Abstract
The beneficial effect of naturally occurring flavonoids in health is believed to be due to their strong antioxidant activity. However, recent laboratory evidence indicates the involvement of a more specific action. Here, we present evidence that, among a number of catechins present in green tea extract, only epigallocatechin-3-gallate (EGCG) exerts a strong inhibitory action on interferon-γ-elicited activation of signal transducer and activator of transcription 1 (STAT1). Protective action of EGCG in ischemia/reperfusion injury in the heart and the molecular mechanism of action, which has nothing to do with its anti-oxidant capacity are described.
Introduction
Green tea is one of the most ancient and widely consumed health drink in the world and has for long time been used as the popular remedy against common pathologies such as headaches, body aches and pains, bad digestion, mild depression and detoxification, and an elixir of long life [2]. Most of the beneficial effects of drinking green tea has been attributed to antioxidant properties of catechins present in it such as epigallocatechin-3-gallate (EGCG, a main polyphenol present in green tea leaves), epicatechin (EC), epigallocatechin (EGC) and epicatechin-3-gallate (ECG) (Fig. 1) [16]. An increasing body of literature evidence furthermore indicates that beneficial action of green tea drinking might be extended to a number of pathologies correlated to inflammation [4, 5, 11, 15, 17, 20, 22, 24, 26, 27, 35, 38]. Since pathogenesis of inflammatory diseases seems not to be exclusively correlated to redox state unbalance, a theory that the beneficial effect of green tea drinking might not simply be achieved by its strong antioxidant properties is emerging.
Fig. 1.
Structure of catechins present in green tea leaves
In 2001, we reported that EGCG but not other catechins present in green tea extract efficiently inhibits interferon-γ (IFN-γ)-elicited phosphorylation of tyrosine residue 701 and DNA-binding activity of signal transducer and activator of transcription 1 (STAT1) in different human carcinoma-derived cell lines [21]. STAT1 almost exclusively mediates the action of macrophage-derived IFN-γ, playing a pivotal role at the early phase of inflammation in regulating the expression of a number of inflammation-related genes [3].
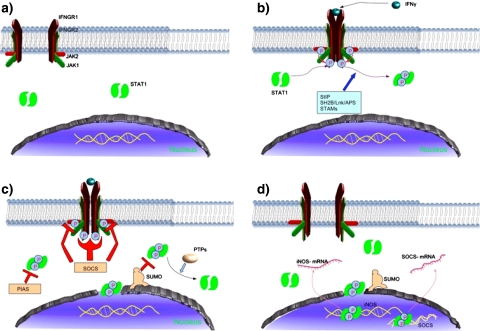
Intracellular pathways activated upon recognition of IFN-γ by its specific receptors (IFNGR1/2) are well elucidated [1, 8]. Briefly, upon dimerisation of IFNGR1/2 followed by IFN-γ binding (Fig. 2b), tyrosine-autophosphorylation of tyrosine kinase 1 and 2 (JAK1/2) occurs. Successively, following SH2 domain-mediated recognition of activated JAK1/2 by IFNGR1/2 JAK1/2 tyrosine-phosphorylate IFNGR1/2 and resting STAT1 present in cytoplasm (Fig. 2b). Phosphorylated dimer of STAT1 enters in the nucleus and binds specifically to regulatory sequences to modulate the expression of target genes (Fig. 2d). Furthermore, up-regulation of JAK/STAT pathway is mediated at least by three different proteins: signal-transducing adapter molecules (STAMs) [19], STAT-interacting protein (StIP) and SH2B/Lnk/APS family (Fig. 2b). Negative regulator of JAK/STAT pathway includes suppressors of cytokine signaling (SOCS), protein inhibitors of activated STAT (PIAS) and protein tyrosine phosphatases (PTPs) such as SHP-1 (Fig. 2c) [6]. Recently discovered sumoylation (Fig. 2c) [10] seems not only to modify PIAS proteins [9[E9]] but also STATs themselves [29]. Despite some reports indicating the critical involvement of the change in redox state in modulating STAT1 activation [7, 12, 23, 25], results obtained by us showing that among all catechins present in green tea extract only EGCG is able to down-regulate STAT1 activation suggest that EGCG may not exert its activity by simply using its antioxidant capacity.
Fig. 2.
JAK1/2-STAT1 pathway. a Normal condition, b activation of IFN-γ-elicited STAT1 and it’s up-regulation, c down-regulation of STAT1, d induction of STAT1-dependent genes
A question arises: which is the molecular mechanism of action of EGCG in inhibiting STAT1 activation? Activated tyrosine-phosphorylated JAK1 and 2 are reported to deeply be involved in STAT1 and STAT3 signal transduction pathways [18]. Considering the fact that EGCG fails to inhibit intereukin-6-elicited activation of STAT3 [21], the possibility that EGCG targets JAK1/2 in inhibiting STAT1 activation is scarce. Involvement of tyrosine phosphatases in EGCG’s STAT1 inhibitory action also seems to be quite improbable, since they may regulate the tyrosine-phosphorylated state of a wide spectrum of proteins including STAT3. SOCS1/3 down-regulate STATs activation, therefore, they could be a target of EGCG. However, the notion that induction of SOCS expression is tightly regulated by STAT1 activation itself renders this possibility unlikely.
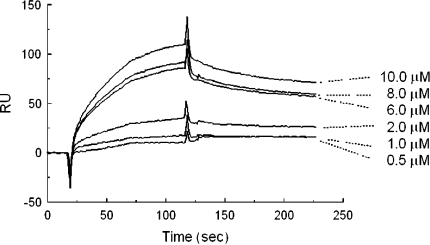
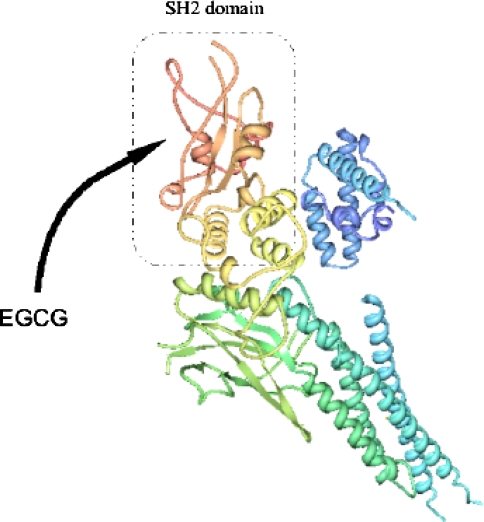
We hypothesize that the STAT1-inhibiting action of EGCG is correlated to its capacity to specifically interact with STAT1 protein. To evaluate this hypothesis we analyzed, by Surface Plasmon Resonance (SPR) method, the interaction between immobilized STAT1 and EGCG or ECG or quercetin, a strong antioxidant flavonoid without anti-STAT1 activity. Not only SPR analysis indicated that only EGCG directly interacts with STAT1 protein (Fig. 3) but also Kd value of EGCG toward STAT1 (640 nM) is far less than that of ECG (5.7 mM) and quercetin (43 μM), indicating that inhibitory action of EGCG on STAT1 DNA-binding activity is, at least partly, due to its capacity to strongly and directly interact with STAT1 protein, the notion in line with computer modeling analysis indicating that EGCG may strongly interact with STAT1 at the site near to SH2 domain, playing a critical role in phospho-tyrosine recognition (Fig. 4).
Fig. 3.
Sensograms indicating the dose-dependent interaction of EGCG with immobilized STAT1. Kd value was estimated by analyzing sensograms
Fig. 4.
Three-dimensional structure of STAT1. SH2 domain and putative EGCG binding region are indicated
To evaluate the possibility that only EGCG but not the other flavonoids without anti-STAT1 activity may act beneficially in animal model of diseases in which hyperactivation of STAT1 plays a deleterious role, we examined the effect of EGCG and quercetin in rat model of ischemia/reperfusion (I/R) injury in the heart. I/R-induced cardiomyocyte death by apoptosis is a pivotal event and STAT1 activation is deeply involved in it [32]. EGCG but not quercetin inhibits efficiently I/R-induced activation of STAT1 and protects heart from I/R-induced tissue and biochemical and functional damages [34]. Since EGCG and quercetin are equally strong antioxidant in this animal model, protective action of EGCG should be ascribed to its capacity to inhibit STAT1 activation. Furthermore, the aforementioned pathologies against which green tea/EGCG show protective action are also characterized by altered activation of STAT1 (Table 1), rendering it quite likely that anti-STAT1 capacity of green tea/EGCG is deeply involved in their beneficial action.
Table 1.
Some pathologies in which STAT1 plays a critical rule
In conclusion, who knows if wisdom of ancient people preferring green tea over myriads of other plant-derived products (one might recall that tea, Camellia sinensis L., is the most drunk beverage only after water in the world) is bringing us to develop a new strategy to prevent and/or treat a number of inflammatory diseases!
Acknowledgments
The authors are thankful for the financial support by CariVerona Project 2001 and FIRB 2001 for the completion of this work.
References
- 1.Aaronson DS, Horvath CM (2002) A road map for those who don’t know JAK–STAT. Science 296:1653–1655 [DOI] [PubMed]
- 2.Cabrera C, Artacho R, Gimenéz R (2006) Beneficial effect of green tea––a review. J Am Coll Nutr 2:79–99 [DOI] [PubMed]
- 3.Carcereri De Prati A, Ciampa AR, Cavalieri E, Zaffini R, Darra E, Menegazzi M, Suzuki H, Mariotto S (2005) STAT1 as a new molecular target of anti-inflammatory treatment. Curr Med Chem 12:1819–1828 [DOI] [PubMed]
- 4.Diepvens K, Westerterp KR, Westerterp-Plantenga MS, Obesity, thermogenesis related to the consumption of caffeine, ephedrine, capsaicin and green tea (2006) Am J Physiol. Regulatory, integrative and comparative physiology [Epub ahead of print] [DOI] [PubMed]
- 5.Dobrzynska I, Sniecinska A, Skrzydlewska E, Figaszewski Z (2004) Green tea modulation of the biochemical and electric properties of rat liver cells that were affected by ethanol and aging. Cell Mol Biol Lett 9:709–721 [PubMed]
- 6.Greenhalgh CJ, Hilton DJ (2001) Negative regulation of cytokine signalling. J Leukoc Biol 70:348–356 [PubMed]
- 7.Grimm M, Spieker M, De Caterina R, Shin WS, Liao JK (2002) Inhibition of major histocompatibility complex class II gene transcription by nitric oxide and antioxidants. J Biol Chem 19:26460–26467 [DOI] [PubMed]
- 8.Ihle JN (1995) The Janus protein tyrosine kinase family and its role in cytokine signaling. Semin Immunol 7:247–254 [DOI] [PubMed]
- 9.Jackson PK (2001) A new RING for SUMO: wrestling transcriptional responses into nuclear bodies with PIAS family E3 SUMO ligases. Genes Dev 153:3053–3058 [DOI] [PubMed]
- 10.Johnson ES, Gupta AA (2001) An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell 21:735–744 [DOI] [PubMed]
- 11.Kao YH, Chang HH, Lee MJ, Chen CL (2006) Tea, obesity, and diabetes. Mol Nutr Food Res 50:188–210 [DOI] [PubMed]
- 12.Kim H, Lee TH, Hwang YS, Bang MA, Kim KH, Suh JM, Chung HK, Yu DY, Lee KK, Kwon OY, Ro HK, Shong M (2001) Methimatazole as an antioxidant and immunomodulator in thyroid cells: mechanism involving interferon gamma signaling and H2O2 scavenging. Mol Pharmacol 60:972–980 [DOI] [PubMed]
- 13.Kitamura Y, Shimohama S, Ota T, Matsuoka Y, Nomura Y, Taniguchi T (1997) Alteration of transcription factors NF-kappaB and STAT1 in Alzheimer’s disease brains. Neurosci Lett 237:17–20 [DOI] [PubMed]
- 14.Klampfer L (2006) Signal transducers and activators of transcription (STATs): novel targets of chemopreventive and chemotherapeutic drugs. Curr Cancer Drug Targets 6:107–121 [DOI] [PubMed]
- 15.Kumar N, Shibata D, Helm J, Coppola D, Malafa M (2007) Green tea polyphenols in the prevention of colon cancer. Front Biosci 1:2309–2315 [DOI] [PubMed]
- 16.Kuroda Y, Hara Y (1999) Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat Res 436:69–97 [DOI] [PubMed]
- 17.Lee H, Bae JH, Lee SR (2004) Protective effect of green tea polyphenol EGCG against neuronal damage and brain edema after unilateral cerebral ischemia in gerbils. J Neurosci Res 77:892–900 [DOI] [PubMed]
- 18.Leonard WJ, O’Shea JJ (1998) Jaks and STATs: biological. Annu Rev Immunol Implications 16:293–322 [DOI] [PubMed]
- 19.Lohi O, Lehto VP (2001) STAM/EAST adapter proteins-integrators of signalling pathways. FEBS Lett 23:287–290 [DOI] [PubMed]
- 20.Mandel S, Maor G, Youdim MB (2004) Iron and apha-synuclein in the substantia nigra of MPTP-treated mice : effect of neuroprotective drugs R-apomorphine and green tea polyphenol (−)epigallocatechin-3-gallate. J Mol Neurosci 24:401–416 [DOI] [PubMed]
- 21.Menegazzi M, Tedeschi E, Dussin D, Carcereri De Prati A, Cavalieri E, Mariotto S, Suzuki H (2001) Anti-interferon gamma action of epigallocatechin-3-gallate mediated by specific inhibition of STAT1 activation. FASEB J 15:1309–1311 [DOI] [PubMed]
- 22.Morley N, Clifford T, Salter L, Campbell S, Gould D, Curnow A (2005) The green tea polyphenols (−) epigallocatechin gallate and green tea can protect human cellular DNA from ultraviolet and visible radiation induced damage. Photodermatol Photoimmunol Photomed 21:15–22 [DOI] [PubMed]
- 23.Pawate S, Shen Q, Fan F, Bhat NR (2004) Redox regulation of glial inflammatory responses to lipopolysaccharide and interferon gamma. J Neurosci Res 15:540–551 [DOI] [PubMed]
- 24.Persson Ingrid AL, Josefsson M, Persson K, Andersson R (2006) Tea flavanols inhibit angiotensin-converting enzyme activity and increase nitric oxide production in human endothelial cells. J Pharm Pharmacol 58:1139–1144 [DOI] [PubMed]
- 25.Pointer ME, Daynes RA (1999) Age-associated alterations in splenic iNOS regulation: influence of constutively expressed IFN-gamma and correction following Oupplementation with PPARalpha activators or vitamin E. Cell Immunol 1:127–136 [DOI] [PubMed]
- 26.Rahman I, Kilty I (2006) Antioxidant therapeutic targets in COPD. Curr Drug Targets 7:707–720 [DOI] [PubMed]
- 27.Ramassamy C (2006) Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol 545:51–64 [DOI] [PubMed]
- 28.Rasschaert J, Ladriere L, Urbain M, Dogusan Z, Katabua B, Sato S, Akira S, Gysemans C, Mathieu C, Eizirik DL (2005) Toll-like receptor 3 and STAT-1 contribute to double-stranded RNA+ interferon-gamma-induced apoptosis in primary pancreatic beta-cells. J Biol Chem 280:33984–33991 [DOI] [PubMed]
- 29.Rogers RS, Horvath CM, Matunis MJ (2003) SUMO modification of STAT1 and its role in PIAS-mediated inhibition of gene activation. J Biol Chem 278:30091–30097 [DOI] [PubMed]
- 30.Rosenblum CI, Tota M, Cully D, Smith T, Collum R, Qureshi S, Hess JF, Phillips MS, Hey PJ, Vongs A, Fong TM, Xu L, Chen HY, Smith RG, Schindler C, Van der Ploeg LH (1996) Functional STAT 1 and 3 signaling by the leptin receptor (OB-R); reduced expression of the rat fatty leptin receptor in transfected cells. Endocrinology 137:5178–5181 [DOI] [PubMed]
- 31.Simon AR, Rai U, Fanburg BL, Cochran BH (1998) Activation of the JAK–STAT pathway by reactive oxygen species. Am J Physiol 275:1640–1652 [DOI] [PubMed]
- 32.Stephanou A (2004) Role of STAT-1 and STAT-3 in ischaemia/reperfusion injury. J Cell Mol Med 8:519–525 [DOI] [PMC free article] [PubMed]
- 33.Takagi Y, Harada J, Chiarugi A, Moskowitz MA (2002) STAT1 is activated in neurons after ischemia and contributes to ischemic brain injury. J Cereb Blood Flow Metab 22:1311–1318 [DOI] [PubMed]
- 34.Townsend PA, Scarabelli TM, Pasini E, Gitti G, Menegazzi M, Suzuki H, Knight RA, Latchman DS, Stephanou A (2004) Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J 18:1621–1623 [DOI] [PubMed]
- 35.Waheed Roomi M, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M (2006) In vivo and in vitro antitumor effect of a unique nutrient mixture on lung cancer cell line a-549. Exp Lung Res 39:441–453 [DOI] [PubMed]
- 36.Wang XQ, Panousis CG, Alfaro ML, Evans GF, Zuckerman SH (2002) Interferon-gamma-mediated downregulation of cholesterol efflux and ABC1 expression is by the Stat1 pathway. Arterioscler Thromb Vasc Biol 22:5–9 [DOI] [PubMed]
- 37.Yoon P, Keylock KT, Hartman ME, Freund GG, Woods JA (2004) Macrophage hypo-responsiveness to interferon-gamma in aged mice is associated with impaired signaling through Jak–STAT. Mech Ageing Dev 125:137–143 [DOI] [PubMed]
- 38.Zaveri NT (2006) Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci 78:2073–2080 [DOI] [PubMed]
- 39.Zhang YM, Rock CO (2004) Evaluation of epigallocatechin gallate and related plant polyphenols as inhibitors of the FabG and FabI reductases of bacterial type II fatty-acid synthase. J Biol Chem 279:30994–31001 [DOI] [PubMed]
- 40.Zykova TA, Zhang Y, Zhu F, Bode AM, Dong Z (2005) The signal transduction networks required for phosphorylation of STAT1 at Ser727 in mouse epidermal JB6 cells in the UVB response and inhibitory mechanisms of tea polyphenols. Carcinogenesis 26:331–342 [DOI] [PubMed]