Introduction
The safest place to store lipids is the white adipose tissue, but its storage capacity may become saturated resulting in excess of fat “overspilled” to non-adipose tissues. This overspill of fat occurs in apparently opposite pathological states such as lipodistrophy or obesity. When the excess of energy is redirected towards peripheral organs, their initial response is to facilitate the storage of the surplus in the form of triacylglycerol, but the limited triacylglycerol buffer capacity becomes saturated soon. Under these conditions excess of lipids enter alternative non-oxidative pathways that result in production of toxic reactive lipid species that induce organ specific toxic responses leading to apoptosis. Reactive lipids can accumulate in non-adipose tissues of metabolically relevant organs such as pancreatic beta cells, liver, heart and skeletal muscle leading to lipotoxicity, a process that contributes substantially to the pathophysiology of insulin resistance, type 2 diabetes, steatotic liver disease and heart failure. Thus, the metabolic syndrome can pathophysiologically be considered a result of the lipid accumulation in non-adipose tissue.
The effects of this lipotoxic insult can be minimised by several strategies:
decreased incorporation of energy,
a less orthodox approach such as increased adipose tissue expandability and/or
increased oxidation of fat in peripheral organs.
The prevalence of obesity has increased dramatically in the last decades and is now considered a major health problem in industrialized and developing countries. In fact, the current epidemic of obesity has been suggested as the leading cause for the decreased life expectancy forecasted for the next generation [22]. When confronting the rather complex clinical problem of obesity, there are several aspects to be taken into account:
the mechanical problems associated with excess body weight (e.g. osteoarthritis, sleep apnea);
the metabolic syndrome typically associated with obesity, which is probably the most important from the point of view of overall survival; and
the aesthetic and psychological elements which can cause extreme distress. Thus, when considering therapeutic strategies for managing obesity, the ideal scenario would involve the use of treatments aimed at all components associated with this condition. However, depending on the predominant clinical phenotype, one may be forced to prioritise specific aspects of the treatment. This approach may in some instances create paradoxical situations, such as, treating obese individuals with agonists of PPARg, agents that will decrease their metabolic complications while potentially increasing their degree of obesity through adipogenesis.
Thermodynamics and obesity
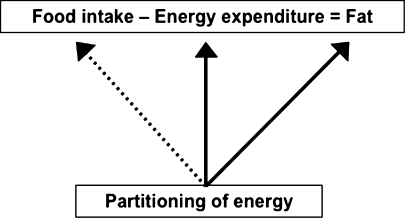
From a thermodynamic perspective, the regulation of body weight can be described as a linear equation (Fig. 1), balancing both food intake and energy expenditure to derive the amount of fat stored. While outwardly this energy balance equation may appear simple, it can be modulated by many other factors, particularly the preferential partitioning of energy towards specific tissues and organs. In fact, it could be argued that some individuals may have an enhanced capacity to extract energy from blood into the white adipose tissue (WAT), thus facilitating fat deposition.
Fig. 1.
Utilization of energy in the regulation of body weight
Adipose tissue expand ability and lipotoxicity
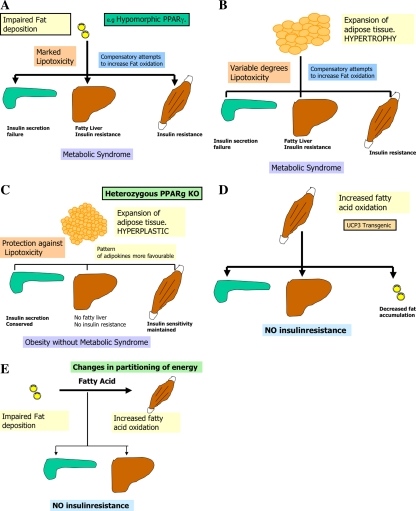
Adipose tissue expandability in response to positive energy balance has been considered traditionally an adaptive passive process. However, recent evidences suggest that the expandability of the adipose tissue is not an unlimited process. In fact adipose tissue expandability may be an important factor determining the appearance of obesity-associated co-morbilities [16]. The importance of the adipose tissue in maintaining energy homeostasis is illustrated by the severity of metabolic defects associated with two apparently opposite syndromes: lipodistrophy and obesity. Lipodystrophy is a syndrome characterized by substantial reduction in fat storage capacity secondary to poor differentiation and expandability of adipose tissue in response to nutritional demands for storage. Such patients lack three crucial adipocyte functions, normal lipid storage capacity, leptin-mediated anorectic effects and leptin-mediated antilipotoxic protection of nonadipose tissues. Despite normal energy intake, lack of adipose tissue results in insulin resistance, elevated serum free fatty acids (FFA) and accumulation of FAs in tissues such as the liver, skeletal muscle and the pancreas (Fig. 2a). Intriguingly, it has been shown recently that patients with lipodystrophy show an increased energy expenditure and FA oxidation after hypercaloric fat load, reflecting a compensatory mechanism to prevent toxic effects [24]. Insufficient attempts by oxidative tissues to dispose the excess of FAs leads to chronic FA deposition and lipotoxicity, followed by insulin resistance, fatty liver and β-cell failure [6]. The other extreme of the spectrum is the obese state, a syndrome resulting from positive energy balance leading to hypertrophic expansion of the WAT (Fig. 2b). Evidence suggests, that hypertrophic obesity is associated with insulin resistance [19], leptin resistance and a spectrum of clinical symptoms that recapitulates the symptomatology observed in lipodystrophy patients. The common link between both of them probably is the defective storage capacity in adipose tissue depots, in the case of lipodistrophy due to lack of proper adipose tissue, in the case of obesity due to saturation of storage capacity due to excessive fat load. Obesity at the expense of hypertrophic adipocytes is associated with insulin resistance (Fig. 2b). Conversely individuals expanding adipose tissue at the expense of increasing the number of adipocytes through a hyperplastic response remain insulin sensitive and healthier (Fig. 2c). Hyperplastic changes have the advantage of retaining insulin sensitivity and a favourable pattern of signalling molecules secreted [8]. In this regard hypertrophy could be a marker of failure in the mechanisms of preadipocyte recruitment [16]. Furthermore, pharmacological remodelation of the adipose tissue facilitating preadipocyte recruitment by thiazolidinediones has been shown at least in rodent models to promote hyperplastic remodulation of the adipose tissue. This is fully supported by the evidence that onset of obesity in young age (<20 years), when the adipocytes are probably able to proliferate and/or differentiate, results in a reduced likelihood of metabolic syndrome [21]. The association between defects in adipose tissue expandability and metabolic complications may depend on other factors, such as the oxidative capacity of skeletal muscle. Apart from increasing storage capacity of fat in “healthy” adipocytes, increasing FA oxidation in muscle can also prevent lipotoxicity. This is illustrated by a transgenic mouse model overexpressing UCP-3 in skeletal muscle [31] or mice overexpressing PGC-1α [20] (Fig. 2d). In this case, the driving force of the phenotype is increased FA oxidation that could act as an energy sink, diverting FA away from storage tissues such as WAT (resulting in a lean phenotype), and also away from other tissues less capable of handling FAs adequately (e.g. liver and beta cells). Evidence from epidemiological studies indicates, that people with an increased FA oxidation, proved by lower RQ (respiratory quotient) show a reduced probability of obesity [3]. Combining both the strategies, prevention of excess fat deposition in WAT and increasing FA oxidation may facilitate safe weight loss and is a target for drug development.
Fig. 2.
Factors affecting fat deposition in different metabolic dysfunctions
Beside form, overall increasing oxidative capacity by e.g. increasing uncoupling mitochondria in muscle, partitioning of fuel can substantially influence energy expenditure. Beneficial metabolic effects might be expected from an agent, which promoted accretion of lean and/or fatty acid oxidation in preference to fat mass. This could be achieved through a reduction in fat deposition and/or an increase in lean tissue (Fig. 2e). While it is still unclear whether defects in the mechanism of fuel partitioning are important causes of human obesity, recently reported genetically modified animal models have provided support for this concept as a potential strategy for the treatment of obesity and its protean complications [15, 30]. In fact mice lacking the enzyme acylCoA: diacylglycerol acyltransferase (DGAT), which catalyses an essential step in triglyceride synthesis, also have an unexplained increase in energy expenditure [15] (Fig. 2e).
SREBPs, gatekeepers of lipotoxicity
Another level of control of the lipotoxic insult in peripheral organs may be the control of lipid transport and functional compartmentalisation into a specific organ. In theory it should be possible in individuals with organ specific susceptibilities to develop beta cell failure, fatty liver or heart failure to devise strategies to spare lipids and protect these organs from lipotoxic insults. This may be accomplished through direct modulation of specific targets involved in lipid related metabolic pathways controlling lipid transport, deposition and specific metabolism in these organs.
Our studies defining the metabolic pathways involved in fatty acid metabolism have identified SREBP1c as a potential gatekeeper for lipotoxicity [1]. SREBP-1c is one of three SREBPs that controls transcription of genes encoding enzymes of lipid biosynthesis in animal cells. We and other groups have established that insulin upregulates SREBP1c mRNA and maturation of SREBP1 protein in parallel with increased expression of FA biosynthesis gene expression [1, 26, 28]. Activation of SREBP increases ACC-catalysed reactions, leading to increased levels of malonyl-CoA, and inhibition of mitochondrial FA oxidation. We have shown that SREBP1c mRNA expression is regulated heterogeneously in adipose tissue and skeletal muscle. In fact, whereas SREBP1 mRNA is reduced in adipose tissue of morbidly obese and diabetic patients [1], levels of expression of SREBP1 mRNA in skeletal muscle are maintained.
PGC and PPARγ
Recent evidence has identified coactiavtors PGC-1α and PGC-1β [25] with its human orthologue PERC [13] as key regulators of the genetic program controlling mitochondrial number and function. These coactivators are involved in processes such as adaptive thermogenesis, glucose and fatty acid oxidation. The results accumulated so far indicate a common function promoting mitochondrial biogenesis to cover basal energy requirements of specific tissues [9, 18].
Peroxisome proliferator-activated receptor-γ (PPARγ) plays a central role in adipogenesis and insulin sensitivity. PPARγ is expressed as two isoforms, PPARγ1 and PPARγ, which differ only in that PPARγ2 has 30 extra amino acids at its NH2 terminus. PPARγ2 is the more potent adipogenic isoform in vitro and is normally restricted to adipose tissues [11], where it is regulated more by nutritional state than PPARγ1 [17]. We have shown that expression of PPARγ isoforms is differentially regulated by nutritional factors [17]. Murine studies showed that PPARγ2 mRNA is markedly downregulated in white adipose tissue (WAT) by fasting and normalized by re-feeding [11]. Similarly, PPARγ2 gene expression is increased in WAT by a high-fat diet (HFD) as well as in mouse models of diet-induced obesity [11]. Studies using genetically modified mouse models have addressed the role of PPARγ in vivo [14]. A proadipogenic role for PPARγ in vivo was supported by the global PPARγ-deficient and the hypomorphic PPARγ mouse models [4, 5, 29]. In addition to a role in promoting adipogenesis, activation of PPARγ also improves insulin sensitivity [2]. However, the characterization of the heterozygous PPARγ knockout mouse provided the paradoxical finding that mice with a 50% reduction in PPARγ gene dosage were resistant to HFD-induced obesity and were more insulin sensitive [10, 23, 27]. Thus, a 50% decrease in PPARγ gene dose at the expense of both isoforms, γ1 and γ2, promoted a similar insulin-sensitizing effect as activating the receptor with a PPARγ-specific agonist.
To elucidate the relevance of the PPARγ2 in vivo, we generated a mouse model in which the PPARγ2 isoform was specifically disrupted. Despite similar weight, body composition, food intake, energy expenditure, and adipose tissue morphology, male mice lacking the γ2 isoform were more insulin resistant than wild-type animals when fed a regular diet [16]. These results indicate that insulin resistance associated with ablation of PPARγ2 is not the result of lipodystrophy and suggests a specific role for PPARγ2 in maintaining insulin sensitivity independently of its effects on adipogenesis. Furthermore, PPARγ2 knockout mice fed with a high-fat diet did not become more insulin resistant than those on a normal diet, despite a marked increase in their mean adipocyte cell size [16]. These findings suggest that PPARγ2 is required for the maintenance of normal insulin sensitivity in mice but also raises the intriguing notion that PPARγ2 may be necessary for the adverse effects of a high-fat diet on carbohydrate metabolism.
Increasing the capacity of storage/fatty acid buffer of the adipose tissue, transforming the structure of adipose tissue
There is evidence that adipocyte cell size [8, 12] may be an important predictor of insulin resistance. Large adipocytes seem to be more insulin resistant, being more susceptible to leak fatty acids inducing lipotoxicity in other organs. Increased secretion of TNFα [7] and resistin and decreased levels of adiponectin are characteristic of large adipocytes from obese individuals. Interestingly, activation of PPARγ induces a remodelling of the adipose tissue structure as a result of changes in the rate of preadipocyte recruitment [23]. The differentiation of new small adipocytes is associated with marked improvement of insulin sensitivity through increased fatty acid buffering capacity, modification of adipokine repertoire and possibly through increases in the amount of cellular membranes that act as a buffering system for cholesterol esters and other lipid signalling molecules. Thus, it is likely that recruitment of new preadipocytes may prevent or reverse some of the metabolic complications associated with obesity [2]. Obviously this strategy should be limited to individuals with strong metabolic risks in whom other strategies aiming to reduce weight have failing.
Acknowledgments
Marc Slawik was supported by a fellowship from the Fritz Thyssen Foundation, Germany.
References
- 1.Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ (2001) Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 291:2613–2616 [DOI] [PubMed]
- 2.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM (1999) PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 4:585–595 [DOI] [PubMed]
- 3.Brochu M, Tchernof A, Dionne IJ, Sites CK, Eltabbakh GH, Sims EA, Poehlman ET (2001) What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab 86:1020–1025 [DOI] [PubMed]
- 4.Cock TA, Houten SM, Auwerx J (2004) Peroxisome proliferator-activated receptor-gamma: too much of a good thing causes harm. EMBO Rep 5:142–147 [DOI] [PMC free article] [PubMed]
- 5.Escher P, Braissant O, Basu-Modak S, Michalik L, Wahli W, Desvergne B (2001) Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology 142:4195–4202 [DOI] [PubMed]
- 6.Garg A (2000) Lipodystrophies. Am J Med 108:143–152 [DOI] [PubMed]
- 7.Gray SL, Dalla Nora E, Vidal-Puig AJ (2005) Mouse models of PPAR-gamma deficiency: dissecting PPAR-gamma’s role in metabolic homoeostasis. Biochem Soc Trans 33:1053–1058 [DOI] [PubMed]
- 8.Holm C, Osterlund T, Laurell H, Contreras JA (2000) Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu Rev Nutr 20:365–393 [DOI] [PubMed]
- 9.Kim JB, Sarraf P, Wright M, Yao KM, Mueller E, Solanes G, Lowell BB, Spiegelman BM (1998) Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J Clin Invest 101:1–9 [DOI] [PMC free article] [PubMed]
- 10.Koutnikova H, Cock TA, Watanabe M, Houten SM, Champy MF, Dierich A, Auwerx J (2003) Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPAR gamma hypomorphic mice. Proc Natl Acad Sci USA 100:14457–14462 [DOI] [PMC free article] [PubMed]
- 11.Kressler D, Schreiber SN, Knutti D, Kralli A (2002) The PGC-1-related protein PERC is a selective coactivator of estrogen receptor alpha. J Biol Chem 277:13918–13925 [DOI] [PubMed]
- 12.Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Kadowaki T et al. (1999) PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell 4:597–609 [DOI] [PubMed]
- 13.Le Lay S, Lefrere I, Trautwein C, Dugail I, Krief S (2002) Insulin and sterol-regulatory element-binding protein-1c (SREBP-1C) regulation of gene expression in 3T3-L1 adipocytes. Identification of CCAAT/enhancer-binding protein beta as an SREBP-1C target. J Biol Chem 277:35625–35634 [DOI] [PubMed]
- 14.Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM (2002a) Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1beta), a novel PGC-1-related transcription coactivator associated with host cell factor. J Biol Chem 277:1645–1648 [DOI] [PubMed]
- 15.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM (2002b) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418:797–801 [DOI] [PubMed]
- 16.Medina-Gomez G, Virtue S, Lelliott C, Boiani R, Campbell M, Christodoulides C, Perrin C, Jimenez-Linan M, Blount M, Dixon J, Zahn D, Thresher RR, Aparicio S, Carlton M, Colledge WH, Kettunen MI, Seppanen-Laakso T, Sethi JK, O’Rahilly S, Brindle K, Cinti S, Oresic M, Burcelin R, Vidal-Puig A (2005) The link between nutritional status and insulin sensitivity is dependent on the adipocyte-specific peroxisome proliferator-activated receptor-gamma2 isoform. Diabetes 54:1706–1716 [DOI] [PMC free article] [PubMed]
- 17.Meirhaeghe A, Crowley V, Lenaghan C, Lelliott C, Green K, Stewart A, Hart K, Schinner S, Sethi JK, Yeo G, Brand MD, Cortright RN, O’Rahilly S, Montague C, Vidal-Puig AJ (2003) Characterization of the human, mouse and rat PGC1 beta (peroxisome-proliferator-activated receptor-gamma co-activator 1 beta) gene in vitro and in vivo. Biochem J 373:155–165 [DOI] [PMC free article] [PubMed]
- 18.Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM (2001) Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci USA 98:3820–3825 [DOI] [PMC free article] [PubMed]
- 19.Molina JM, Ciaraldi TP, Brady D, Olefsky JM (1989) Decreased activation rate of insulin-stimulated glucose transport in adipocytes from obese subjects. Diabetes 38:991–995 [DOI] [PubMed]
- 20.Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H, Yazaki Y, Kadowaki T (1998) Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest 101:1354–1361 [DOI] [PMC free article] [PubMed]
- 21.Olefsky JM (1977) Insensitivity of large rat adipocytes to the antilipolytic effects of insulin. J Lipid Res 18:459–464 [PubMed]
- 22.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS (2005) A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 352:1138–1145 [DOI] [PubMed]
- 23.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM (1999) PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4:611–617 [DOI] [PubMed]
- 24.Savage DB, Murgatroyd PR, Chatterjee VK, O’Rahilly S (2005) Energy expenditure and adaptive responses to an acute hypercaloric fat load in humans with lipodystrophy. J Clin Endocrinol Metab 90:1446–1452 [DOI] [PubMed]
- 25.Sewter C, Berger D, Considine RV, Medina G, Rochford J, Ciaraldi T, Henry R, Dohm L, Flier JS, O’Rahilly S, Vidal-Puig AJ (2002) Human obesity and type 2 diabetes are associated with alterations in SREBP1 isoform expression that are reproduced ex vivo by tumor necrosis factor-alpha. Diabetes 51:1035–1041 [DOI] [PubMed]
- 26.Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV Jr (2000) Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet 25:87–90 [DOI] [PubMed]
- 27.Spiegelman BM (1998) PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes 47:507–514 [DOI] [PubMed]
- 28.Tabarin A, Diz-Chaves Y, Carmona Mdel C, Catargi B, Zorrilla EP, Roberts AJ, Coscina DV, Rousset S, Redonnet A, Parker GC, Inoue K, Ricquier D, Penicaud L, Kieffer BL, Koob GF (2005) Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: evidence for a “thrifty gene”. Diabetes 54:3510–3516 [DOI] [PubMed]
- 29.Vidal-Puig A, Jimenez-Linan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE (1996) Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest 97:2553–2561 [DOI] [PMC free article] [PubMed]
- 30.Wang S, Subramaniam A, Cawthorne MA, Clapham JC (2003) Increased fatty acid oxidation in transgenic mice overexpressing UCP3 in skeletal muscle. Diabetes Obes Metab 5:295–301 [DOI] [PubMed]
- 31.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE (2000) Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 43:1498–1506 [DOI] [PubMed]