Abstract
Background
It is generally appreciated that gestational diabetes is a risk factor for type 2 diabetes. However, the precise relation between these 2 conditions remains unknown. We sought to determine the incidence of diabetes mellitus after diagnosis of gestational diabetes.
Methods
We used a population-based database to identify all deliveries in the province of Ontario over the 7-year period from Apr. 1, 1995, to Mar. 31, 2002. We linked these births to mothers who had been given a diagnosis of gestational diabetes through another administrative database that records people with diabetes on the basis of either physician service claims or hospital admission records. We examined database records for these women from the time of delivery until Mar. 31, 2004, a total of 9 years. We determined the presence of diabetes mellitus according to a validated administrative database definition for this condition.
Results
We identified 659 164 pregnant women who had no pre-existing diabetes. Of these, 21 823 women (3.3%) had a diagnosis of gestational diabetes. The incidence of gestational diabetes rose significantly over the 9-year study period, from 3.2% in 1995 to 3.6% in 2001 (p < 0.001). The probability of diabetes developing after gestational diabetes was 3.7% at 9 months after delivery and 18.9% at 9 years after delivery. After adjustment for age, urban or rural residence, neighbourhood income quintile, whether the woman had a previous pregnancy, whether the woman had hypertension after the index delivery, and primary care level before the index delivery, the most significant risk factor for diabetes was having had gestational diabetes during the index pregnancy (hazard ratio 37.28, 95% confidence interval 34.99–40.88; p < 0.001). Age, urban residence and lower income were also important factors. When analyzed by year of delivery, the rate of development of diabetes was higher among the latest subcohort of women with gestational diabetes (delivery during 1999–2001) than among the earliest subcohort (delivery during 1995 or 1996) (16% by 4.7 years after delivery v. 16% by 9.0 years).
Interpretation
In this large population-based study, the rate of development of diabetes after gestational diabetes increased over time and was almost 20% by 9 years. This estimate should be used by clinicians to assist in their counselling of pregnant women and by policy-makers to target these women for screening and prevention
Recently, the US Centers for Disease Control and Prevention predicted a 3-fold rise in the prevalence of diabetes mellitus in the United States between 2005 and 2050, from 16.2 million to 48.3 million.1 Although evidence to support population-based screening as an approach to stem this epidemic is lacking, targeted screening of high-risk populations has been advocated.2–5 One group at very high risk for diabetes consists of women with a history of gestational diabetes.
During pregnancy, women with gestational diabetes display metabolic abnormalities similar to those of people with type 2 diabetes mellitus, such as insulin resistance and reduced β-cell compensation for that resistance.6 After delivery, most of these women return to a euglycemic state, but they are at increased risk for overt type 2 diabetes in the future. The rates of development of type 2 diabetes among women with previous gestational diabetes quoted in the literature have been extremely variable, between 3% and 70%.7–11 Aside from genetic differences among populations, this large variation in the subsequent development of type 2 diabetes may also be due to the use of diverse tests for glucose tolerance in pregnancy, selection bias and, in particular, duration of follow-up.9
In light of a growing body of evidence that it is possible to delay the development of diabetes among those at high risk,12–16 it is important to determine the true risk of type 2 diabetes by means of a population-based study; this will allow accurate assessment of the cost-effectiveness and appropriateness of postpartum case management and screening. We sought to determine the incidence of diabetes mellitus in the years following a diagnosis of gestational diabetes.
Methods
Data sources
We used a national database of hospital discharge information prepared by the Canadian Institute for Health Information to identify all deliveries that occurred in Ontario over the 7-year period from Apr. 1, 1995, to Mar. 31, 2002. For women with multiple deliveries during the period of interest, one delivery was selected at random as the index pregnancy. We identified women with a diagnosis of diabetes before the index pregnancy using the Ontario Diabetes Database,17 a validated administrative data registry of Ontario residents with diagnosed diabetes; these women were excluded from the study cohort. The Ontario Diabetes Database includes all patients for whom a diagnosis of diabetes is recorded in hospital discharge information or in claims for outpatient physician services (through the Ontario Health Insurance Plan). For patients meeting these criteria, the earliest record referring to a diagnosis of diabetes is deemed to be the date of diagnosis. The database has been validated against data taken from primary care charts and was found to have a sensitivity of 86% and a specificity of greater than 97%.17
Study population and eligibility
We identified women with gestational diabetes by examining administrative data records for the latter half of each pregnancy or the immediate postpartum period, when follow-up visits for gestational diabetes could be expected to occur. Although specific diagnosis codes for gestational diabetes exist within the International Classification of Diseases (9th and 10th revisions), they are not reliably used, and many women with gestational diabetes are simply coded as having diabetes. Accordingly, any woman with a diagnosis code for gestational diabetes or diabetes in either a physician service claim to the Ontario Health Insurance Plan or Canadian Institute for Health Information discharge information during the period from 150 days before the index delivery to 90 days after was deemed to have had gestational diabetes. Women were excluded if they were younger than 16 or older than 50 years of age, if there was no recorded postal code or local health integration network code, or if they died or left Ontario before the start of follow-up.
Data collection and outcomes
We examined database records for women in the study cohort from the time of delivery until Mar. 31, 2004, and recorded a diagnosis of postpartum diabetes for any women who were entered into the Ontario Diabetes Database during that period. The Ontario Diabetes Database does not distinguish between type 1 and type 2 diabetes; however, the majority of women in whom diabetes develops after gestational diabetes tend to have type 2 diabetes.8–11,18–22 We obtained a range of related diagnostic and demographic characteristics and indicators of health-service use for adjustment of multivariable models. We assessed general comorbidity for the index delivery using the Charlson Comorbidity Index.23 This index, a simple, valid method of estimating the risk of death from comorbid disease, has been used in many previous studies. We examined physician service claims for the 2 years before the index delivery to determine the frequency of primary care visits (including prenatal visits) and whether there was a regular provider of primary care (defined as a single provider accounting for at least half of the woman's primary care visits). Income data were not available on an individual basis but were estimated on the basis of neighbourhood characteristics in census data obtained from Statistics Canada. We used residential postal code data to link the patients to their census subdivisions, and we then attributed the median household income for that census unit to the individuals living within it. We identified hypertension on the basis of any physician service claim or hospital discharge information that recorded a diagnosis of hypertension following the index delivery. We used census definitions to determine urban or rural residential status.
Statistical analysis
We calculated descriptive statistics to describe the study cohort. We used the Cochran Armitage test24 to assess changes in the rate of gestational diabetes over time and the Kaplan–Meier method to calculate the cumulative incidence rate for the development of postpartum diabetes. We evaluated risk factors leading to the development of diabetes after the index pregnancy using a Cox proportional hazards model. For all analyses we used a 2-tailed p value of 0.05 as the threshold for statistical significance.
Results
From Apr. 1, 1995, to Mar. 31, 2002, we identified 914 971 deliveries involving 674 647 women in Ontario. Using the eligibility criteria for the study, we included 659 164 of the women in our analysis (Figure 1). Women who were removed from the Ontario Registered Persons Database (i.e., those who moved from the province and were therefore excluded) were slightly younger (mean 28.4 [standard deviation (SD) 5.6] years) than those who were entered into the Ontario Diabetes Database (mean 30.5 [SD 5.5] years) and those whose records were followed until the end of the study period (mean 29.3 [SD 5.5] years) (p < 0.001).
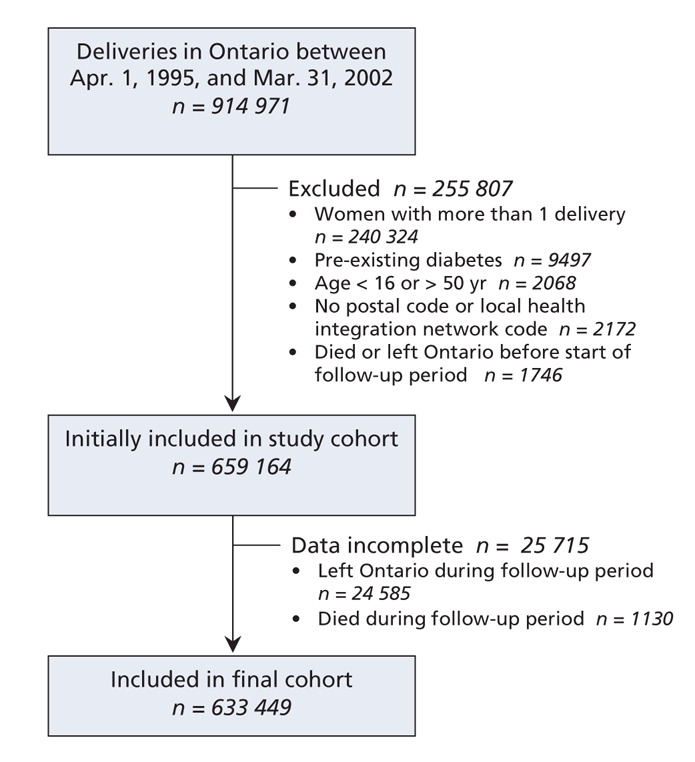
Figure 1: Identification of cohort (using a national database of hospital discharge information prepared by the Canadian Institute for Health Information) and loss to follow-up.
Development of gestational diabetes
Incidence of gestational diabetes
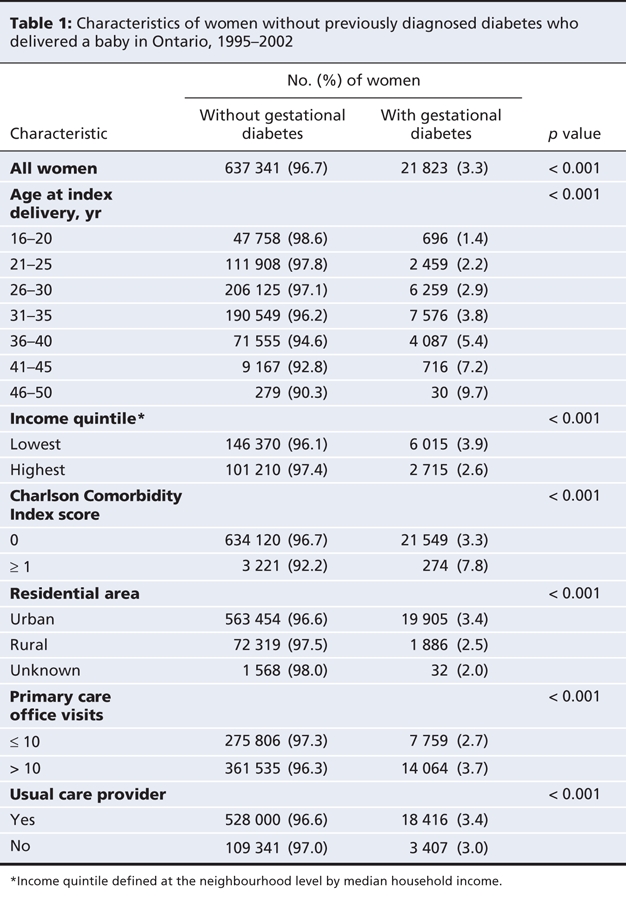
The overall incidence of gestational diabetes in Ontario rose from 3.2% in 1995 to 3.6% in 2001 (p < 0.001). The incidence of gestational diabetes increased with increasing age of the women at delivery (Table 1). In particular, the rate of gestational diabetes was 9.7% among women 46–50 years of age but only 1.4% among those 16–20 years of age (p < 0.001). The likelihood of having gestational diabetes was higher among women with a Charlson Comorbidity Index score of 1 or greater than among women whose comorbidity index was 0 (7.8% v. 3.3%; p < 0.001). Women from higher-income neighbourhoods were less likely to have gestational diabetes (2.6% for those in the highest income quintile v. 3.9% for those in the lowest income quintile; p < 0.001). Women from urban areas were more likely than women living in rural areas to have gestational diabetes (3.4% v. 2.5%; p < 0.001).
Table 1
Access to care
Women with 10 or fewer primary care visits within the 2 years before the index delivery were less likely to receive a diagnosis of gestational diabetes than women with more than 10 visits (2.7% v. 3.7%; p < 0.001). As well, women without a usual care provider were less likely to receive a diagnosis of gestational diabetes than women who had a usual care provider (3.0% v. 3.4%; p < 0.001).
Development of diabetes after delivery
We examined database records for the women included in the study for a maximum of 9 years and a median of 5.4 years after delivery. The rate of development of diabetes among women who had had gestational diabetes increased rapidly during the first 9 months postpartum (Figure 2) and remained reasonably constant thereafter. The probability of development of diabetes was 3.7% at 9 months, 4.9% at 15 months and 13.1% at 5.2 years. By the end of the 9 years of follow-up, 3.9% of the women had been lost to follow-up (Figure 1). Among those remaining, 2874 women (18.9%) with prior gestational diabetes had received a diagnosis of diabetes; in contrast, the rate was only 2.0% among women who did not have gestational diabetes. When analyzed by year group, those who had gestational diabetes in the latest period (delivery during 1999–2001) had a higher risk of diabetes (hazard ratio 47.43, 95% confidence interval [CI] 40.59–55.43) than those who had gestational diabetes in the earliest period (delivery during 1995 or 1996) (hazard ratio 31.83, 95% CI 26.70–37.94). The rate of development of diabetes was also higher among those with a later delivery. In the latest subcohort, diabetes developed in 16% of women with gestational diabetes by 4.7 years, whereas it took 9.0 years for the earliest subcohort to reach a rate of 16% (Figure 3).
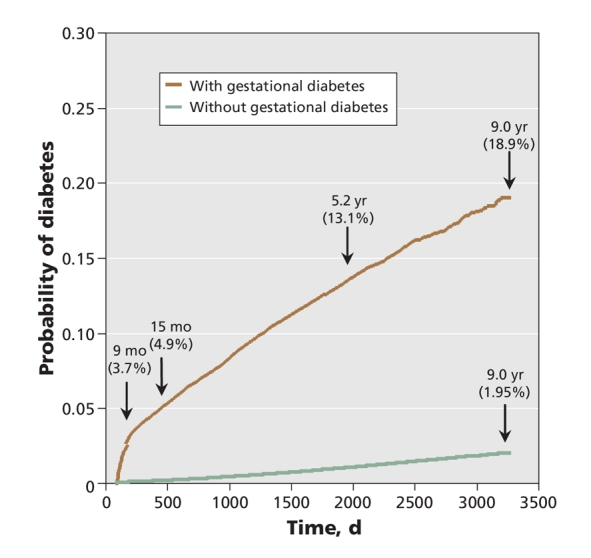
Figure 2: Cumulative incidence rate of diabetes mellitus.
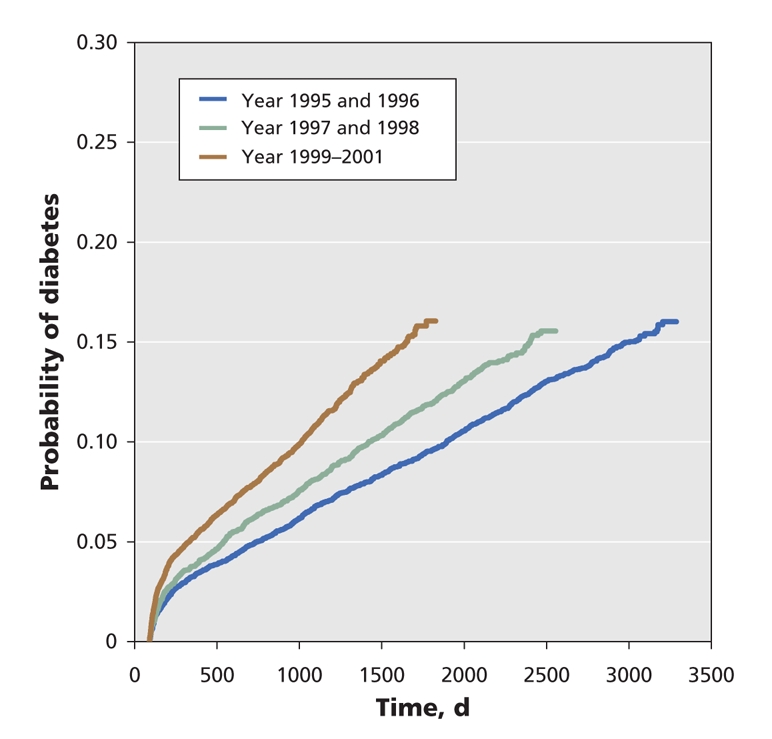
Figure 3: Cumulative incidence rate of diabetes mellitus for women with gestational diabetes by year group.
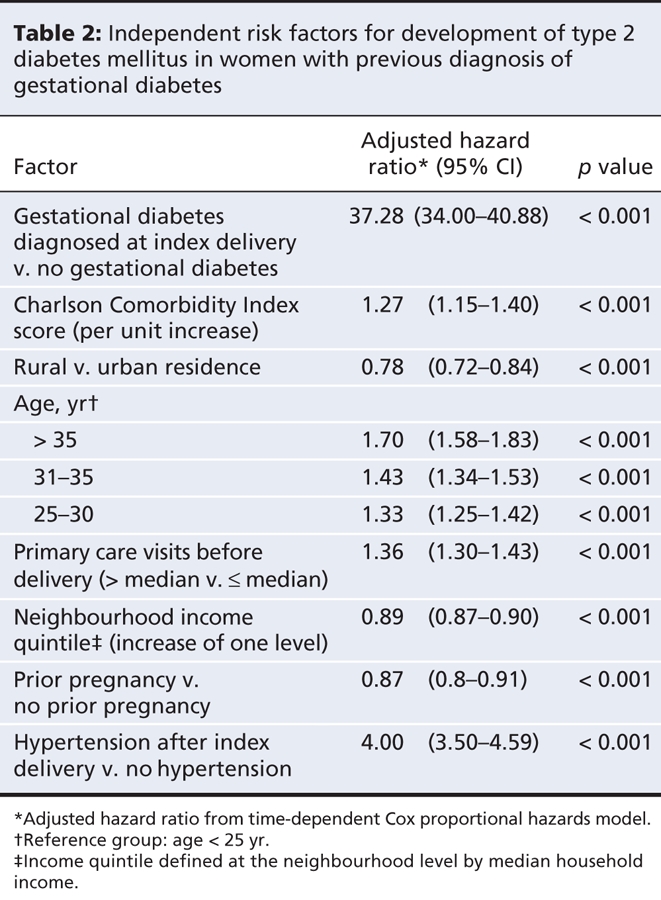
In the multivariable analysis, the most significant factor associated with the risk of development of diabetes after delivery was having had gestational diabetes during the index pregnancy (hazard ratio 37.28, 95% CI 34.00–40.88; p < 0.001) (Table 2). Other factors increasing the risk of development of diabetes after delivery included higher Charlson Comorbidity Index score, greater age, having a greater number of primary care visits in the 2 years before the index delivery and the development of hypertension after the index delivery. Factors that decreased the risk of development of diabetes after the index pregnancy were living in a rural area rather than an urban area, having a higher income and having had a prior pregnancy within 4 years of the index pregnancy.
Table 2
Interpretation
In this large, population-based study, we found that diabetes developed within 9 years after the index pregnancy in 18.9% of women with previous gestational diabetes; this rate was much higher than the rate among women without gestational diabetes (2.0%). This cumulative incidence is also higher than what has been reported for certain populations followed for a similar period8 but lower than for other populations known to have high rates of diabetes.25–27 For example, these observations are similar to the rate of 17% noted among white Australian women28 and the rate of 22% observed over 10 years in a small study involving Nova Scotian women.29 Although others found that the rate increased markedly until 5 years post partum and plateaued at 10 years,9 the cumulative incidence rate in our study showed no signs of a plateau.
The rate of development of diabetes was rapid in the first 9 months post partum and remained relatively constant thereafter. More specifically, diabetes had been diagnosed in 3.7% of the women by 9 months. These women probably had previously undiagnosed type 2 diabetes that was discovered through screening for gestational diabetes in pregnancy. A study in Spain found a similar rate of diabetes immediately post partum (5.4% by 6 months).30 In that study, there was an association between postpartum glucose intolerance and other cardiovascular risk factors such as triglyceride levels, blood pressure, obesity and regional distribution of body fat, which underscores the potential risk that these women carry for cardiovascular disease. Another study revealed that women with undiagnosed type 2 diabetes in pregnancy had worse perinatal outcomes than women known to have type 2 diabetes,31 most likely because of lack of proper care before the diagnosis. For this reason it is imperative to identify this high-risk group as early in the pregnancy as possible, and ideally before pregnancy.
The most significant risk factor for the development of diabetes was previous gestational diabetes. This finding is reasonable, given that the presence of gestational diabetes identifies women with a defect in β-cell function, in whom insulin secretion does not increase adequately in response to the insulin-resistant state of pregnancy. The same defect in β-cell function predisposes some women to overt diabetes in the ensuing years.32 We found that the latest subcohort of women with gestational diabetes (delivery during 1999–2001) had a higher rate of development of diabetes than those of the earliest subcohort (delivery during 1995 or 1996). Women with gestational diabetes in the latest subcohort reached a cumulative incidence rate of 16% by 4.66 years, whereas it took the earliest subcohort 9 years to reach a similar incidence rate. This result implies that the risk of development of diabetes among those with a history of gestational diabetes is rising over time. A similar phenomenon was seen in a study performed in Denmark, where the incidence of diabetes was higher in a recent cohort (delivery during 1987–1996) than in an earlier one (delivery during 1978–1985).33 The authors speculated that this shift related to the significantly higher prepregnancy body mass index in the more recent group. However, the explanation for the finding in our cohort is unclear and merits further study.
In accordance with other studies from the United States,34,35 we found that the incidence of gestational diabetes is increasing in Ontario. Women were more likely to have gestational diabetes and were more likely to have a subsequent diagnosis of diabetes if they had a lower income and lived in an urban setting. This may reflect the large South and East Asian and black populations living in urban areas, who have a higher risk of type 2 diabetes. Women were less likely to receive a diagnosis of gestational diabetes if they had suboptimal care (i.e., 10 or fewer visits to a physician in the 2 years before delivery) and if they did not have a physician providing usual care, probably because these situations indicate less opportunity to make the diagnosis.
The main strength of our study lies in the fact that it was a large population-based study involving more than 21 000 women with gestational diabetes, with up to 9 years of follow-up. Unlike other studies, it covered a large, well-defined geographic region with a population of about 13 million, which allowed us to make a more robust assessment of the risk of type 2 diabetes after gestational diabetes than has been possible in previous studies. The validated Ontario Diabetes Database, with its high sensitivity and specificity, provides confidence that these data accurately reflect the rate of development of clinical diabetes after a diagnosis of gestational diabetes. In addition, our attrition rate was much lower than that noted in many of the other studies that have attempted to follow women with gestational diabetes.4–6
Some important limitations of our study include our inability to assess the effect of ethnicity, obesity and level of fasting glucose during pregnancy, risk factors that are clearly associated with the development of diabetes but that are unavailable for population-based administrative data. If we had had access to these data, the independent risk related to gestational diabetes might have been less striking. In addition, we might have underestimated the true incidence of diabetes because some women moved out of the province; however, outmigration was a censoring variable in the survival models, so its impact should be small. Given the very large sample, some of the hazard ratios are of uncertain clinical significance, even though statistical significance was achieved (e.g., the increased risk of diabetes with higher Charlson Comorbidity Index score).
Our study has important implications. Although population-based screening for diabetes has been rejected in many jurisdictions as inefficient, targeted screening in high-risk populations has been widely accepted. The risk level for women with prior gestational diabetes as defined by this study suggests that these women may benefit from both preventive interventions and regular screening. In addition, more robust estimates of risk may allow policy-makers to evaluate more accurately the cost and potential impact of such programs.
@@ See related commentary by Simmons, page 215
Supplementary Material
Acknowledgments
We thank the Banting and Best Diabetes Centre for its funding of this project. Janet Hux receives salary support from the Institute for Clinical Evaluative Sciences. The opinions, results and conclusions are those of the authors, and no endorsement by the Ontario Ministry of Health and Long-Term Care or by the Institute for Clinical Evaluative Sciences is intended or should be inferred.
Footnotes
Une version française de ce résumé est disponible à l'adresse www.cmaj.ca/cgi/content/full/179/3/229/DC1
This article has been peer reviewed.
Contributors: Denice Feig designed the study, participated in the analysis and interpretation of data, and wrote the manuscript. Bernard Zinman participated in the design of the study and the interpretation of data and contributed to the writing of the manuscript. Xuesong Wang participated in the design of the study, performed the analysis of the data, participated in its interpretation, and contributed to revision of the manuscript. Janet Hux participated in the design of the study and in the analysis and interpretation of data and contributed to the writing of the manuscript.
Competing interests: None declared.
Correspondence to: Dr. Denice S. Feig, Mount Sinai Hospital, Ste. 5027, Lebovic Building, 60 Murray St., Toronto ON M5T 3L9; fax 416 361-2657; d.feig@utoronto.ca
REFERENCES
- 1.Narayan KM, Boyle JP, Geiss LS, et al. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care 2006;29:2114-6. [DOI] [PubMed]
- 2.Janssen PG, Gorter KJ, Stolk RP, et al. Low yield of population-based screening for type 2 diabetes in the Netherlands: the ADDITION Netherlands study. Fam Pract 2007;24:555-61. [DOI] [PubMed]
- 3.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2003 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2003:27 Suppl 2:S1-152. [DOI] [PubMed]
- 4.American Diabetes Association. Standards of medical care in diabetes. II. Screening for diabetes. Diabetes Care 2007;30 Suppl 1:S5-7. [DOI] [PubMed]
- 5.US Preventive Services Task Force. Screening for type 2 diabetes mellitus in adults: recommendations and rationale. Ann Intern Med 2003;138:212-4. [DOI] [PubMed]
- 6.Xiang AH, Peters RK, Trigo E, et al. Multiple metabolic defects during late pregnancy in women at high risk for type 2 diabetes mellitus. Diabetes 1999;48:848-54. [DOI] [PubMed]
- 7.O'Sullivan JB. Diabetes mellitus after GDM. Diabetes 1991;40 Suppl 2:131-5. [DOI] [PubMed]
- 8.Albareda M, Caballero A, Badell G, et al. Diabetes and abnormal glucose tolerance in women with previous gestational diabetes. Diabetes Care 2003;26:1199-205. [DOI] [PubMed]
- 9.Kim C, Newton K, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes. Diabetes Care 2002;25:1862-8. [DOI] [PubMed]
- 10.Damm P. Diabetes following gestational diabetes mellitus. In: Dornhorst A, Hadden DR, editors. Diabetes and pregnancy. Chichester (UK): Wiley; 1996. p. 341-50.
- 11.Schaefer-Graf UM, Buchanan TA, Xiang AH, et al. Clinical predictors for a high risk for the development of diabetes mellitus in the early puerperium in women with recent gestational diabetes mellitus. Am J Obstet Gynecol 2002;186:751-6. [DOI] [PubMed]
- 12.Xiang AH, Peters RK, Kjos SL, et al. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes 2006;55:517-22. [DOI] [PMC free article] [PubMed]
- 13.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. Finnish Diabetes Prevention Study. N Engl J Med 2001;344:1343-50. [DOI] [PubMed]
- 14.Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403. [DOI] [PMC free article] [PubMed]
- 15.Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomized trial. Lancet 2002;359:2072-7. [DOI] [PubMed]
- 16.DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators, Gerstein HC, Yusuf S, Bosch J et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096-105. [DOI] [PubMed]
- 17.Hux JE, Ivis F, Flintoft V, et al. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002;25:512-6. [DOI] [PubMed]
- 18.Lobner K, Knopff A, Baumgarten A, et al. Predictors of postpartum diabetes in women with gestational diabetes. Diabetes 2006;55:792-7. [DOI] [PubMed]
- 19.Kjos SL, Buchanan TA, Greenspoon JS, et al. Gestational diabetes mellitus: the prevalence of glucose intolerance and diabetes mellitus in the first two months postpartum. Am J Obstet Gynecol 1990;163:93-8. [DOI] [PubMed]
- 20.Mohamed N, Dooley J. Gestational diabetes and subsequent development of NIDDM in aboriginal women of northwestern Ontario. Int J Circumpolar Health 1998;57 Suppl 1:355-8. [PubMed]
- 21.Catalano PM, Vargo KM, Bernstein IM, et al. Incidence and risk factors associated with abnormal postpartum glucose tolerance in women with gestational diabetes. Am J Obstet Gynecol 1991;165:914-9. [DOI] [PubMed]
- 22.Henry OA, Beischer NA. Long-term implications of gestational diabetes for the mother. Baillieres Clin Obstet Gynaecol 1991;5:461-83. [DOI] [PubMed]
- 23.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. [DOI] [PubMed]
- 24.Agresti A. Categorical data analysis. 2nd ed. New York: Wiley-Interscience; 2002.
- 25.Kjos SL, Peters R, Xiang A, et al. Predicting future diabetes in Latino women with gestational diabetes: utility of early postpartum glucose tolerance testing. Diabetes 1995;44:586-91. [DOI] [PubMed]
- 26.Steinhart JR, Sugarman JR, Connell FA. Gestational diabetes is a herald of NIDDM in Navajo women. High rate of abnormal glucose tolerance after GDM. Diabetes Care 1997;20:943-7. [DOI] [PubMed]
- 27.Benjamin E, Mayfield J, Winters D, et al. Diabetes in pregnancy in Zuni Indian women: prevalence and subsequent development of clinical diabetes after gestational diabetes. Diabetes Care 1993;16:1231-5. [DOI] [PubMed]
- 28.Lee AJ, Hiscock RJ, Wein P, et al. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes. Diabetes Care 2007;30:878-83. [DOI] [PubMed]
- 29.Russell C, Dodds L, Armson BA, et al. Diabetes mellitus following gestational diabetes: role of subsequent pregnancy. BJOG 2008;115:253-60. [DOI] [PubMed]
- 30.Pallardo F, Herranz L, Garcia-Ingelmo T, et al. Early postpartum metabolic assessment in women with prior gestational diabetes. Diabetes Care 1999;22:1053-8. [DOI] [PubMed]
- 31.Cundy T, Gamble G, Townend K, et al. Perinatal mortality in type 2 diabetes mellitus. Diabet Med 2000;17:33-9. [DOI] [PubMed]
- 32.Buchanan TA. Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab 2001;86:989-93. [DOI] [PubMed]
- 33.Lauenborg J, Hansen T, Moller Jensen D, et al. Increasing incidence of diabetes after gestational diabetes. Diabetes Care 2004;27:1194-9. [DOI] [PubMed]
- 34.Dabelea D, Snell-Bergeon JK, Hartsfield CL, et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort. Diabetes Care 2005;28:579-84. [DOI] [PubMed]
- 35.Ferrara A, Kahn HS, Quesenberry CP, et al. An increase in the incidence of gestational diabetes mellitus: northern California, 1991–2000. Obstet Gynecol 2004;103:526-33. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


