Abstract
Observational studies with healthy persons demonstrated an inverse association of vitamin E with the risk of coronary heart disease or cancer, the outcome of large-scale clinical trials conducted to prove a benefit of vitamin E in the recurrence and/or progression of such disease, however, was disappointing. Vitamin E did not provide benefits to patients with cardiovascular diseases, cancer, diabetes or hypertension. Even harmful events and worsening of pre-existing diseases were reported, which are hard to explain. Since vitamin E is metabolized along the same routes as xenobiotics and induces drug-metabolizing enzymes in rodents, it is hypothesized that a supplementation with high dosages of vitamin E may also lead to an induction of the drug-metabolizing system in patients that depend on drug therapy. Compromising essential therapy might therefore outweigh any benefit of vitamin E in patients. It is recommended to work out at which threshold the drug-metabolizing system can be induced in humans before new trials with high dosages of vitamin E are started.
Keywords: Vitamin E, Clinical trial, Cardiovascular disease, Drug metabolism, Interference with drug metabolism, Adverse effects
Introduction
Based on the hypothesized association of various chronic diseases with oxidative stress and on observational epidemiological studies indicating that individuals with a high intake of fruits and vegetables rich in antioxidants are better protected, a number of large clinical trials were conducted to prove the benefit of antioxidants in prevention and progression of such diseases. Special emphasis was laid on vitamin E since its major biological function has for long been considered to act as the most important chain breaking, lipid-soluble antioxidant. The outcome of almost all of these studies, however, was disappointing. Vitamin E did not provide benefits to patients with cardiovascular diseases, cancer or diabetes. Therefore, the oxidative modification theory has been questioned even by the father of the hypothesis who recommends to halt trials until a basic information about criteria for selection of patients and markers for oxidative events influenced by antioxidants are available [72]. In some studies even harmful effects were reported. It is not the intention of the paper to summarize the individual trials in detail again. For this, the reader is referred to recent comprehensive reviews [12, 33, 43, 63, 69]. Instead, the paper will focus on very recent trials and meta-analyses summarising adverse effects of α-tocopherol supplementation in patients. Being still unable to provide a final explanation for vitamin E-mediated side effects, it is suggested to consider the recently discovered functions of vitamin E in future clinical trials. An increasing number of papers describe an influence of α-tocopherol on gene activities [15, 59, 80]. Genes which have been shown to be induced by α-tocopherol in vitro and in vivo, although not yet in humans, are genes encoding drug-metabolizing enzymes, like CYP3A4 which metabolizes 60% of all the prescription drugs, [19, 25]. An induction of drug metabolism by the high dosages of vitamin E generally used in the trials would weaken the therapeutic potential of the drugs the subjects participating in these studies had to be treated for their pre-existing diseases.
Observational studies in apparently healthy persons
Most studies focused on the effect of vitamin E on cardiovascular diseases and cancer as the most frequent chronic diseases that are widely believed to be associated with, or even result from, oxidative stress.
The IOWA Women’s Healthy Study [44] observed an inverse association of vitamin E in food with the risk of death due to coronary heart disease in postmenopausal women. The Health Professionals’ Follow-Up-Study [66] showed a similar result. Whereas in both studies vitamin E was administered via the diet, the Nurses’ Health Study [71] revealed a result of better protection from coronary heart disease when vitamin E was taken as supplement. In a Finnish study [40] dietary vitamin E consumption was linked to a reduction in CVD mortality. The Established Populations for Epidemiologic Studies of the Elderly reported a decrease in mortality in particular due to coronary artery disease in elderly people taking vitamin E supplements [51]. As an exception, the Rotterdam study [38] did not reveal a beneficial effect of vitamin E on myocardial infarction. These epidemiological studies were considered as preliminary observations due to potential limitations such as reliance on food questionnaires and lack of validation of historical data. Therefore, randomized controlled intervention trials were required.
Intervention studies in apparently healthy persons
The Linxian study was one of the first nutritional intervention trials and revealed a decreased mortality in subjects receiving β-carotene, vitamin E and selenium, especially mortality due to cancer [9]. Supplementation for patients in nursing homes with α-tocopherol led to a significant lower incidence of respiratory tract infections, whereas no difference in all-cause mortality was observed [54]. In the ATBC study [2] on male smokers for the first time, adverse effects of “antioxidants” were reported. The most important adverse effect was an increase in lung cancer due to β-carotene supplementation. However, also a significantly higher incidence of hemorrhagic stroke was observed in the α-tocopherol group. There were no side effects of 600 IU α-tocopherol every second day given over 10 years to healthy women in the Women’s Health Study. However, despite of a reduction in cardiovascular mortality no overall benefit was observed for major cardiovascular events or cancer [46] and also not for type 2 diabetes [50]. The authors of the study conclude that their data do not support a recommendation for vitamin E supplementation to prevent CVD, cancer or diabetes in initially healthy women.
Intervention studies in patients
The CHAOS [73] reported a significantly lower incidence of nonfatal myocardial infarctions (MI), but an increase in fatal MI which was not significant. The study did not differentiate between patients receiving either 400 or 800 IU α-tocopherol day−1. However, a comment to the meta-analysis by Miller et al. [56] (see below) contained the information that CVD death was observed in 2% of patients in the 400 IU group, whereas it was 3.1% in the 800 IU group [8].
In the GISSI study, patients with a former myocardial infarction were supplemented with 300 mg vitamin E [23] without any effect.
The HOPE study [29[t1]] revealed that the diabetic CVD patients did not experience any positive effect of supplementation with 400 IU vitamin E from natural sources for 4.5 years.
In contrast, the secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE) [10] demonstrated a striking 50% reduction in cardiac events by supplementation with 800 IU vitamin E day−1 in renal failure patients. The study indicates that under certain circumstances vitamin E might have beneficial effects.
In the primary prevention project (PPP) [20], which investigated patients with a history of CVD, vitamin E supplementation in contrast to aspirin intake was not associated with any benefit.
Meta-analyses of the intervention studies
In a comment to the PPP trial a meta-analysis of observational and intervention studies was performed and the results of both kinds of studies were compared [28]. Whereas the former showed a significant protective association with a higher intake of “synthetic vitamin E”, the mortality from cardiovascular disease was essentially unchanged in the latter. The contradictory results were explained by a putative modification of vitamin E effects in the presence of a disease, pointing for the first time, a link between vitamin E effects and the type of subjects investigated, i.e. patients or healthy people [28]. In fact, the discrepancies between the studies might equally lead to the conclusion that vitamin E might have modified the disease.
Vivekananthan et al. [77] stated that the “lack of benefit combined with the lack of mechanistic data for an efficacy of vitamin E do not support the routine use of vitamin E”. As discussed in this report, the potential benefit of vitamin E has mostly been investigated in secondary prevention, which may account for the negative results. It was therefore recommended to test α-tocopherol in primary prevention since in the animal studies that corroborated a benefit, the medication had been administered prophylactically, i.e. before the disease was initiated.
Also the meta-analysis by Eidelman et al. [22] resulted in the conclusion that “vitamin E supplementation has no statistically significant or clinically important effects on cardiovascular disease”.
The first meta-analysis reporting an increase in mortality after the supplementation with antioxidants appeared in 2004. Antioxidants, including α-tocopherol, given alone or in combination did not prevent gastrointestinal cancer, in contrast they seemed to increase the overall mortality [6].
The meta-analysis by Miller et al. [56] even demonstrated an increase in all-cause mortality in patients taking more than 400 IU α-tocopherol. The analysis was highly disputed and criticized for inconsistencies, biased selection of studies included in the meta-analysis, and even for inappropriate handling of data [3, 8, 18, 21, 27, 34, 42, 49, 53, 55, 64]. However, the authors might not be completely wrong in view of recent reports on harmful effects of α-tocopherol supplementation. Their findings have recently been supported by a meta-analysis of 68 trials in which the effect of antioxidants (vitamins A, E, C and selenium) was summarized [7]. It was found that α-tocopherol given alone or in combination, significantly increased the mortality when highly biased (low methodology) risk trials were excluded from the calculation.
Adverse effects of α-tocopherol
The HOPE-TOO trial, the HOPE study extended to 7 years, did not find differences in cancer incidences or deaths and major cardiovascular events but found higher rates of congestive heart failure and hospitalizations for heart failure associated with α-tocopherol supplementation [30]. The authors finally concluded that “vitamin E supplements should not be used in patients with vascular disease or diabetes”.
Stimulated by these results, the GISSI-Preventione Investigators measured the development of congestive heart failure (CHF) in their patients and found a 50% increase in risk to develop CHF in the α-tocopherol group [52].
Also smaller studies observed side effects of α-tocopherol which might be linked to a persisting disease. In a randomized controlled trial performed with 652 elderly Dutch persons, a greater severity of respiratory infections, a higher number of symptoms and larger total illness duration were observed in subjects receiving α-tocopherol [24]. During infections, patients underwent episode-related medication. In haemodialysis patients, anticardiolipin antibodies which are related to increased atherosclerosis progression and thrombolytic episodes were generally increased and even more after 1 year α-tocopherol supplementation [1]. Treatment of type 2 diabetes patients with 500 mg RRR-α-tocopherol day−1 for 6 weeks in contrast to the expected outcome significantly increased systolic and diastolic blood pressure [78].
A very recent study differentiated between patients and healthy individuals. It reports a null effect of α-tocopherol on mortality in a defined population aged 65 years or older with or without taking vitamin E supplements [26]. The authors; however, state that the null effect “seems to represent a combination of increased mortality in those with severe CVD and a possible protective effect in those without”. They conclude: “potential interactions between vitamin E and medications commonly used for cardiac illness should be investigated further”.
Many possibilities are there to explain the failure of α-tocopherol to prevent CVD and cancer in large clinical trials, e.g., the selection of subjects, geographical (and hence dietary) differences, stage of the disease, selected endpoints, dosage, mode of application and chemical nature of vitamin E (discussed in [12]). Even pro-oxidant properties of α-tocopherol have been discussed [11, 41]. As a redox active compound α-tocopherol can react anti- or pro-oxidatively depending on the reaction partner, the concentrations of all partners, and the cellular milieu in which the reactions take place [11, 41]. Thus, α-tocopherol was thought to require co-antioxidants to become beneficial. This was one of the reasons to administer α-tocopherol together with vitamin C in the ASAP study. With this approach retardation in the progression of atherosclerosis was observed after 3 [67] and 6 years [68] in smoking men, at least. In this context it might be interesting that in smokers suffering from an elevated oxidation state, the disappearance of α-tocopherol from plasma was faster when the plasma vitamin C level was low [16, 17]. Whether vitamin C can indeed prevent adverse effects of α-tocopherol remains to be investigated. An alternative explanation for the adverse effects observed in patients might be the metabolism of vitamin E and related regulatory phenomena which will be discussed in search of an answer to the challenging problem.
Vitamin E is metabolized like xenobiotics
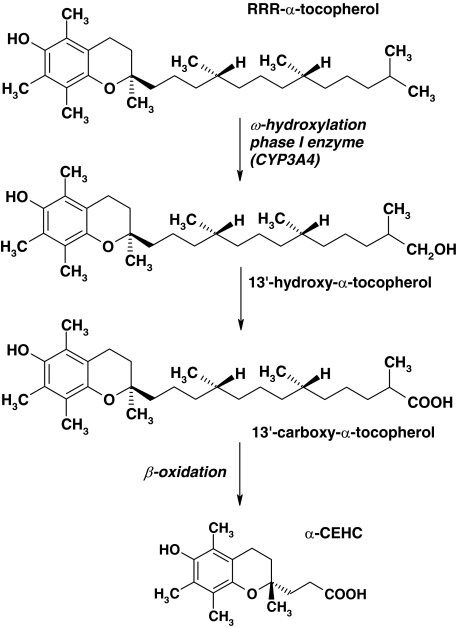
All forms of vitamin E are degraded by the same mechanism, an initial ω-hydroxylation followed by β-oxidation (reviewed in [15, 62]). Hydroxylation is catalyzed by cytochrome P450 enzymes (CYPs), of which CYP3A4 [4, 61] and CYP4F2 [70] are the most likely candidates. Then β-oxidation follows the route that is used for branched-chain or unsaturated fatty acids [5]. The final products of all forms are the respective carboxyethyl hydroxychromans (CEHC), which have the intact chroman structure and the side chain shortened to three carbon atoms (Fig. 1).
Fig. 1.
Structure of α-tocopherol, intermediate metabolites and the terminal product α-carboxyethyl hydroxychroman (α-CEHC)
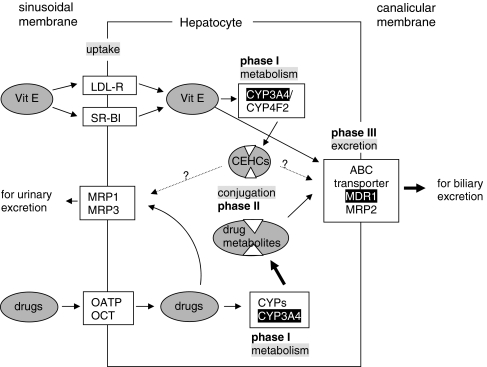
CEHCs are conjugated with glucuronic acid or sulphate and eliminated via the urine. Thus, enzymes/proteins of all three phases of the xenobiotic metabolism are involved in the breakdown of vitamin E: phase I enzymes for hydroxylation, phase II enzymes for conjugation and phase III proteins for excretion (Fig. 2).
Fig. 2.
Metabolism of vitamin E follows the metabolism of xenobiotics. Drugs are mainly taken up in the liver by the organic anion transporting peptide (OATP) or the organic cation transporters (OCT) at the sinusoidal membrane. All forms of chylomicron-remnant-bound vitamin E enter the hepatocyte via the LDL-receptor, HDL-bound α-tocopherol via the scavenger receptor BI (SR-BI). Within the cell the mainly lipophilic drugs become “activated” by phase I enzymes which oxidize, reduce or hydrolyze drugs before they are subjected to phase II metabolism. Oxidation/hydroxylation is catalyzed by cytochrome P450 enzymes, mainly by CYP3A4 in humans, Cyp3a11 in mice and CYP3A1 in rats. Tocopherols and tocotrienols are hydroxylated in human cells by CYP3A4 or CYP4F2. The hydroxy group is oxidized by ω-oxidation to the carboxy group, then the side chain is degraded by β-oxidation ending up in carboxyethyl hydroxychromans (CEHC), see Fig. 1. Metabolites of drugs and vitamin E are sulphated or glucuronidated during phase II metabolism to make them more hydrophilic for the excretion via the biliary or the renal route. Efflux into the bile occurs via the ABC transporters MDR1 or MRP2. Intact vitamin E appears to take this route, too. MRP1 and MRP3 are located at the sinusoidal membrane and pump drugs and their metabolites into the circulation for renal excretion. Whether also the CEHCs are transported via ABC transporters remains to be investigated. Enzymes/transporters shown to be up-regulated by α-tocopherol are indicated white on black background, stimulated pathways by bold arrows. It is obvious that induction of CYP3A4 expression increases the phase I metabolism of drugs. Moreover, induction of MDR-1 is followed by an enhanced excretion of drug metabolites. In consequence, the efficacy of a given dosage of the respective drug will decrease
The degradation of vitamin E by an enzyme system evolutionarily developed to eliminate xenobiotics was unexpected and raised the question whether vitamin E can behave like a xenobiotic [13, 14, 75]. Many xenobiotics induce their metabolizing enzymes. The best-investigated examples in this context are the phase I enzymes, CYPs [65]. The CYP3A family is responsible for the metabolism of about 60% of all prescription drugs [19]. Homologues to the human CYP3A4 are Cyp3a11 in mice and CYP3A1, 2 and 23 in rats [60]. CYP3A4 and CYP3A5 could indeed be induced in HepG2 cells after treatment with γ-tocotrienol and to a weaker extent by α-tocopherol [45]. Induction of CYP3As is mediated by the nuclear pregnane X receptor (PXR), which binds to its responsive element as a heterodimer with RXR [37, 47]. The activation of a PXR-driven CAT reporter in HepG2 cells by different forms of vitamin E has recently been described [45]. The in vivo relevance was demonstrated by an up-regulation of Cyp3a11 by α-tocopherol in mice [39, 75]. Cyp3a11 expression correlated with the hepatic α-tocopherol content. In rats, CYP3A, CYP2B and MDR1, members of the ABC transporter family were up-regulated by α-tocopherol [58]. These studies clearly show that in laboratory animals at least, α-tocopherol can induce enzymes playing a major role in the metabolism of drugs. However, the metabolism of vitamin E is indeed very similar in humans and animals. In both, the mechanism of side chain degradation is identical, as is the low degradation rate of α-tocopherol when compared to other forms of vitamin E (reviewed in [15]); all rac-α-tocopherol is degraded faster than RRR-α-tocopherol [35, 74]; γ-tocopherol levels are similarly decreased by high dosages of α-tocopherol [36, 57] and γ-tocopherol metabolism is equally impaired in humans and rats by inhibition of CYP3A [48, 31]. In view of these observations, the possibility that α-tocopherol also induces enzymes involved in drug metabolism in humans can not be disregarded, but needs to be investigated, because the system displays species specificity, with particular in respect to the response to inducers.
Conclusions
If the induction of drug-metabolizing enzymes by individual forms of vitamin E proves to be valid also for humans, the consequences may be both beneficial and detrimental. Maintenance of an optimum xenobiotic metabolizing system may protect against harmful food ingredients and environmental poisons, but induction of CYPs also weakens the therapeutic efficacy of drugs. Recent experience with herbal remedies reveals that unforeseen interaction of natural products with drug metabolism which is not just an academic problem [32]. A striking example is hyperforin, a constituent of Saint John’s wort. It decreases plasma levels and thus the efficiency of the immunosuppressant cyclosporin, protease inhibitor indinavir used for HIV therapy, oral contraceptives and anticoagulants [32], a scope of complications previously known from risky prescription drugs like rifampicin.
A similar action of α-tocopherol can obviously not be that severe as the action of hyperforin, otherwise it would have been recognized before. But certainly, the demonstration of induction of CYPs and other components of the drug-metabolizing system in rodents, sheds new light on the still mysteriously unfavorable outcome of high-dosed α-tocopherol intervention trials in patients. It may be safely assumed that the overwhelming majority of patients enrolled in the large-scale secondary intervention trials depended on drugs to treat their pre-existing diseases (Table 1), and again a substantial part of those drugs is metabolized by enzymes that are up-regulated by supra-nutritional dosages of α-tocopherol in rodents. An interference with the metabolism of therapeutically required drugs in secondary prevention trials is likely the better working hypothesis than the assumption of any unsubstantiated immuno-compromising, heart damaging or hypertensive efficacy of α-tocopherol itself. With these considerations in mind, it is no longer justified to supplement patients with high α-tocopherol dosages. High dosages should be avoided all the more because a follow-up analysis of the ATBC study revealed that circulating α-tocopherol concentrations at the upper level of the normal range, as it is induced by a well-balanced diet [76], were associated with significantly lower total and cause-specific mortality in older male smokers [79]. High dosages might seriously compromise mandatory therapy of pre-existing diseases and thus outweigh the expected benefits. Instead, it appears mandatory to work out the threshold at which the individual tocopherols and tocotrienols start to induce the drug-metabolizing system in humans, before intakes far beyond the nutritional level are uncritically recommended to prevent progression of diseases that are only suspected to respond to an “antioxidant therapy”.
Table 1.
Diseases and medication of patients participating in the large clinical trials and adverse effects observed by vitamin E supplementation
| Trial | Patients suffering from | Medication | Adverse effect of vitamin E |
|---|---|---|---|
| CHAOS | Coronary atherosclerosis | Calcium antagonists, β-blockers, nitrate, aspirin | Non-significant increase in fatal MI (but significant decrease in non-fatal MI) |
| GISSI | Recent myocardial infarction | Antiplatelet drugs, β-blockers, ACE inhibitors | Increase in heart failure |
| HOPE, HOPE-TOO | Cardiovascular disease, hypertension, diabetes | Aspirin, β-blockers, calcium channel blockers, diuretics, lipid-lowering drugs | Increase in heart failure in HOPE-TOO |
| Ward et al. [78] | Type 2 diabetes | “Usual medication” including oral hypoglycaemics and antihypertensives | Increase in blood pressure |
| Cache County Study | Pre-existing CVD | Nitrate, warfarin | Increased mortality only in patients |
Acknowledgment
The work was supported by the German Research Foundation (DFG), grant Br 778/6-3.
Conflict of interest The author does not have any conflict of interest.
References
- 1.Antoniadi G, Eleftheriadis T, Liakopoulos V, Kakasi E, Vayonas G, Kortsaris A, Vargemezis V (2005) Effect of 1 year oral alpha-Tocopherol administration on anticardiolipin antibodies in heamodialysis patients. Ren Fail 27:193–198 [DOI] [PubMed]
- 2.ATBC prevention study group (1994) The effect of vitamin E and beta-Carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 330:1029–1035 [DOI] [PubMed]
- 3.Baggott JE (2005) Comment on: high-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med 143:155–156 author reply 156–158 [DOI] [PubMed]
- 4.Birringer M, Drogan D, Brigelius-Flohé R (2001) Tocopherols are metabolized in HepG2 cells by side chain (omega oxidation and consecutive beta-oxidation. Free Radic Biol Med 31:226–232 [DOI] [PubMed]
- 5.Birringer M, Pfluger P, Kluth D, Landes N, Brigelius-Flohé R (2002) Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J Nutr 132:3113–3118 [DOI] [PubMed]
- 6.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C (2004) Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 364:1219–1228 [DOI] [PubMed]
- 7.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C (2007) Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 297:842–857 [DOI] [PubMed]
- 8.Blatt DH, Pryor WA (2005) Comment on: high-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med 143:150–151 author reply 156–158 [DOI] [PubMed]
- 9.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, Yang CS, Zheng SF, Gail M, Li GY et al (1993) Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst 85:1483–1492 [DOI] [PubMed]
- 10.Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M, Green MS (2000) Secondary prevention with antioxidants of cardiovascular disease in end-stage renal disease (SPACE): randomised placebo-controlled trial. Lancet 356:1213–1218 [DOI] [PubMed]
- 11.Bowry VW, Stocker R (1993) Tocopherol-mediated peroxidation. The pro-oxidant effect of vitamin E on the radical-initiated oxidation of human low-density lipoprotein. J Am Chem Soc 115:6029–6044 [DOI]
- 12.Brigelius-Flohé R, Kelly FJ, Salonen J, Neuzil J, Zingg J-M, Azzi A (2002) The European perspective on vitamin E: current knowledge and future research. Am J Clin Nutr 76:703–716 [DOI] [PubMed]
- 13.Brigelius-Flohé R (2003) Vitamin E and drug metabolism. Biochem Biophys Res Commun 305:737–740 [DOI] [PubMed]
- 14.Brigelius-Flohé R (2005) Induction of drug metabolizing enzymes by vitamin E. J Plant Physiol 162:797–802 [DOI] [PubMed]
- 15.Brigelius-Flohé R (2006) Bioactivity of vitamin E. Nutr Res Rev 19:174–186 [DOI] [PubMed]
- 16.Bruno RS, Ramakrishnan R, Montine TJ, Bray TM, Traber MG (2005) α-Tocopherol disappearance is faster in cigarette smokers and is inversely related to their ascorbic acid status. Am J Clin Nutr 81:95–103 [DOI] [PubMed]
- 17.Bruno RS, Leonard SW, Atkinson J, Montine TJ, Ramakrishnan R, Bray TM, Traber MG (2006) Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Rad Biol Med 40:689–697 [DOI] [PubMed]
- 18.Carter T (2005) Comment on: high-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med 143:155 author reply 156–158 [DOI] [PubMed]
- 19.Cholerton S, Daly AK, Idle JR (1992) The role of individual human cytochromes P450 in drug metabolism and clinical response. Trends Pharmacol Sci 13:434–439 [DOI] [PubMed]
- 20.Collaborative group of the primary prevention project (2001) Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Lancet 357:89–95 [DOI] [PubMed]
- 21.DeZee KJ, Shimeall W, Douglas K, Jackson JL (2005) Comment on: high-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med 143:153–154 author reply 156–158 [DOI] [PubMed]
- 22.Eidelman RS, Hollar D, Hebert PR, Lamas GA, Hennekens CH (2004) Randomized trials of vitamin E in the treatment and prevention of cardiovascular disease. Arch Intern Med 164:1552–1556 [DOI] [PubMed]
- 23.GISSI Gruppo Italiano per lo Studio della Soprawivenza nell’Infarto miocardico (1999) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 354:447–455 [DOI] [PubMed]
- 24.Graat JM, Schouten EG, Kok FJ (2007) Effect of daily vitamin E and multivitamin-mineral supplementation on acute respiratory tract infections in elderly persons. JAMA 288:715–721 [DOI] [PubMed]
- 25.Guengerich FP (1999) Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol 39:1–17 [DOI] [PubMed]
- 26.Hayden KM, Welsh-Bohmer KA, Wengreen HJ, Zandi PP, Lyketsos CG, Breitner JC (2007) Risk of mortality with vitamin E supplements: the Cache county study. Am J Med 120:180–184 [DOI] [PubMed]
- 27.Hemila H (2005) Comment on: high-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med 143:151–152 author reply 156–158 [DOI] [PubMed]
- 28.Hooper L, Ness AR, Smith GD (2001) Antioxidant strategy for cardiovascular diseases. Lancet 357:1705–1706 [DOI] [PubMed]
- 29.HOPE study investigators (2000) Vitamin E supplementation and cardiovascular events in high-risk patients. N Engl J Med 342:154–160 [DOI] [PubMed]
- 30.HOPE and HOPE-TOO trial investigators (2005) Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 293:1338–1347 [DOI] [PubMed]
- 31.Ikeda S, Tohyama T, Yamashita K (2002) Dietary sesame seed and its lignans inhibit 2,7,8-trimethyl-2 (2′-carboxyethyl)-6-hydroxychroman excretion into urine of rats fed gamma-Tocopherol. J Nutr 132:961–966 [DOI] [PubMed]
- 32.Ioannides C (2002) Pharmacokinetic interactions between herbal remedies and medicinal drugs. Xenobiotica 32:451–478 [DOI] [PubMed]
- 33.Jialal I, Traber MG, Devaraj S (2001) Is there a vitamin E paradox? Curr Op Lipidiol 12:49–53 [DOI] [PubMed]
- 34.Jialal I, Devaraj S (2005) Comment on: high-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med 143:155 author reply 156–158 [DOI] [PubMed]
- 35.Kaneko K, Kiyose C, Ueda T, Ichikawa H, Igarashi O (2000) Studies of the metabolism of a alpha-Tocopherol stereoisomers in rats using [5-methyl-(14)C]SRR- and RRR-alpha-Tocopherol. J Lipid Res 41:357–367 [PubMed]
- 36.Kiyose C, Saito H, Kaneko K, Hamamura K, Tomioka M, Ueda T, Igarashi O (2001) Alpha-Tocopherol affects the urinary and biliary excretion of 2,7,8-trimethyl-2 (2′-carboxyethyl)-6-hydroxychroman, gamma tocopherol metabolite, in rats. Lipids 36:467–472 [DOI] [PubMed]
- 37.Kliewer SA (2003) The nuclear pregnane X receptor regulates xenobiotic detoxification. J Nutr 133:2444S–2447S [DOI] [PubMed]
- 38.Klipstein-Grobusch K, Geleijnse JM, den Breeijen JH, Boeing H, Hofman A, Grobbee DE, Witteman JC (1999) Dietary antioxidants and risk of myocardial infarction in the elderly: the Rotterdam study. Am J Clin Nutr 69:261–266 [DOI] [PubMed]
- 39.Kluth D, Landes N, Pfluger P, Müller-Schmehl K, Weiss K, Bumke-Vogt C, Ristow M, Brigelius-Flohé R (2005) Modulation of CYP3a11 expression by α-tocopherol but not γ-tocotrienol in mice. Free Rad Biol Med 38:507–514 [DOI] [PubMed]
- 40.Knekt P, Reunanen A, Jarvinen R, Seppanen R, Heliovaara M, Aromaa A (1994) Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol 139:1180–1189 [DOI] [PubMed]
- 41.Kontush A, Finckh B, Karten B, Kohlschütter A, Beisiegel U (1996) Antioxidant and prooxidant activity of α-tocopherol in human plasma and low density lipoprotein. J Lipid Res 37:1436–1448 [PubMed]
- 42.Krishnan K, Campbell S, Stone WL (2005) Comment on: high-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med 143:151 author reply 156–158 [DOI] [PubMed]
- 43.Kritharides L, Stocker R (2002) The use of antioxidant supplements in coronary heart disease. Atherosclerosis 164:211–219 [DOI] [PubMed]
- 44.Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM (1996) Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N Engl J Med 334:1156–1162 [DOI] [PubMed]
- 45.Landes N, Pfluger P, Kluth D, Birringer M, Ruhl R, Bol GF, Glatt H, Brigelius-Flohé R (2003) Vitamin E activates gene expression via the pregnane X receptor. Biochem Pharmacol 65:269–273 [DOI] [PubMed]
- 46.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE (2005) Vitamin E in the primary prevention of cardiovascular disease and cancer: the women’s health study: a randomized controlled trial. JAMA 294:56–65 [DOI] [PubMed]
- 47.Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA (1998) The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest 102:1016–1023 [DOI] [PMC free article] [PubMed]
- 48.Lemcke-Norojärvi M, Kamal-Eldin A, Appelqvist LA, Dimberg LH, Öhrvall M, Vessby B (2001) Corn and sesame oils increase serum gamma-tocopherol concentrations in healthy Swedish women. J Nutr 131:1195–1201 [DOI] [PubMed]
- 49.Lim WS, Liscic R, Xiong C, Morris JC (2005) Comment on: high-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med 143:152 author reply 156–158 [DOI] [PubMed]
- 50.Liu S, Lee IM, Song Y, Van Denburgh M, Cook NR, Manson JE, Buring JE (2006) Vitamin E and risk of type 2 diabetes in the women’s health study randomized controlled trial. Diabetes 55:2856–2862 [DOI] [PubMed]
- 51.Losonczy KG, Harris TB, Havlik RJ (1996) Vitamin E and vitamin C supplement use and risk of all-cause and coronary heart disease mortality in older persons: the established populations for epidemiologic studies of the elderly. Am J Clin Nutr 64:190–196 [DOI] [PubMed]
- 52.Marchioli R, Levantesi G, Macchia A, Marfisi RM, Nicolosi GL, Tavazzi L, Tognoni G, Valagussa F (2006) Vitamin E increases the risk of developing heart failure after myocardial infarction: results from the GISSI-Prevenzione trial. J Cardiovasc Med (Hagerstown) 7:347–350 [DOI] [PubMed]
- 53.Marras C, Lang AE, Oakes D, McDermott MP, Kieburtz K, Shoulson I, Tanner CM, Fahn S (2005) Comment on: high-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med 143:152–153 author reply 156–158 [DOI] [PubMed]
- 54.Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MF, Hamer DH (2004) Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA 292:828–836 [DOI] [PMC free article] [PubMed]
- 55.Meydani SN, Lau J, Dallal GE, Meydani M (2005) Comment on: high-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med 143:153 author reply 156–158 [DOI] [PubMed]
- 56.Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E (2005) Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 142:37–46 [DOI] [PubMed]
- 57.Morinobu T, Yoshikawa S, Hamamura K, Tamai H (2003) Measurement of vitamin E metabolites by high-performance liquid chromatography during high-dose administration of alpha-Tocopherol. Eur J Clin Nutr 57:410–414 [DOI] [PubMed]
- 58.Mustacich DJ, Leonard SW, Devereaux MW, Sokol RJ, Traber MG (2006) Alpha-Tocopherol regulation of hepatic cytochrome P450s and ABC transporters in rats. Free Rad Biol Med 41:1069–1078 [DOI] [PubMed]
- 59.Nell S, Bahtz R, Bossecker A, Kipp A, Landes N, Bumke-Vogt C, Halligan E, Lunec J, Brigelius-Flohé R (2007) PCR-verified microarray analysis and functional in vitro studies indicate a role of α-tocopherol in vesicular transport. Free Rad Res, in press [DOI] [PubMed]
- 60.Nelson DR (1999) Cytochrome P450 and the individuality of species. Arch Biochem Biophys 369:1–10 [DOI] [PubMed]
- 61.Parker RS, Sontag TJ, Swanson JE (2000) Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochem Biophys Res Commun 277:531–534 [DOI] [PubMed]
- 62.Pfluger P, Kluth D, Landes N, Bumke-Vogt C, Brigelius-Flohé R (2004) Vitamin E: underestimated as an antioxidant. Redox Rep 9:249–254 [DOI] [PubMed]
- 63.Pham DQ, Plakogiannis R (2005) Vitamin E supplementation in cardiovascular disease and cancer prevention: part 1. Ann Pharmacother 39:1870–1878 [DOI] [PubMed]
- 64.Possolo AM (2005) Comment on: high-dosage vitamin E supplementation and all-cause mortality. Ann Intern Med 143:154 author reply 156–158 [DOI] [PubMed]
- 65.Quattrochi LC, Guzelian PS (2001) Cyp3A regulation: from pharmacology to nuclear receptors. Drug Metabol Dispos 29:615–622 [PubMed]
- 66.Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC (1993) Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med 328:1450–1456 [DOI] [PubMed]
- 67.Salonen JT, Nyyssonen K, Salonen R, Lakka HM, Kaikkonen J, Porkkala-Sarataho E, Voutilainen S, Lakka TA, Rissanen T, Leskinen L, Tuomainen TP, Valkonen VP, Ristonmaa U, Poulsen HE (2000) Antioxidant supplementation in atherosclerosis prevention (ASAP) study: a randomized trial of the effect of vitamins E and C on 3 year progression of carotid atherosclerosis. J Intern Med 248:377–386 [DOI] [PubMed]
- 68.Salonen RM, Nyyssonen K, Kaikkonen J, Porkkala-Sarataho E, Voutilainen S, Rissanen TH, Tuomainen TP, Valkonen VP, Ristonmaa U, Lakka HM, Vanharanta M, Salonen JT, Poulsen HE (2003) Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: the antioxidant supplementation in atherosclerosis prevention (ASAP) study. Circulation 107:947–953 [DOI] [PubMed]
- 69.Shekelle PG, Morton SC, Jungvig LK, Udani J, Spar M, Tu W, Suttorp MJ, Coulter I, Newberry SJ, Hardy M (2004) Effect of supplemental vitamin E for the prevention and treatment of cardiovascular disease. J Gen Intern Med 19:380–389 [DOI] [PMC free article] [PubMed]
- 70.Sontag TJ, Parker RS (2002) Cytochrome P450 omega hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J Biol Chem 277:25290–25296 [DOI] [PubMed]
- 71.Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC (1993) Vitamin E consumption and the risk of coronary disease in women. N Engl J Med 328:1444–1449 [DOI] [PubMed]
- 72.Steinberg D, Witztum JL (2002) Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation 105:2107–2111 [DOI] [PubMed]
- 73.Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ (1996) Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge heart antioxidant study (CHAOS). Lancet 347:781–786 [DOI] [PubMed]
- 74.Traber MG, Elsner A, Brigelius-Flohé R (1998) Synthetic as compared with natural vitamin E is preferentially excreted as alpha-CEHC in human urine: studies using deuterated Ω-tocopheryl acetates. FEBS Lett 437:145–148 [DOI] [PubMed]
- 75.Traber MG, Siddens LK, Leonard SW, Schock B, Gohil K, Krueger K, Cross CE, Williams D (2005) α-Tocopherol modulates Cyp3a expression increases γ-CEHC production, and limits tissue γ-tocopherol accumulation in mice fed high γ-tocopherol diets. Free Rad Biol Med 38:773–785 [DOI] [PubMed]
- 76.Traber MG (2006) How much vitamin E? ... Just enough! Am J Clin Nutr 84:959–960 [DOI] [PubMed]
- 77.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topo,l EJ (2003) Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 361:2017–2023 [DOI] [PubMed]
- 78.Ward NC, Wu JH, Clarke MW, Puddey IB, Burke V, Croft KD, Hodgson JM (2007) The effect of vitamin E on blood pressure in individuals with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. J Hypertens 25:227–234 [DOI] [PubMed]
- 79.Wright ME, Lawson KA, Weinstein SJ, Pietinen P, Taylor PR, Virtamo J, Albanes D (2006) Higher baseline serum concentrations of vitamin E are associated with lower total and cause-specific mortality in the omega-Tocopherol, beta-Carotene cancer prevention study. Am J Clin Nutr 84:1200–1207 [DOI] [PubMed]
- 80.Zingg JM, Azzi A (2004) Non-antioxidant activities of vitamin E. Curr Med Chem 11:1113–1133 [DOI] [PubMed]