Abstract
Low selenium (Se) status has been associated with increased risk of colorectal cancer (CRC). Se is present as the amino acid selenocysteine in selenoproteins, such as the glutathione peroxidases. Se incorporation requires specific RNA structures in the 3′ untranslated region (3′UTR) of the selenoprotein mRNAs. A single nucleotide polymorphism (SNP) occurs at nucleotide 718 (within the 3′UTR) in the glutathione peroxidase 4 gene. In the present study, Caco-2 cells were transfected with constructs in which type 1 iodothyronine deiodinase coding region was linked to the GPx4 3′UTR with either C or T variant at position 718. Higher reporter activity was observed in cells expressing the C variant compared to those expressing the T variant, under either Se-adequate or Se-deficient conditions. In addition, a disease association study was carried out in cohorts of patients with either adenomatous polyps, colorectal adenocarcinomas and in healthy controls. A higher proportion of individuals with CC genotype at the GPx4 T/C 718 SNP was present in the cancer group, but not in the polyp group, compared with the control group (P < 0.05). The present data demonstrate the functionality of the GPx4 T/C 718 SNP and suggest that T genotype is associated with lower risk of CRC.
Keywords: Colorectal cancer, GPx4, Reporter gene, Selenium, SNP, 3′Untranslated region
Introduction
Selenium (Se) is a dietary micronutrient that is essential for human health [12, 34] and low Se status has been associated with increased mortality [1]. Worldwide, mortality from cancers, including colorectal cancer (CRC), is inversely correlated with estimated Se intake [11]. Additionally in case-control studies, low Se intake is associated with increased risk of CRC [35] and, in a 10-year prospective study, initial plasma Se was inversely correlated with subsequent risk of CRC [9]. Moreover, a clinical trial in the United States indicated that a daily supplement of 200 μg Se led to reduced mortality from CRC. Carcinogenesis in the colon is a multistage process and CRC develops via an adenoma–carcinoma sequence from microscopic dysplastic aberrant crypt foci within the epithelium to benign adenomatous polyps, which can then potentially, but not necessarily, undergo malignant change. The sequence of biochemical changes underlying this sequence is not known but oxidant damage to DNA is a critical event in tumour formation [13, 20, 39]. Se is necessary for the function of ∼25 selenoproteins, including the antioxidant enzymes cytosolic glutathione peroxidase (GPx1), gastrointestinal specific glutathione peroxidase (GPx2) and phospholipid hydroperoxide glutathione peroxidase (GPx4) [8, 41] which provide defence against oxidative damage.
In selenoproteins, Se is present as the amino-acid selenocysteine (Sec) and its incorporation occurs during synthesis of the proteins. This incorporation requires the recoding of a UGA codon that involves the formation of a complex between an RNA stem-loop structure (SECIS) located in the 3′untranslated region (3′UTR) of selenoprotein mRNAs and various trans-acting proteins [6]. Thus selenoprotein expression can depend on both dietary Se intake and genetic factors. Furthermore, variants in genes encoding any of the GPxs affecting antioxidant capacity, may change susceptibility to genomic damage by oxidants and thus influence neoplastic change. A SNP has been identified in the GPx1 gene which results in a proline to leucine substitution at codon 198 [18, 19] and this SNP is associated with lung and bladder cancer risk [23, 24]. Two SNPs within the Sep15 gene occur in a region of the 3′UTR and, although the function of the encoded protein is not known, there is an association of alleles at these two positions with susceptibility to breast cancer [22]. Despite the report that Se supplementation reduces CRC mortality [10], there is little information on whether SNPs in GPx genes affect susceptibility to CRC.
A SNP (rs713041) has been found at position 718 of the GPx4 gene mRNA in the 3′UTR region; the regulatory region required for incorporation of Se into selenoproteins. The C allele is associated with increased levels of lymphocyte lipoxygenase products [42]. However, since the effects of the SNP on Se incorporation are not known, the first objective of this work was to use a reporter gene assay in transfected Caco-2 cells [4] to assess the functionality of this SNP. The effect of the SNP on levels of lymphocyte lipoxygenase products implies that the SNP may affect inflammatory signalling, and, since inflammation in the colon is regarded as a frequent initiating event in carcinogenesis [26, 28], the second objective was to carry out a disease-association study focusing on the GPx4 T/C 718 SNP in patients with CRC and in controls. The data indicate that the T/C 718 SNP has functional effects on Se incorporation and that the C variant is more common in patients diagnosed with CRC.
Materials and methods
Plasmid construction
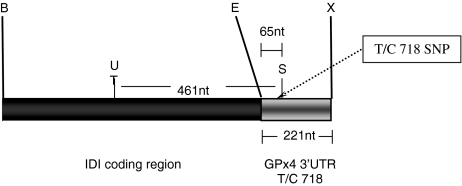
Constructs containing the rat type 1 iodothyronine deiodinase (IDI) coding region linked to GPx4 3′UTR with either a T or C at position 718, were cloned to assess functionality of the T/C polymorphism in the human GPx4 3′UTR at position 718 [42]. The rat IDI coding region was amplified by PCR from IDI–IDI construct in pCDNA3 [4] and the human GPx4 3′UTR (T variant) was amplified from pRc/CMV [45]. PCR primers used for the amplification reactions are shown in Table 1. Following ligation of the rat IDI coding region (BamHI/EcoRI) to the human GPx4 3′UTR (EcoRI/XbaI) (Fig. 1), the product was cloned into pCDNA3.1/Zeo (+) (Invitrogen, Paisley, UK) for expression in mammalian cells. PCR products were purified using QIAquick PCR purification kit (Qiagen; Crawley, UK) according to the manufacturers’ instructions. The construct in which the IDI coding region was linked to the C variant (position 718) of the GPx4 3′UTR was made by site-directed mutagenesis using QuickChange Site-Directed Mutagenesis Kit (Stratagene, Amsterdam, The Netherlands) and the primers are shown in Table 1. All constructs were confirmed by sequencing.
Table 1.
Primer sequences used for plasmid construction, mutagenesis and genotyping
| Cloning | Primers 5′–3′ |
|---|---|
| rIDI coding region | |
| BamHI | CGGGATCCATGGGGCTGTCCCAG |
| EcoRI | CGGAATTCCTAGAACTGAGGCATGTGTG |
| hGPx4 3′UTR | |
| EcoRI | CGGAATTCCTCCACAAGTGTGTGGC |
| XbaI | GCTCTAGACTAATTTGTCTGTTTATTCCC |
| Mutagenesis | |
| Sense | CTGCCCACGCCCTCGGAGCCTTCCACC |
| Antisense | GGTGGAAGGCTCCGAGGGCGTGGGCAG |
| Genotyping | |
| hGPx4 3′UTR | GACCTGCCCCACTATTTCTAGTCTGTTTATTCCCACAAGG |
X: Nucleotides added, X: restriction enzyme recognition sequence added, h: human, r: rat
Fig. 1.
Schematic diagram of IDI-GPx4 T/C 718 reporter constructs. The T/C 718 SNP within the GPx4 3′UTR is indicated by a dotted line at the base of the SECIS element. The size of the 3′UTR is indicated, as well as the distance of the SECIS element from the UGA codon for Se incorporation [data obtained from the NCBI database (Accession number NM_002085)] and the restriction sites used for cloning. U UGA codon for selenocysteine incorporation, S SECIS element, BBamHI, EEcoRI, XXbaI
DNA transfection
Endotoxin-free pCDNA3.1/Zeo (+) constructs were stably transfected into human colon adenocarcinoma cells (Caco-2) using Lipofectamine 2000 reagent (1 mg/ml; Invitrogen, Paisley, UK) according to the manufacturer’s instructions. Briefly, 2.5 × 105 cells were plated out in a 60 mm dish, and transfected after 1 day when they were 90–95% confluent. The ratio of DNA (μg) to Lipofectamine 2000 (μl) used was 1:3.5. The day after transfection, cells were split at a 1:5 ratio and grown in normal growth medium for further 24 h. A selective medium containing 200 μg/ml of the antibiotic zeocin (Invitrogen; Paisley, UK) was added to the cells 48 h after the start of the transfection. Stable transfected colonies were identified after about 1 week but were allowed to grow for an extra 2–3 weeks in the selective medium before a mixed population of transfected cells was obtained.
Cell culture conditions
Stock of stable transfected Caco-2 cells were grown in normal Dulbecco’s Modified Eagle’s Medium (with 4.5 g/l glucose and Glutamax) supplemented with 10% (v/v) foetal calf serum (Sigma; Poole, UK), 60 μg/ml gentamycin, 1% (v/v) non-essential amino acids and penicillin/streptomycin (100 units/ml and 100 μg/ml, respectively), until they reached 80% confluence. Cells were then transferred to serum-free medium to which either insulin (5 μg/ml) and transferrin (5 μg/ml) (Se-deficient cells), or insulin, transferrin and selenium as sodium selenite (7 ng/ml; 40.5 nM) (Se-adequate cells) were added. Two days after the transfer to serum-free medium, cells were harvested for RNA extraction and for measurement of IDI activity.
RNA extraction and Northern hybridization
Total RNA was extracted using Trizol reagent (Invitrogen; Paisley, UK) and following the manufacturer’s instructions. Northern blotting was carried out as described previously [3, 32]. The rat IDI probe was a 2.1 kb fragment excised with BstXI/NotI from IDI–IDI construct in pCDNA3 [4] whereas the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe was a 780 bp PstI–XbaI fragment obtained from human foetal liver as previously described [3]. Quantification of the bound probe was carried out using the Canberra Packard Instantimager (Perkin Elmer; Boston, USA), and results were expressed per unit of hybridisation achieved with the GAPDH cDNA.
Type 1 iodothyronine deiodinase (IDI) activity assay
IDI enzyme activity was measured in cells that had been washed and scraped in ice cold PBS (pH 7.4). Cells were collected by centrifugation at 10,000g for 15 min at 4°C. The cell pellets were then resuspended in 200 μl phosphate buffer (0.125 M KPO4/1 mM EDTA pH 7.4 containing 0.1% (v/v) Triton X-100), and sonicated (10% power; Status 200, Philip Harris Scientific; Hyde, UK) three times for 10 s. Sonicated samples were centrifuged at 2,400g for 10 min at 4°C, and the supernatant fluids were used for the assay; 5 μl of cell lysate were incubated with [125I] reverse T3 (l-3,3’,5’-[125I]-triodothyronine (rT3) (1 μM) and 1 mM dithiothreitol (DTT) in phosphate buffer (0.125 M K3PO4/1 mM EDTA pH 7.4), in a final volume of 300 μl at 37°C for 30 min. The reaction was terminated, by precipitating rT3, with the addition of heat-inactivated horse serum and 10% (v/v) trichloroacetic acid (TCA). The free 125I released by the activity of IDI in both the supernatant fluid and the precipitate was counted using a gamma counter (Cobra counter, Canberra Instruments/Perkin Elmer; Boston, USA) and the release of 125I was quantified as described by Arthur and co-workers [2]. Protein content of the cell lysates was measured by the Bicinchoninic acid (BCA) method [40].
Study subjects
Participants in the study were recruited through two centres: the Gastro-Intestinal Unit at Aberdeen Royal Infirmary, and Newcastle and North Tyneside Health district. The study was approved by the Grampian Ethics Committee and by the Newcastle and North Tyneside Health Authorities Joint Ethics Committee. Written informed consent was obtained from each study participant. Individuals attending clinic for colonoscopy were invited to participate in the study and, if consent was given, blood samples were obtained at the time of colonoscopy. A total number of 546 participants were recruited and identified as having colorectal adenocarcinoma, adenomatous polyps or no evidence of tumour or polyp at the time of colonoscopy. Subsequently, diagnosis was confirmed histologically.
Genotyping
DNA was extracted from whole blood; 35 ml cell lysis buffer pH 8 (10 mM Tris–HCl, 320 mM sucrose, 5 mM MgCl2, 1% (v/v) Triton X-100) was added to 5–10 ml blood and centrifuged at 900g for 10 min at 4°C. The pellet was then resuspended in 2 ml of nuclear lysis buffer (400 mM Tris–HCl, 60 mM EDTA, 150 mM NaCl, 1% (w/v) SDS), 0.5 ml of 5 M sodium perchlorate added and the sample mixed at room temperature for 15 min and then incubated at 65°C for 30 min; 2.5 ml of chloroform were then added, the sample mixed for 10 min and the aqueous phase separated by centrifugation at 250g for 10 min at 4°C. The aqueous phase containing the DNA was transferred to a fresh tube, two volumes of 100% (v/v) ethanol were added and the sample gently rotated. The DNA precipitated to form a white pellet, which was spooled with a microbiological loop. The DNA was air dried for 10 min and resuspended in 200 μl of 1 mM Tris–HCl pH 8, overnight at 60°C. For a small number of samples, only a limited amount of blood (<1 ml) was available. For these samples, DNA was extracted using the QIAamp DNA Blood Mini kit (Qiagen; Crawley, UK), according to the manufacturers’ instructions.
One hundred nanogram of DNA was subjected to PCR to amplify the 221 bp fragment corresponding to the 3′UTR of the human GPx4 RNA (Acc. N. NCBI: NM_002085; nt 654–874). The PCR primers used are shown in Table 1 and conditions were as follows: one cycle at 94°C for 2 min, 53.5°C for 30 s, 72°C for 1 min, followed by 30 cycles at 94°C for 30 s, 53.5°C for 30 s and 72°C for 1 min and a final elongation step at 72°C for 7 min. PCR products were purified by adsorption to a polyethylene glycol (PEG) mix (26.2% (w/v) PEG 8000, 6.6 mM MgCl2, 0.6 M Sodium Acetate pH 5.2), washed with 70% (v/v) ethanol, air-dried and resuspended in 18 μl H2O. The integrity of the PCR product was confirmed by agarose gel electrophoresis and the T/C variation at position 718 determined by direct sequencing (MWG Biotech; Ebersberg, Germany) or restriction fragment analysis using the restriction enzyme Sty1 to generate either two fragments of 159 and 62 bp (C allele) or three fragments of 97, 62 and 62 bp (T allele). Genotype frequencies in patient groups were compared by a chi-squared test and Fischer’s exact test and odds ratios calculated using binary logistic regression and corrected for age and gender.
Results
Reporter gene constructs and cell culture
Incorporation of Sec into selenoproteins depends on the SECIS structure within the 3′UTR and reporter gene studies show that the 3′UTR sequences from the various selenoprotein mRNAs differ in their ability to allow Sec incorporation [4, 6, 44]. To study the effect of the T/C variation at position 718 in the GPx4 3′UTR, reporter gene constructs were made (Fig. 1) in which the rat type 1 IDI coding region was linked to either the T or C variant of the human GPx4 3′UTR, and stable cell populations expressing the constructs were generated in Caco-2 cells by transfection. IDI is not expressed at detectable levels in Caco-2 cells and therefore using IDI as a reporter linked to the GPx4 3′UTR variants rather than over-expressing the full GPx4 transcripts avoided any complications due to endogenous transcripts.
Levels of the IDI mRNA and its enzyme activity were then measured to assess Se incorporation into active selenoprotein. Untransfected cells did not express IDI as judged by Northern hybridisation or enzyme activity (data not shown). Cells expressing the chimaeric constructs in which the T or C GPx4 variants were linked to the IDI coding region exhibited closely similar levels of IDI transcripts (Table 2). However, the cells expressing IDI linked to the C variant 3′UTR showed a considerably higher (3.8-fold) IDI reporter activity when the cells were grown under Se-adequate conditions (Table 2). Furthermore, the ratio of activity/mRNA, a measure of Sec incorporation during translation that also accounts for any differences in level of mRNA expression [4, 5], was 2.7-fold higher with the C variant than with the T in cells grown under Se-adequate culture conditions. This suggests that the C variant is more effective in incorporating Se under Se-adequate conditions. When cells were grown under Se-deficient conditions [4], the levels of IDI mRNA did not change but IDI activity fell by 69 and 78% in IDI-GPx4 T and in IDI-GPx4 C transfected cells, respectively (P < 0.05, Mann–Whitney U test, Table 2). Under these conditions IDI activity was still greater in the cells expressing the C variant but the difference between T and C variants was less marked. Indeed, ratio of IDI activity in Se-deficient conditions compared with that in Se-adequate conditions was significantly (P < 0.05) smaller in the cells expressing the C variant (mean ± SEM 0.22 ± 0.01) compared with cells expressing the T variant (0.31 ± 0.01). These data show that the change from T to C at position 718 in the GPx4 3′UTR altered the ability of the 3′UTR to drive Se incorporation in a reporter protein and that, regardless of Se conditions, the C variant led to greater Se incorporation, although the expression driven by the T variant is relatively less sensitive to Se depletion.
Table 2.
Type 1 iodothyronine deiodinase (IDI) reporter studies to assess the effect of T to C mutation at position 718 in GPx4 3′UTR
| Cell line | IDI mRNA levels (mean ± SEM) n = 4 | IDI enzyme activity (mean ± SEM) n = 4 | Ratio activity/mRNA levels (mean) | |||
|---|---|---|---|---|---|---|
| +Se | −Se | +Se | −Se | +Se | −Se | |
| IDI-GPx4 T | 1.01 ± 0.27 | 0.93 ± 0.16 | 46.88 ± 3.00 | 14.43 ± 0.82 | 46.4 | 15.5 |
| IDI-GPx4 C | 1.44 ± 0.24 | 1.32 ± 0.14 | 177.90 ± 19.00 | 39.80 ± 5.30 | 123.4 | 30.0 |
Caco-2 cells were stably transfected with constructs in which the IDI coding region was linked to either the T or C variant of the GPx4 3′UTR. Cells were grown under Se-adequate or Se-deficient conditions as described in the text. RNA was extracted for measurement of IDI mRNA levels by Northern hybridisation and protein extracts prepared for assay of IDI activity. IDI mRNA levels are expressed in arbitrary units and activity expressed as pmol iodine released/min/mg protein. Se deficiency did not induce any changes in the levels of IDI mRNA abundance for the two different 3′UTRs (T or C). IDI activity levels decreased significantly (P < 0.05) in Se-deficient conditions. An account of the translational efficiency (selenocysteine read-through) of the different 3′UTRs used (ratio of activity/mRNA abundance) [4, 5] is shown in the last column. Statistical analysis was performed using Mann–Whitney U test
Clinical study
A total of 546 participants took part to the study: 252 with adenocarcinoma, 107 with adenomatous polyps and 187 controls. The three groups were comparable with respect to mean age and the control and cancer groups were similar in gender distribution (Table 3). A 221 bp sequence of the GPx4 gene corresponding to the 3′UTR was amplified by PCR from DNA extracted from blood taken from all the participants. Sequencing of the PCR products showed a T/C variation at position 718, confirming the presence of the SNP previously identified at this position in a group of healthy volunteers [42]. The frequency distribution of the T/C 718 SNP in the control group showed a high number of heterozygotes (60%) with both homozygotes being relatively common (20% CC, 20% TT) (Table 3). The distribution of the three genotypes in the group of patients with adenomatous polyps was similar to that of the control group. In contrast, in the group with adenocarcinoma, TT individuals represented a smaller proportion (17%), and those with CC a larger proportion (30%), of the cohort. Indeed, statistical analysis using a chi square test showed that the frequency of the CC genotype was significantly higher in the cancer group compared with the control group (P < 0.05), and higher in the cancer group compared with the group with adenomatous polyps (P < 0.05). A multivariable analysis indicated that the odds ratio for the protective effect of carriage of one or more T alleles at the GPx4 T/C 718 SNP, independent of age and gender, was 0.60 (95% CI 0.37–0.96, P = 0.033) in the cancer group compared with controls and 0.54 (95% CI 0.30–0.96, P = 0.035) in the cancer group compared with the polyp group. There was no statistically significant difference between genotype frequencies in the control and polyp groups. In view of the reporter gene data indicating functionality of the SNP and a lack of haplotype and other functional SNP data on the GPx4 gene, the focus of the study was restricted to the GPx4 T/C 718 SNP rather than extending it to include other variants by using a tagging or haplotype approach.
Table 3.
Distribution of GPx4 T/C 718 genotype among patients with cancer and polyps, and controls
| GPx4 T/C 718 genotype | Cancer (n = 252) | Polyp (n = 107) | Control (n = 187) |
|---|---|---|---|
| CC | n = 76 (30%)* | n = 20 (19%) | n = 38 (20%) |
| CT | n = 132 (53%) | n = 60 (56%) | n = 111 (60%) |
| TT | n = 44 (17%) | n = 27 (25%) | n = 38 (20%) |
| Age (mean ± SD) | 68 ± 10 | 64 ± 6 | 63 ± 13 |
| Gender distribution (% of males:females) | 55:45 | 78:22 | 51:49 |
Results are expressed as number of individuals of each specific genotype with the % frequency in parentheses. Groups were compared by a chi-squared test and *indicates a statistically significant difference from controls and polyp groups (both P < 0.05)
Discussion
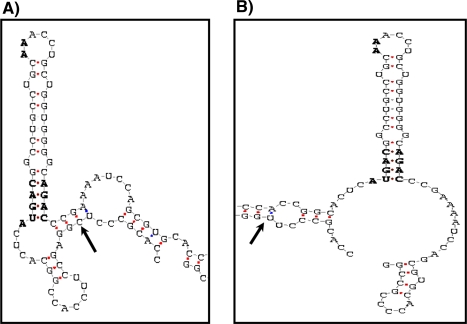
The T/C allelic variants at position 718 investigated in the present study occur in a region of the GPx4 gene that corresponds to the 3′UTR of the mRNA [42] and, notably, is close to the base of the selenocysteine insertion sequence (SECIS) element (Fig. 2), a region of the mRNA required for incorporation of the amino acid Sec into GPx4. Higher reporter activity was observed in cells transfected with the IDI-GPx4 C variant and grown under either Se-adequate or Se-deficient conditions compared with the corresponding activities observed with the T variant. However, the activity driven by T variant was significantly less sensitive to Se depletion. This may reflect the much lower starting level for activity for the T variant in Se-adequate conditions but it suggests that, in vivo, the C variant leads to GPx4 synthesis that is greater at optimal Se intake but that is more sensitive to Se depletion. In contrast, there was no significant difference between the mRNA levels of IDI-GPx4 T 718 and IDI-GPx4 C 718 constructs in Se-deficient and Se-adequate conditions, suggesting that any effect of the 3′UTR on Se incorporation occurs at a translational, rather than transcriptional level. The present data, therefore, indicate that the T and C variants of the GPx4 3′UTR differ in their ability to drive synthesis of a reporter selenoprotein.
Fig. 2.
Prediction of the secondary structure of the region containing the SECIS element in the GPx4 3′UTR. The C (a) and T (b) variants of the polymorphism at position 718 (indicated by arrow) were predicted to result into two patterns of the RNA folding. Both predicted structures contain the same consensus sequence (Non-Watson–Crick quartet), as well as the same number of stems and loops in the core of the SECIS but there were differences in the region close to the SECIS. Prediction patterns were obtained using the SECIS-search secondary structure prediction program [30]
These results are the first direct evidence that the SNP T/C 718 in the GPx4 3′UTR has functional consequences. They are supported by computational analysis that suggests that there may be structural differences around the two core SECIS elements (Fig. 2) in the GPx4 T and GPx4 C 3′UTRs. The ability of a small change in 3′UTR sequence to affect Se incorporation is compatible with previous studies showing that the efficiency of SECIS elements to incorporate Se differs between selenoproteins in vitro [29] and that two SNPs within the 3′UTR of the 15 kDa selenoprotein influence the ability of the 3′UTR to support Se incorporation [22].
In addition, the present data also provide evidence for a link between the SNP T/C 718 and disease risk. Since GPx4 has been implicated in the regulation of leukotriene metabolism [8, 25] and individuals of CC and TT genotype for the GPx4 T/C 718 SNP have been reported to have different levels of lymphocyte 5-lipoxygenase products [42], it is possible that the SNP has functional consequences in vivo and it may cause differences in inflammatory responses. The lower frequency of TT genotypes for the GPx4 T/C 718 SNP observed in patients suffering from ulcerative colitis [33] and the evidence for a link between longstanding ulcerative colitis and colon cancer [36, 38] led us to investigate the frequency of this particular SNP, in patients diagnosed with CRC or adenomatous polyps and healthy controls. The frequency of the TT, TC and CC variations at this position was found to be different in patients with CRC from those patients with adenomatous polyps or controls with no colo-rectal neoplasia, suggesting that the risk of CRC, but not the risk of having adenomatous polyps, is affected by genotype at the T/C 718 SNP in the GPx4 gene. It is possible that the GPx4 T/C 718 SNP causes differences in inflammatory responses in the colon, responses that have been suggested to affect progression of polyps to tumour formation [26, 28].
However, the link between the C variant, possibly higher GPx4 activity and higher levels of lipoxygenase products and disease risk has not been defined. One possibility is that alterations in GPx4 lead to changes in 5- and 15-lipoxygenase activity, cycloxygenase-2 activity and activation of NF-κB pathways [7, 21, 37), reflecting the role of GPx4 as a regulator of inflammatory responses. A second possible mechanism is based on the hierarchy of selenoprotein synthesis in which selenoproteins essentially compete for available Se [6] and, on such a model, increased synthesis of GPx4 due to the C variant would be expected to cause a withdrawal of Se from synthesis of other selenoproteins such as GPx1 and so lead to an altered pattern of overall selenoprotein synthesis. The observed colitis in the combined GPx1/GPx2 knock-out mice where GPx1 has a minor role in counteracting inflammation [16, 17) is compatible with this hypothesis. Furthermore, such indirect effects, through changes in the selenoprotein hierarchy, not only could account for the link between the C variant and disease risk but are also consistent with the observed beneficial effect of increased Se intake on CRC risk [27]. However, a third possibility is that high GPx4 expression may lead directly to lower apoptosis [31].
Several lines of evidence suggest that low Se status increases risk of mortality from CRC. Firstly, colon cancer mortality has been inversely correlated with estimated Se intake [12]. Secondly, low Se intake or low plasma Se is associated with increased risk of CRC [12, 35]. Thirdly, a recent meta-analysis indicates that higher Se status is associated with lower risk of a recurrence of colonic tumours [27]. Finally, a clinical trial indicates that Se supplementation reduces CRC mortality rates [10]. In view of these reported effects of low Se status on CRC risk [12, 27, 35, 43], it is possible that the impact of genotype at the 718 SNP is modulated by Se status. Plasma Se and erythrocyte GPx1 activity are commonly used measures of functional Se status [14]. Interestingly, preliminary data indicate that in a sub-group of the cancer patients studied here both plasma Se levels and erythrocyte GPx1 activity were significantly lower in individuals with CC genotype (mean ± SEM plasma Se = 0.80 ± 0.06 μmol/l, mean ± SEM GPx1 activity = 4.5 ± 0.5 U/ml) than in individuals with CT or TT genotype (mean plasma Se = 0.99 ± 0.04 and 1.00 ± 0.05 μmol/l, respectively and mean GPx1 activity = 5.3 ± 0.2 and 6.1 ± 0.7 U/ml respectively). In contrast, in the polyp group, plasma Se concentration and erythrocyte GPx1 activity were not significantly affected by genotype. The observed differences in plasma Se and erythrocyte GPx1 activity with genotype and clinical diagnosis suggest that the combination of Se status and GPx4 T/C 718 genotype affects development of colorectal cancer but not the risk of developing polyps. This is consistent with the previous suggestion that patients with polyps and the lowest Se levels are at higher risk of malignant transformation [15].
In summary, the present study demonstrates the functionality of the SNP T/C 718 in the GPx4 gene and provides evidence that genotype at this SNP is associated with altered risk of CRC. Our hypothesis is that a combination of sub-optimal Se status and a particular genotype at the GPx4 T/C 718 SNP increases susceptibility to CRC but not to the risk of developing adenomatous polyps. Further work is required to define the link between GPx4 T/C 718 variants, selenoprotein synthesis and cell function.
Acknowledgments
We thank the World Cancer Research Fund for financial support (grants 2000/10, 2002/41), clinical staff for assistance with sampling and the patients for consenting to take part in this study. We greatly acknowledge Brian Burtle, Rebecca Lamb, Leanne Boulding and Rachel Ledward for help with genotyping and Fergus Nicol for help with IDI activity assay. JRA’s lab is funded by The Scottish Executive Environment and Rural Affairs Department (SEERAD). Conflict of interest-None declared.
Footnotes
G. Bermano and V. Pagmantidis have contributed equally to this work.
References
- 1.Akbaraly NT, Arnaud J, Hininger-Favier I, Gourlet V, Roussel AM, Berr C (2005) Selenium and mortality in the elderly: results from the EVA study. Clin Chem 51:2117–2123 [DOI] [PubMed]
- 2.Arthur JR, Nicol F, Beckett GJ (1990) Hepatic type 1 iodothyronine 5′-deiodinase. The role of selenium. Biochem J 272:537–540 [DOI] [PMC free article] [PubMed]
- 3.Bermano G, Nicol F, Dyer JA, Sunde RA, Beckett GJ, Arthur JR, Hesketh JE (1995) Tissue-specific regulation of selenoenzyme gene expression during selenium deficiency in rats. Biochem J 311:425–430 [DOI] [PMC free article] [PubMed]
- 4.Bermano G, Arthur JR, Hesketh JE (1996) Role of the 3′ untranslated region in the regulation of cytosolic glutathione peroxidase and phospholipid-hydroperoxide glutathione peroxidase gene expression by selenium supply. Biochem J 320:891–895 [DOI] [PMC free article] [PubMed]
- 6.Berry MJ (2005) Insights into the hierarchy of selenium incorporation. Nat Genet 37:1162–1163 [DOI] [PubMed]
- 5.Berry MJ, Banu L, Chen YY, Mandel SJ, Kieffer JD, Harney JW, Larsen PR (1991) Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3′ untranslated region. Nature 353:273–276 [DOI] [PubMed]
- 8.Brigelius-Flohe R (1999) Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med 27:951–965 [DOI] [PubMed]
- 7.Brigelius-Flohe R, Friedrichs B, Maurer S, Schultz M, streicher R (1997) Interleukin-1-induced nuclear factor kappa B activation is inhibited by overexpression of phospholipid hydroperoxide glutathione peroxidase in a human endothelial cell line. Biochem J 328:199–203 [DOI] [PMC free article] [PubMed]
- 9.Clark LC, Hixson LJ, Combs GF, Reid ME, Turnbull BW, Sampliner RE (1993) Plasma selenium concentration predicts the prevalence of colorectal adenomatous polyps. Cancer Epidemiol Biomark Prev 2:41–46 [PubMed]
- 10.Clark LC, Combs GF Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL Jr, Park HK, Sanders BB Jr, Smith CL, Taylor JR (1996) Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. J Am Med Assoc 276:1957–1963 [DOI] [PubMed]
- 12.Combs GF (2005) Current evidence and research needs to support a health claim for selenium and cancer prevention. J Nutr 135:343–347 [DOI] [PubMed]
- 11.Combs GF Jr, Gray WP (1998) Chemopreventive agents: selenium. Pharmacol Ther 79:179–192 [DOI] [PubMed]
- 13.Cummings OW (2000) Pathology of the adenoma–carcinoma sequence: from aberrant crypt focus to invasive carcinoma. Semin Gastrointest Dis 11:229–237 [PubMed]
- 14.Diplock AT (1993) Indexes of selenium status in human populations. Am J Clin Nutr 57:256S–258S [DOI] [PubMed]
- 15.Dworkin BM, Rosenthal WS, Mittelman A, Weiss L, Applebee-Brady L, Arlin Z (1988) Selenium status and the polyp-cancer sequence: a colonoscopy controlled study. Am J Gastroenterol 83:748–751 [PubMed]
- 16.Esworthy RS, Aranda R, Martin MG, Doroshow JH, Binder SW, Chu FF (2001) Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol 281:G848–55 [DOI] [PubMed]
- 17.Esworthy RS, Yang L, Frankel PH, Chu FF (2005) Epithelium-specific glutathione peroxidase, Gpx2, is involved in the prevention of intestinal inflammation in selenium-deficient mice. J Nutr 135:740–745 [DOI] [PubMed]
- 18.Forsberg L, de Faire U, Morgenstern R (1998) To identify genetic polymorphisms in the “Expressed Sequence Tag” (EST) database. Technical tips on-line http://www.elsevier.com_/locate_/tto, T01440
- 19.Forsberg L, de Faire U, Marklund SL, Andersson PM, Stegmayr B, Morgenstern R (2000) Phenotype determination of a common Pro-Leu polymorphism in human glutathione peroxidase 1. Blood Cells Mol Dis 26:423–426 [DOI] [PubMed]
- 20.Haklar G, Sayin-Ozveri E, Yuksel M, Aktan AO, Yalcin AS (2001) Different kinds of reactive oxygen and nitrogen species were detected in colon and breast tumors. Cancer Lett 165:219–224 [DOI] [PubMed]
- 21.Heirman I, Ginneberge D, Brigelius-Flohe R, Hendrickx N, Agostinis P, Brouckaert P, Rottiers P. And Grooten J (2006) Blocking tumor cell eicosanoid synthesis by GPx4 impedes tumor growth and malignancy. Free Radic Biol Med 40:285–94 [DOI] [PubMed]
- 23.Hu YJ, Diamond AM (2003) Role of glutathione peroxidase 1 in breast cancer: loss of heterozygosity and allelic differences in the response to selenium. Cancer Res 63:3347–3351 [PubMed]
- 22.Hu YJ, Korotkov KV, Mehta R, Hatfield DL, Rotimi CN, Luke A, Prewitt TE, Cooper RS, Stock W, Vokes EE, Dolan ME, Gladyshev VN, Diamond AM (2001) Distribution and functional consequences of nucleotide polymorphisms in the 3′-untranslated region of the human Sep15 gene. Cancer Res 61:2307–2310 [PubMed]
- 24.Ichimura Y, Habuchi T, Tsuchiya N, Wang L, Oyama C, Sato K, Nishiyama H, Ogawa O, Kato T (2004) Increased risk of bladder cancer associated with a glutathione peroxidase 1 codon 198 variant. J Urol 172:728–732 [DOI] [PubMed]
- 25.Imai H, Nakagawa Y (2003) Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med 34:145–169 [DOI] [PubMed]
- 26.Itzkowitz SH, Yio X (2004) Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol 287:G7–G17 [DOI] [PubMed]
- 27.Jacobs ET, Jiang R, Alberts DS, Greenberg ER, Gunter EW, Karagas MR, Lanza E, Ratnasinghe L, Reid ME, Schatzkin A, Smith-Warner SA, Wallace K, Martinez ME (2004) Selenium and colorectal adenoma: results of a pooled analysis. J Natl Cancer Instit 96:1669–1675 [DOI] [PubMed]
- 28.Jacobson-Brown P, Neuman MG (2004) Colorectal polyposis and immune-based therapies. Canad J Gastroenterol 18:239–249 [DOI] [PubMed]
- 29.Kollmus H, Flohe L, McCarthy JE (1996) Analysis of eukaryotic mRNA structures directing cotranslational incorporation of selenocysteine. Nucleic Acids Res 24:1195–1201 [DOI] [PMC free article] [PubMed]
- 30.Kryukov GV, Kryukov VM, Gladyshev VN (1999) New mammalian selenocysteine-containing proteins identified with an algorithm that searches for selenocysteine insertion sequence elements. J Biol Chem 274:33888–33897 [DOI] [PubMed]
- 31.Nomura K, Imai H, Koumura T, Arai M, Nakagawa Y (1999) Mitochondrial phospholipid hydroperoxide glutathione peroxidase suppresses apoptosis mediated by a mitochondrial death pathway. J Biol Chem 274:22294–29302 [DOI] [PubMed]
- 32.Pagmantidis V, Bermano G, Villette S, Broom J, Arthur J, Hesketh JE (2005) Effects of Se-depletion on glutathione peroxidase and SelW gene expression in the colon. FEBS Lett 579:792–796 [DOI] [PubMed]
- 33.Qatatsheh A, Seal CJ, Jowett SL, Welfare MR, Hesketh JE (2005) Patients with ulcerative colitis show an altered frequency distribution of a single nucleotide polymorphism in the gene encoding the phospholipid hydroperoxide glutathione peroxidase. Proceedings of the Nutrition Society 64, OCA, 20A (Abstract)
- 34.Rayman MP (2005) Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc 64:527–542 [DOI] [PubMed]
- 35.Russo MW, Murray SC, Wurzelmann JI, Woosley JT, Sandler RS (1997) Plasma selenium levels and the risk of colorectal adenomas. Nutr Cancer 28:125–129 [DOI] [PubMed]
- 36.Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A (2004) Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 126:451–459 [DOI] [PubMed]
- 37.Schnurr K, Belkner J, Ursini F, Schewe T, Kuhn H (1996) The selenoenzyme phospholipid hydroperoxide glutathione peroxidase controls the activity of the 15-lipoxygenase with complex substrates and preserves the specificity of the oxygenation products. J Biol Chem 271:4653–4658 [DOI] [PubMed]
- 38.Seril DN, Liao J, Yang GY, Yang CS (2003) Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis 24:353–362 [DOI] [PubMed]
- 39.Shamberger RJ, Tytko SA, Willis CE (1976) Antioxidants and cancer. Part VI. Selenium and age-adjusted human cancer mortality. Arch Environ Health 31:231–235 [DOI] [PubMed]
- 40.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Ann Biochem 150:76–85 [DOI] [PubMed]
- 41.Stadtman TC (1996) Selenocysteine. Ann Rev Biochem 65:83–100 [DOI] [PubMed]
- 42.Villette S, Kyle JA, Brown KM, Pickard K, Milne JS, Nicol F, Arthur JR, Hesketh JE (2002) A novel single nucleotide polymorphism in the 3′ untranslated region of human glutathione peroxidase 4 influences lipoxygenase metabolism. Blood Cells Mol Dis 29:174–178 [DOI] [PubMed]
- 43.Whanger PD (2004) Selenium and its relationship to cancer: an update dagger. Br J Nutr 91:11–28 [DOI] [PubMed]
- 44.Wingler K, Böcher M, Flohe L, Kollmus H, Brigelius-Flohe R (1999) mRNA stability and selenocysteine insertion sequence efficiency rank gastrointestinal glutathione peroxidase high in the hierarchy of selenoproteins. Eur J Biochem 259:149–157 [DOI] [PubMed]
- 45.Yagi K, Komura S, Kojima H, Sun Q, Nagata N, Ohishi N, Nishikimi M (1996) Expression of human phospholipid hydroperoxide glutathione peroxidase gene for protection of host cells from lipid hydroperoxide-mediated injury. Biochem Biophys Res Commun 219:486–491 [DOI] [PubMed]