect (control versus ablation), but side (left versus right) has no effect for either dependent variable.
Although there was no consistent interaction of the periodicity factor (R2) and no effect of overall baseline (overall density) with condition (control versus ablation), it should be pointed out that, in fact, the line plots representing the spatial distribution of labeling in the experimental cases were relatively flat with less difference in labeling within and between peaks in the experimental group compared to controls. That there was no pairwise interaction of the baseline for the curves in the control group and ablated group (Tukey post hoc, p>0.05) supports the qualitative observation that overall the density of labeled fibers in the IC in the control and ablation group was not different.
Thus, a more homogeneous spread of labeled DNLL fibers across the cochleotopic axis of IC could be seen just three days following bilateral ablation. Disruption of the organized afferent bands in bilateral ablated cases was similar to that reported for cases with P9 unilateral cochlear ablation (Franklin et al., 2006) but with symmetrical effects in the right and left IC.
DISCUSSION
In the present study, projections from the DNLL to the IC were labeled with DiI in P12 rat pups that had had the cochlea ablated bilaterally at P9. At the time the cochleas were ablated, a pattern of banded DNLL projections is already apparent in the central nucleus of the IC (Gabriele et al., 2000a). Just three days after bilateral ablation at approximately the time of hearing onset in rat pups, however, labeled DNLL projections were present in IC, but axons were no longer distributed in distinct, dense bands. Any periodic spacing in the dense labeling pattern across IC layers was significantly less than in the control group. There was no change in volume of IC after ablations, however, that would influence such spacing. Shrinkage of CN volume was symmetrical after bilateral ablation indicating the likelihood that effects on cochlear influence in developing ascending pathways were balanced in the IC on each side.
Effects of cochlear ablation on development of ascending auditory projections
Removal of the cochlea has been shown previously to alter many structural features of development of neurons and their connections in the auditory brainstem (e.g., Rubel et al., 1990; Cant, 1997; Friauf and Lohmann, 1999). A number of studies have shown that changes observed after unilateral ablations result from a competitive advantage of intact pathways over deprived ones. For instance, unilateral cochlear ablation in neonatal ferret kits resulted in expansion of projections from the CN on the intact side to the ipsilateral IC (Moore and Kitzes, 1985; Moore and Kowalchuk, 1988) although there was no observable influence of bilateral ablation on the number of cells in the CN that project to the ipsilateral IC (Moore, 1990). Similarly, the balance of ipsilateral excitatory and contralateral inhibitory synaptic contacts on neurons in the lateral superior olivary nucleus (LSO) was altered by unilateral cochlear ablation (Koch and Sanes, 1998) and ectopic projections of the CN to the contralateral LSO developed in place of the normal ipsilateral projection to the LSO after ablation of the contralateral cochlea (Kitzes et al., 1995).
Development of inhibitory projections in the auditory system
Though less is known about mechanisms involved in development of inhibitory than about excitatory connections, long GABAergic projections such as those of the DNLL and glycinergic projections of the medial nucleus of the trapezoid body (MNTB) provide excellent models for such investigation. Elegant studies by several groups (e.g., Kandler and Gillespie, 2005; Gillespie et al., 2005; Kim and Kandler, 2003; Kotak and Sanes 2003; Sanes and Friauf, 2000) have detailed important aspects development of such inhibitory connections in the superior olive and in the IC. Interestingly, early inhibitory projections have an excitatory effect on postsynaptic neurons, co-release glutamate, and possibly utilize mechanisms for synapse stabilization or elimination similar to glutamatergic ones. However, in the present experimental paradigm cochlear ablations were performed after the early postnatal period of co-release of GABA and glutamate. Thus, either co-release is initiated again after cochlear ablations or some other synaptic mechanisms account for reshaping of axonal distribution.
In another related study, Sanes and Takács (1993) showed that MNTB axons fail to undergo normal developmental pruning to form narrow target areas in the LSO after unilateral cochlear ablation in postnatal gerbils and as a result the axonal arbors are wider in the tonotopic axis of the LSO. However, total axon length was not altered and the total number of endings was largely unaffected in this condition. Similar structural plasticity of DNLL axons deprived of normal input from ascending pathways may account for failure of afferent axons to segregate into dense bands in IC layers and still proliferate broadly in the central nucleus of the IC in the present study as well as in the IC ipsilateral to unilateral cochlear ablation in previous studies (summarized in Figure 6). Total axonal length and synaptic contacts may even be similar in spite of disruption of the banded pattern.
Figure 6.
Summary diagram illustrating the patterns of afferent projections from DNLL to the central nucleus of IC in three groups: control (A), unilateral cochlear ablation (B), and bilateral cochlear ablation (C). Only a simplified schematic of ascending projections is included simplicity. Crossed projections from DNLL are shown as black symbols and shaded bands in IC and uncrossed projections as open symbols and bands. The hypothesized affects of cochlear influence on presynaptic and postsynaptic activity in the left and right IC in each condition are indicated by the direction of the arrows. The DNLL projections in the bilateral ablation condition, both deprived of cochlear influence, and the deprived DNLL projections to the IC ipsilateral to the unilateral cochlear ablation are not segregated into distinct bands as in the control condition. Together, results of bilateral and unilateral cochlear ablation are consistent with a role of non-Hebbian spontaneous activity-dependent regulation of afferent segregation. Other abbreviations: Co, cochlea; SOC, superior olivary complex.
Clarification of results in relation to previous studies
In contrast to the findings above, our previous studies showed that the pattern of disruption of DNLL afferent bands in IC following unilateral cochlear ablation in neonatal rat pups was not consistent with a Hebbian model of competition between intact and deprived inputs (Gabriele et al., 2000b; Franklin et al., 2006). As illustrated in Figure 6, after unilateral cochlear ablation, both the major crossed and the lesser uncrossed projections from DNLL to IC are affected. In that case, in the IC contralateral to unilateral ablation the unaffected crossed projection must compete with affected ipsilateral DNLL projections. If Hebbian rules apply, then some ipsilateral connections would be displaced by crossed projections influenced by the intact cochlea. Instead the results suggest that the affected crossed projections expanded into space occupied by unaffected uncrossed projections.
Potential mechanisms
Before the onset of hearing, rhythmic bursts of spontaneous activity originate across the basilar membrane of the cochlea (Walsh and McGee, 1987; Kotak and Sanes, 1995; Lippe, 1995). Thus, patterns of spontaneous activity propagated through ascending pathways from the cochlear may influence development of synaptic circuits in central pathways (Friauf and Lohmann, 1999; Illing, 2001). Cochlear ablation may influence activity of both presynaptic and postsynaptic components in IC circuits as indicated in Figure 6. In the present experimental paradigm the influence on presynaptic and postsynaptic activity in IC would be symmetric after bilateral cochlear ablation. This state is contrasted with the imbalances in the unilateral cochlear ablation paradigm studied previously (Franklin et al., 2007; Gabriele et al., 2001). Disruption of bands in both the present study (bilateral ablation group) and the previous unilateral cochlear ablation studies is associated with the reduction of the influence of the cochlea on IC presynaptic activity and not on an imbalance of presynaptic activity.
Together with results of previous unilateral cochlear ablation studies (Gabriele et al., 2000b; Franklin et al., 2006), the present observation that bilateral ablation in neonatal rat pups disrupts the banded pattern of DNLL projections prior to hearing onset suggest that spontaneous activity has a permissive role in development of neural circuits in IC layers. That is, differences in the level or pattern of spontaneous activity between various inputs may not regulate which afferent projections end in specific banded compartments. Instead it may be that spontaneous activity is required for axons that have reached their basic targets to recognize spatially distributed molecular markers and/or to be regulated by trophic signaling (e.g., Friauf and Lohmann, 1999).
Similar permissive roles for spontaneous-activity in development of neural circuits have been supported by findings in other sensory systems (McLaughlin and O’Leary, 2005; Ruthazer and Cline, 2004). It is likely that mechanisms influencing the development and refinement of neural circuits in the IC are conserved between sensory systems (Pallas et al., 2006). A small but growing body of information describing the expression of pattern forming molecules has addressed the development of the inner ear and the connections between the cochlea and spiral ganglion in mammals (Lee et al., 1996; Bianchi and Gale, 1998; Cramer, 2005). Indeed there is preliminary evidence that members of the Eph-ephrin family of guidance molecules are distributed in banded layers in postnatal rat pups (Shahmoradian et al., 2005; Gabriele et al., 2005). Once confirmed, additional studies will be needed to show whether spontaneous activity is required for afferent axons to respond to these cues.
In conclusion, deprived of normal input to central auditory pathways from the cochlea as in previous unilateral cochlear ablation studies or the present bilateral cochlear ablation study, the banded pattern of DNLL projections to the central nucleus of the IC is not maintained or refined. It will be important for future experiments to determine whether or not the changes observed at P12 in afferent band organization in IC after earlier postnatal cochlear ablation simply reflect a delay in maturation or persist with age. The possible impact of cochlear ablation on spontaneous activity in IC circuits and the spontaneous activity-dependent and independent mechanisms that guide formation and refinement of afferent projections to IC remain to be further elucidated.
Acknowledgments
Supported in part by NIH grant DC004412 and the Mr. and Mrs. A. Tab Williams, Jr. and Family Neuroscience Research and Program Development Endowment.
The authors would like to thank Stephanie Evans for her technical support and Dr. John McHaffie for his comments of the text.
ABBREVIATIONS
- CNIC
central nucleus of the inferior colliculus
- CN
cochlear nucleus
- Co
cochlea
- DCIC
dorsal cortex of the inferior colliculus
- DiI
1,1′-dioctadecyl-3,3,3′,3′tetramethylindocarbocyanine perchlorate
- DNLL
dorsal nucleus of the lateral lemniscus
- ECIC
external cortex of the inferior colliculus
- IC
inferior colliculus
- LSO
lateral superior olivary nucleus
- P
postnatal day
- SOC
superior olivary complex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.Aitkin LM, Moore DR. Inferior colliculus. II Development of tuning characteristics and tonotopic organization in central nucleus of the neonatal cat. J Neurophysiol. 1975;38:1208–1216. doi: 10.1152/jn.1975.38.5.1208. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi LM, Gale NW. Distribution of Eph-related molecules in the developing and mature cochlea. Hear Res. 1998;117:161–172. doi: 10.1016/s0378-5955(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 3.Brunso-Bechtold JK, Henkel CK. Development of auditory afferents to the central nucleus of the inferior colliculus. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. Springer-Verlag; New York: 2005. pp. 537–558. [Google Scholar]
- 4.Cant NB. Structural development of the mammalian central auditory pathways. In: Rubel EW, Popper AN, Fay RR, editors. Development of the auditory system. Vol. 9. Springer-Verlag; New York: 1997. pp. 315–413. [Google Scholar]
- 5.Cramer KS. Eph proteins and the assembly of auditory circuits. Hear Res. 2005;206:42–51. doi: 10.1016/j.heares.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Ehret G, Romand R. Development of tone response thresholds, latencies and tuning in the mouse inferior colliculus. Dev Brain Res. 1992;67:317–326. doi: 10.1016/0165-3806(92)90233-m. [DOI] [PubMed] [Google Scholar]
- 7.Franklin SR, Brunso-Bechtold JK, Henkel CK. Bilateral cochlea ablation disrupts the development of banded DNLL afferent projections in the rat inferior colliculus. Assoc Res Otolaryngol Abs. 2004:871. [Google Scholar]
- 8.Franklin SR, Brunso-Bechtold JK, Henkel CK. Unilateral cochlear ablation before hearing onset disrupts the maintenance of dorsal nucleus of the lateral lemniscus projection patterns in the rat inferior colliculus. Neurosci. 2006;143:105–115. doi: 10.1016/j.neuroscience.2006.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friauf E, Lohmann C. Development of auditory brainstem circuitry. Activity-dependent and activity-independent processes. Cell Tissue Res. 1999;297:187–195. doi: 10.1007/s004410051346. [DOI] [PubMed] [Google Scholar]
- 10.Gabriele ML, Brunso-Bechtold JK, Henkel CK. Development of afferent patterns in the inferior colliculus of the rat: projection from the dorsal nucleus of the lateral lemniscus. J Comp Neurol. 2000a;416:368–382. doi: 10.1002/(sici)1096-9861(20000117)416:3<368::aid-cne8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.Gabriele ML, Brunso-Bechtold JK, Henkel CK. Plasticity in the development of afferent patterns in the inferior colliculus of the rat after unilateral cochlear ablation. J Neurosci. 2000b;20:6939–6949. doi: 10.1523/JNEUROSCI.20-18-06939.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabriele ML, Robenolt J, Laz A, Jaynes D. Involvement of ephrins and Eph receptors in establishing early pattern formation in the auditory midbrain. Assoc Res Otolaryngol Abs. 2005:317. [Google Scholar]
- 13.Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci. 2005;8:332–8. doi: 10.1038/nn1397. [DOI] [PubMed] [Google Scholar]
- 14.Hardie NA, Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hear Res. 1999;128:147–165. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- 15.Hashisaki GT, Rubel EW. Effects of unilateral cochlea removal on anteroventral cochlear nucleus neurons in developing gerbils. J Comp Neurol. 1989;283:465–473. doi: 10.1002/cne.902830402. [DOI] [PubMed] [Google Scholar]
- 16.Henkel CK, Gabriele ML, McHaffie JG. Quantitative assessment of developing afferent patterns in the cat inferior colliculus revealed with calbindin immunohistochemistry and tract tracing methods. Neurosci. 2005;136:945–955. doi: 10.1016/j.neuroscience.2005.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henkel CK, Keiger CJ, Franklin SR, Brunso-Bechtold JK. Development of banded afferent compartments in the inferior colliculus before onset of hearing in ferrets. Neurosci. 2007;146:225–235. doi: 10.1016/j.neuroscience.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Illing RB. Activity-dependent plasticity in the adult auditory brainstem. Audiol Neurootol. 2001;6:319–345. doi: 10.1159/000046844. [DOI] [PubMed] [Google Scholar]
- 19.Kandler K. Activity-dependent organization of inhibitory circuits: lessons from the auditory system. Curr Opin Neurobiol. 2004;14:96–104. doi: 10.1016/j.conb.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Kandler K, Gillespie DC. Developmental refinement of inhibitory sound-localization circuits. Trends Neurosci. 2005;28:290–296. doi: 10.1016/j.tins.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci. 2003;6:282–290. doi: 10.1038/nn1015. [DOI] [PubMed] [Google Scholar]
- 22.Kitzes LM, Kageyama GH, Semple MN, Kil J. Development of ectopic projections from the ventral cochlear nucleus to the superior olivary complex induced by neonatal ablation of the contralateral cochlea. J Comp Neurol. 1995;353:341–363. doi: 10.1002/cne.903530303. [DOI] [PubMed] [Google Scholar]
- 23.Koch U, Sanes DH. Afferent regulation of glycine receptor distribution in the gerbil LSO. Microsc Res Tech. 1998;41:263–269. doi: 10.1002/(SICI)1097-0029(19980501)41:3<263::AID-JEMT9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 24.Kotak VC, Sanes DH. Synaptically evoked prolonged depolarizations in the developing auditory system. J Neurophysiol. 1995;74:1611–1620. doi: 10.1152/jn.1995.74.4.1611. [DOI] [PubMed] [Google Scholar]
- 25.Kotak VC, Sanes DH. Gain adjustment of inhibitory synapses in the auditory system. Biol Cybern. 2003;89:363–370. doi: 10.1007/s00422-003-0441-7. [DOI] [PubMed] [Google Scholar]
- 26.Lippe WR. Relationship between frequency of spontaneous bursting and tonotopic position in the developing avian auditory system. Brain Res. 1995;703:205–213. doi: 10.1016/0006-8993(95)01096-3. [DOI] [PubMed] [Google Scholar]
- 27.McLaughlin T, O’Leary DD. Molecular gradients and development of retinotopic maps. Annu Rev Neurosci. 2005;28:327–355. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- 28.Moore DR, Irvine DR. Development of binaural input, response patterns, and discharge rate in single units of the cat inferior colliculus. Exp Brain Res. 1980;38:103–108. doi: 10.1007/BF00237936. [DOI] [PubMed] [Google Scholar]
- 29.Moore DR, Irvine DR. Development of responses to acoustic interaural intensity differences in the cat inferior colliculus. Exp Brain Res. 1981;41:301–309. doi: 10.1007/BF00238887. [DOI] [PubMed] [Google Scholar]
- 30.Moore DR, Kitzes LM. Projections from the cochlear nucleus to the inferior colliculus in normal and neonatally cochlea-ablated gerbils. J Comp Neurol. 1985;240:180–195. doi: 10.1002/cne.902400208. [DOI] [PubMed] [Google Scholar]
- 31.Moore DR, Kowalchuk NE. Auditory brainstem of the ferret: effects of unilateral cochlear lesions on cochlear nucleus volume and projections to the inferior colliculus. J Comp Neurol. 1988;272:503–515. doi: 10.1002/cne.902720405. [DOI] [PubMed] [Google Scholar]
- 32.Moore DR. Auditory brainstem of the ferret: bilateral cochlear lesions in infancy do not affect the number of neurons projecting from the cochlear nucleus to the inferior colliculus. Brain Res Dev Brain Res. 1990;54:125–130. doi: 10.1016/0165-3806(90)90072-7. [DOI] [PubMed] [Google Scholar]
- 33.Moore DR, Russell FA, Cathcart NC. Lateral superior olive projections to the inferior colliculus in normal and unilaterally deafened ferrets. J Comp Neurol. 1995;357:204–216. doi: 10.1002/cne.903570203. [DOI] [PubMed] [Google Scholar]
- 34.Moore DR, Rogers NJ, O’Leary SJ. Loss of cochlear nucleus neurons following aminoglycoside antibiotics or cochlear removal. Ann Otol Rhinol Laryngol. 1998;107:337–343. doi: 10.1177/000348949810700413. [DOI] [PubMed] [Google Scholar]
- 35.Nordeen KW, Killackey HP, Kitzes LM. Ascending auditory projections to the inferior colliculus in the adult gerbil, Meriones unquiculatus. J Comp Neurol. 1983;214:131–143. doi: 10.1002/cne.902140203. [DOI] [PubMed] [Google Scholar]
- 36.Oliver DL, Shneiderman A. The anatomy of the inferior colliculus: a cellular basis for integration of monaural and binaural information. In: Altschuler RA, et al., editors. Neurobiology of hearing: The central auditory system. Raven Press; New York: 1989. [Google Scholar]
- 37.Oliver DL, Beckius GE, Bishop DC, Kuwada S. Simultaneous anterograde labeling of axonal layers from lateral superior olive and dorsal cochlear nucleus in the inferior colliculus of cat. J Comp Neurol. 1997;382:215–229. doi: 10.1002/(sici)1096-9861(19970602)382:2<215::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Pallas SL, Wenner P, Gonzalez-Islas C, Fagiolini M, Razak KA, Kim G, Sanes D, Roerig B. Developmental plasticity of inhibitory circuitry. J Neurosci. 2006;41:10358–61. doi: 10.1523/JNEUROSCI.3516-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubel EW, Hyson RL, Durham D. Afferent regulation of neurons in the brain stem auditory system. J Neurobiol. 1990;21:169–196. doi: 10.1002/neu.480210112. [DOI] [PubMed] [Google Scholar]
- 40.Ruthazer ES, Cline HT. Insights into activity-dependent map formation from the retinotectal system: a middle-of-the-brain perspective. J Neurobiol. 2004;59:134–146. doi: 10.1002/neu.10344. [DOI] [PubMed] [Google Scholar]
- 41.Sanes DH, Friauf E. Development and influence of inhibition in the lateral superior olivary nucleus. Hear Res. 2000:46–58. doi: 10.1016/s0378-5955(00)00119-2. 2000. [DOI] [PubMed] [Google Scholar]
- 42.Sanes DH, Takács C. Activity-dependent refinement of inhibitory connections. Eur J Neurosci. 1993;5:570–574. doi: 10.1111/j.1460-9568.1993.tb00522.x. [DOI] [PubMed] [Google Scholar]
- 43.Schreiner CE, Langner G. Periodicity coding in the inferior colliculus of the cat. II Topographical organization. J Neurophysiol. 1988;60:1823–1840. doi: 10.1152/jn.1988.60.6.1823. [DOI] [PubMed] [Google Scholar]
- 44.Semple MN, Aitkin LM. Representation of sound frequency and laterality by units in central nucleus of cat inferior colliculus. J Neurophysiol. 1979;42:1626–1639. doi: 10.1152/jn.1979.42.6.1626. [DOI] [PubMed] [Google Scholar]
- 45.Shahmoradian SH, James RL, Simpson NS, Gabriele ML. Program No. 942.2. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience, Online; 2005. Banded ephrin-B3 expression patterns in the neonatal rat inferior colliculus. [Google Scholar]
- 46.Shatz CJ. Emergence of order in visual system development. Proc Natl Acad Sci USA. 1996;93:602–608. doi: 10.1073/pnas.93.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shneiderman A, Henkel CK. Banding of lateral superior olivary nucleus afferents in the inferior colliculus: a possible substrate for sensory integration. J Comp Neurol. 1987;266:519–534. doi: 10.1002/cne.902660406. [DOI] [PubMed] [Google Scholar]
- 48.Shneiderman A, Oliver DL, Henkel CK. Connections of the dorsal nucleus of the lateral lemniscus: an inhibitory parallel pathway in the ascending auditory system? J Comp Neurol. 1988;276:188–208. doi: 10.1002/cne.902760204. [DOI] [PubMed] [Google Scholar]
- 49.Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- 50.Tierney TS, Russell FA, Moore DR. Susceptibility of developing cochlear nucleus neurons to deafferentation-induced death abruptly ends just before the onset of hearing. J Comp Neurol. 1997;378:295–306. doi: 10.1002/(sici)1096-9861(19970210)378:2<295::aid-cne11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 51.Trune DR. Influence of neonatal cochlear removal on the development of mouse cochlear nucleus: I. Number, size, and density of its neurons. J Comp Neurol. 1982;209:409–424. doi: 10.1002/cne.902090410. [DOI] [PubMed] [Google Scholar]
- 52.Walsh EJ, Mcgee J. Postnatal development of auditory nerve and cochlear nucleus neuronal responses in kittens. Hearing Res. 1987;28:97–116. doi: 10.1016/0378-5955(87)90157-2. [DOI] [PubMed] [Google Scholar]
- 53.Wenstrup JJ, Ross LS, Pollak GD. Binaural response organization within a frequency-band representation of the inferior colliculus: implications for sound localization. J Neurosci. 1986;6:963–973. doi: 10.1523/JNEUROSCI.06-04-00962.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]