Introduction
Knowledge about the biological role of carotenoids is still very poor because of the bioaviability of carotenoids is complicated. Multiple factors influence tissues ability to: absorb, transport, metabolise and storage of carotenoids. Beta-carotene (BC) as well as fatty acids coming from food act as a source of energy and a regulator of metabolic processes [1–3]. About 10% of characterized natural carotenoids can be metabolized in human tissues to vitamin A, the precursor of retinoid acid (RA). Different type of carotenoids are present in human tissues and function as: free radical scavengers, immunomodulators, enhancers of the gap junction proteins, apoptosis regulators for many cell types, cancer preventive agents [4–9] Angiogenesis is the important process for the ischemic tissue growth and remodeling including the cancer as well as adipose tissue [10,11]. The main result of presented study demonstrated chemotactic activity of BC in vitro in relation to observed changes in gene expression in human endothelial cells and human umbilical cord blood originated endothelial progenitor cells.
Methods
Cell cultures
Human umbilical vascular endothelial cells (HUVEC) were isolated from human umbilical veins using collagenase digestion and cultured in EBM with supplement. Experiments were performed on 70% confluent cell cultures (up to fifth passage). AC133 positive cells were isolated from the mononuclear fraction of human umbilical blood using the magnetic bits. The cells, after 5th day of growing in proangiogenic conditions (VEGF 50 ng/ml, SCF 100 ng/ml), when about 80% of cells were VE-cadherin positive were called endothelial cell progenitors (EPCs) and used for the further study. HUVECs and EPCs were incubated with BC 3 mM for 24 h.
Isolation of total RNA
Total RNA was isolated by the guanidine thiocyanate–caesium chloride method [12] and purified using the SV total RNA Isolation System Kit.
Microarray hybridization
For the hybridization on HG-U133A GeneChips, RNA was prepared according to manufacturer recomentations. Changes in relative gene expression were calculated versus control. Only spots with significant differences in signal intensity were included in the analysis.
cDNA synthesis and real-time PCR
Microarray results were confirmed, by real-time PCR using GAPDH as the reference gene. For the cDNA synthesis 1 mg of total RNA, oligo(dT), SUPERSCRIPT reverse transcriptase were used. cDNA was subjected to real-time PCR in a reaction mixture containing QuantiTect SYBR Green PCR mix and primers. Data expressed as relative gene expression calculated versus control.
Results
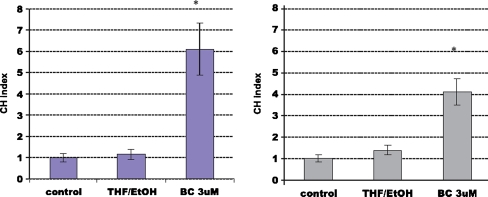
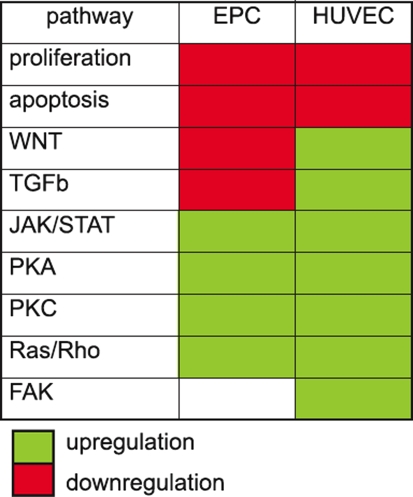
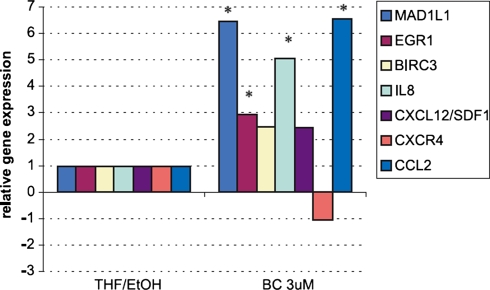
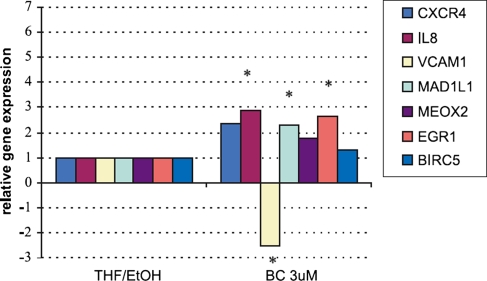
To investigate the influence of beta-carotene on angiogenesis two type of cell lines were used: not finally differentiated endothelial cell line isolated from human umbilical veins and endothelial progenitor cells isolated from the mononuclear cell fraction of the human umbilical cord blood cultured five days in proangiogenic conditions. Beta-carotene did not influence proliferation, apoptosis and differentiation of HUVEC and EPC in in vitro experiments [12,13]. Migration of HUVEC and EPC was the only one observed biological effect of BC in cell culture experiments. BC (3 μM) caused a four-fold increase of HUVEC migration and also (fivefold) increased migration of EPC (Fig. 1). in the gene expression pattern in cells that explain the biological effects of beta-carotene. Microarray results demonstrated that a huge number of BC regulated genes participated in cell–cell and cell–matrix adhesion, matrix proteins, proteases, related to G protein mediated signaling pathway and involved in activation of Ras signaling pathway. General overview of pathways regulated by BC were presented in Table 1 As it was previously reported beta-carotene in non toxic concentration (3 mM) did not effect proliferation, apoptosis and differentiation of HUVEC and EPC in in vitro experiments [13, 14] (Figs. 2, 3). Up-regulation by beta-carotene, of two transcription factors MEOX2 and MAD1L1 could explain mentioned lack of effect. MEOX2 and MAD1L1 has been reported as proliferation inhibitors. It has been shown that MEOX2 upregulated by betacarotene in HUVEC, strongly inhibits endothelial cell activation and tube formation in vitro in response to proangiogenic growth factor-VEGF [15]. MAD1L1 up-regulated in HUVEC and in EPC has been proposed as natural antagonist of Myc [16]. MAD can effectively compete with Max and repress transcriptional activity of Myc by binding to the same CACGTG elements of Ebox region [16]. MAD1L1 through effectively competition Myc–Max inhibits Myc activated transcription of molecules participating in proliferation [15, 16]. EGR-1 transcription factor related to differentiation of endothelial cells was up-regulated by BC [17, 18]. This could suggest that BC can regulate differentiation of endothelium. Further analysis of microarray data revealed that BC induces also two potent EGR-1 repressors: DR 1 and NAB-1. The microarray results were selectively verified by Real-Time PCR as described above. Expression of the most regulated genes related to observed biological effects of beta-carotene on HUVEC or EPC e.g.: proliferation (MAD1L1 [16], MEOX2 [15]), differentiation (EGR1[17, 18], MEOX2), apoptosis (BIRC3, BIRC5 [19]), chemotaxis (VCAM 1 [20], IL-8 [21], CCL2 [22]) and homing (CXCR4, CXCL12 [23]) were analysed (Fig. 2). The real-time PCR confirmed the BC-induced regulation of gene expression obtained previously by microarray.
Fig. 1.
Beta-carotene-induced chemotaxis of EPC and HUVEC. a (left) Influence of beta-carotene on EPC migration. b (right) Influence of beta-carotene on HUVEC migration. The chemotactic activity of used cells was expressed as the chemotaxis index (CHI). Values are mean values ± SD, n = 3 done in triplicates. *Significantly different from the corresponding control, *P < 0.05, **P < 0.005
Table 1.
General overview of pathways regulated by beta-carotene in EPC and HUVEC
Fig. 2.
Influence of beta-carotene on gene expression in EPC. The expression of selected genes verified by the quantitative real-time PCR. MAD1L1 (MAD1 mitotic arrest deficient-like 1); EGR-1 (early growth response 1) BIRC3 (baculoviral IAP repeat-containing 3) apoptosis inhibitor; IL-8 (interleukin 8);CXCL12/SDF-1 (stromal cell-derived factor 1); CXCR4 (chemokine (C-X-C motif ) receptor 4); CCL2 (MCP-1 monocyte chemoattractant protein-1). Data expressed as relative gene expression calculated versus control value (EPCs incubated in medium with 0.075%THF/EtOH). The mean values ± SEM of n = 3 done in triplicates are shown. Significance P < 0.05 versus control
Fig. 3.
Influence of beta-carotene on gene expression in HUVEC. The expression of selected genes verified by the quantitative real-time PCR. CXCR4 (chemokine (C-X-C motif ) receptor 4), IL-8 (interleukin 8); VCAM-1 (vascular cell adhesion molecule 1); MAD1L1 (MAD1 mitotic arrest deficient-like 1); MEOX2 (mesenchyme homeo box 2 (growth arrest-specific homeo box)); EGR-1 (early growth response 1) and BIRC5 (baculoviral IAP repeat-containing 5) apoptosis inhibitor. Data expressed as relative gene expression calculated versus control (HUVECs incubated in medium with 0.075%THF/EtOH). The mean values ± SEM ; significance P < 0,05 at least , n = 3 done in triplicates are shown
Discussion
We have demonstrated the BC induced migration of differentiated endothelial cells and also endothelial progenitor cells. Confirmed by microarray and real-time PCR activation of the important for homing and chemotaxis genes underlines the BC stimulatory effect on migration. Changes in expression of extracellular matrix proteins (collagens, FBN1, FBN2, LAMB1, MATN2, MGP, CSPG6, COL6A3, FGL2, LAMC1, as well as proteinases (ADAMTS1, ADAMTS18, MMP10, MMP12, MMP14, MMP24, MMP9, MMP2, PLAU, TIMP) receptors mediating cell/matrix and cell/cell interactions CELSR1, CTNNA1L, CTNNB1, VCAM1, SELP, CD24 ITGA6, ICAM, CEACAM8, LGALS1, CTNND1, ALCAM, NEDD9, JAGGED-1, vWF, SCARB1) were found in microarray results. All such events may decide about priming of endothelial cell and its progenitors for migration[24, 25]. Beta-carotene, also by stimulation GPCRs (G-protein coupled receptors) and its activators could lead to activation of Rho/Rac/CDC42 small GTPases, and as result of this activation regulate cytoskeletal changes involved in cell migration [26, 27].
Acknowledgments
This work was supported by the F5 EU DLARFID project QLK1-CT-2001-00183.
References
- 1.Repa JJ, Mangelsdorf DJ (1999) Nuclear receptor regulation of cholesterol and bile acid metabolism. Curr Opin Biotechnol 10:557–563 [DOI] [PubMed]
- 2.Heuvel JP (1999) Peroxisome proliferator-activated receptors: a critical link among fatty acids, gene expression and carcinogenesis. J Nutr 129:575S–580S [DOI] [PubMed]
- 3.Duplus E, Glorian M, Forest C (2000) Fatty acid regulation of gene transcription. J Biol Chem 275:30749–30752 [DOI] [PubMed]
- 4.Ross SA, McCaffery PJ, Drager UC, De Luca LM (2000) Retinoids in embryonal development. Physiol Rev 80:1021–1054 [DOI] [PubMed]
- 5.Ehinger M, Bergh G, Johnsson E, Baldetorp B, Olsson I, Gullberg U (1998) p53-dependent and -independent differentiation of leukemic U-937 cells: relationship to cell cycle control. Exp Hematol 26:1043–1052 [PubMed]
- 6.Ferrari N, Pfahl M, Levi G (1998) Retinoic acid receptor gamma1 (RARgamma1) levels control RARbeta2 expression in SK-N-BE2(c) neuroblastoma cells and regulate a differentiation apoptosis switch. Mol Cell Biol 18:6482–6492 [DOI] [PMC free article] [PubMed]
- 7.Trosko JE, Chang CC (2001) Mechanism of upregulated gap junctional intercellular communication during chemoprevention andchemotherapy of cancer. Mutat Res 480–481:219–229 [DOI] [PubMed]
- 8.Costa SL, Paillaud E, Fages C, Rochette-Egly C, Plassat JL, Jouault H, Perzelova A, Tardy M (2001) Effects of a novel synthetic retinoid on malignant glioma in vitro: inhibition of cell proliferation, induction of apoptosis and differentiation. Eur J Cancer 37:520–530 [DOI] [PubMed]
- 9.Simoni D, Tolomeo M (2001) Retinoids, apoptosis and cancer. Curr Pharm Des 7:1823–1837 [DOI] [PubMed]
- 10.Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9:653–660 [DOI] [PubMed]
- 11.Weinmann M, Belka C, Plasswilm L (2004) Tumour hypoxia: impact on biology, prognosis and treatment of solid malignant tumors. Onkologie 27:83–90 [DOI] [PubMed]
- 12.Chomczyñski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate–phenolchloroform extraction. Ann Biochem 162:156–159 [DOI] [PubMed]
- 13.Dembinska-Kiec A, Polus A, Kiec-Wilk B, Grzybowska J, Mikolajczyk M, Hartwich J, Razny U, Szumilas K, Banas A, Bodzioch M, Stachura J, Dyduch G, Laidler P, Zagajewski J, Langman T, Schmitz G (2005) Proangiogenic activity of betacarotene is coupled with the activation of endothelial cell chemotaxis. Biochim Biophys Acta 1740:222–239 [DOI] [PubMed]
- 14.Kiec-Wilk B, Polus A, Grzybowska J, Mikolajczyk M, Hartwich J, Pryjma J, Skrzeczynska J, Dembinska-Kiec A (2005) Beta-Carotene stimulates chemotaxis of human endothelial progenitor cells. Clin Chem Lab Med 43:488–498 [DOI] [PubMed]
- 15.Gorski DH, Leal AJ (2003) Inhibition of endothelial cell activation by the homeobox gene Gax. J Surg Res 111:91–99 [DOI] [PubMed]
- 16.Poortinga G, Hannan KM, Snelling H, Walkley CR, Jenkins A, Sharkey K, Wall M, Brandenburger Y, Palatsides M, Pearson RB, McArthur GA, Hannan RD (2004) MAD1 and c-MYC regulate UBF and rDNA transcription during granulocyte differentiation. EMBO J 23:3325–3335 [DOI] [PMC free article] [PubMed]
- 17.Fu M, Zhu X, Zhang J, Liang J, Lin Y, Zhao L, Ehrengruber MU, Chen YE (2003) Egr-1 target genes in human endothelial cells identified by microarray analysis. Gene 315:33–41 [DOI] [PubMed]
- 18.Fahmy RG, Dass CR, Sun LQ, Chesterman CN, Khachigian LM (2003) Transcription factor Egr-1 supports FGFdependent angiogenesis during neovascularization and tumor growth. Nat Med 9:1026–1032 [DOI] [PubMed]
- 19.Schultz DR, Harrington WJ (2003) Apoptosis: programmed cell death at a molecular level. Semin Arthritis Rheum 32:345–369 [DOI] [PubMed]
- 20.Hordijk PL (2003) VCAM-1-mediated Rac signaling controls endothelial cell–cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol 285:C343–C352 [DOI] [PubMed]
- 21.Schraufstatter IU, Trieu K, Zhao M, Rose DM, Terkeltaub RA, Burger M (2003) IL-8-mediated cell migration in endothelial cells depends on cathepsin B activity and transactivation of the epidermal growth factor receptor. J Immunol 171:6714–6722 [DOI] [PubMed]
- 22.Maslin CL, K Kedzierska, Webster NL, Muller WA, Crowe SM (2005) Transendothelial migration of monocytes: the underlying molecular mechanisms and consequences of HIV-1 infection. Curr HIV Res 3:303–317 [DOI] [PubMed]
- 23.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T (2003) Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 107:1322–1328 [DOI] [PubMed]
- 24.Bazzoni G, Dejana E, Lampugnani MG (1999) Endothelial adhesion molecules in the development of the vascular tree: the garden of forking paths. Curr Opin Cell Biol 11:573–581 [DOI] [PubMed]
- 25.Collen A, Hanemaaijer R, Lupu F, Quax PH, van Lent N, Grimbergen J, Peters E, Koolwijk P, van Hinsbergh VW (2003) Membrane-type matrix metalloproteinase-mediated angiogenesis in a fibrin–collagen matrix. Blood 101:1810–1817 [DOI] [PubMed]
- 26.Hoang MV, Whelan MC, Senger DR (2004) Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc Natl Acad Sci USA 101:1874–1879 [DOI] [PMC free article] [PubMed]
- 27.van Nieuw Amerongen GP, van Hinsbergh VW (2001) Cytoskeletal effects of rho-like small guanine nucleotide-binding proteins in the vascular system. Arterioscler Thromb Vasc Biol 21:300–311 [DOI] [PubMed]