Abstract
Diabetes is associated with accelerated atherosclerosis and macrovascular complications are a major cause of morbidity and mortality in this disease. Although our understanding of vascular pathology has lately greatly improved, the mechanism(s) underlying enhanced atherosclerosis in diabetes remain unclear. Endothelial cell dysfunction is emerging as a key component in the pathophysiology of cardiovascular abnormalities associated with diabetes. Although it has been established that endothelium plays a critical role in overall homeostasis of the vessels, vascular smooth muscle cells (vSMC) in the arterial intima have a relevant part in the development of atherosclerosis in diabetes. However, high glucose induced alterations in vSMC behaviour are not fully characterized. Several studies have reported that impaired nitric oxide (NO) synthesis and/or actions are often present in diabetes and endothelial dysfunction. Furthermore, although endothelial cells are by far the main site of vascular NO synthesis, vSMC do express nitric oxyde synthases (NOSs) and NO synthesis in vSMC might be important in vessel’s function. Although it is known that vSMC contribute to vascular pathology in diabetes by their change from a quiescent state to an activated proliferative and migratory phenotype (termed phenotypic modulation), whether this altered phenotypic modulation might also involve alterations in the nitrergic systems is still controversial. Our recent data indicate that, in vivo, chronic hyperglycemia might induce an increased number of vSMC proliferative clones which persist in culture and are associated with increased eNOS expression and activity. However, upregulation of eNOS and increased NO synthesis occur in the presence of a marked concomitant increase of O2− production. Since NO bioavailabilty might not be increased in high glucose stimulated vSMC, it is tempting to hypothesize that the proliferative phenotype observed in cells from diabetic rats is associated with a redox imbalance responsible quenching and/or trapping of NO, with the consequent loss of its biological activity. This might provide new insight on the mechanisms responsible for accelerated atherosclerosis in diabetes.
Keywords: Hyperglicemia, Nitric oxide, Atherosclerosis
Chronic hyperglycemia and risk of cardiovascular disease
Diabetes mellitus is characterized by accelerated atherosclerosis and increased risk of cardiovascular disease (CVD) [1, 2]. Although a number of conventional cardiovascular risk (CVR) factors are altered in diabetic patients, several studies have demonstrated that the increased CVD risk in diabetes cannot be explained by the concomitant increase in CVR factors alone [3]. Infact, recent evidences strongly suggest that hyperglycemia, probably through increased oxidative stress [4], can induce endothelial dysfunction [1], which plays a central role in the development of atherosclerosis [5–7] and can be considered an early sign of diabetic vascular disease.
On the other hand, although glycemic control remains the major intervention for prevention of micro- and macrovascular disease, stronger predictors of macrovascular complications are factors such as LDL cholesterol and blood pressure that may be related more to insulin resistance than to glycemic control [8]. Nonetheless, since hyperglycemia associated with insulin resistance can lead to modification of macromolecules (as advanced glycation end products that bind surface receptors) which augment the production of proinflammatory cytokines in vascular endothelial cells, the correction of both hyperglycemia and insulin resistance improves endothelial functions.
Moreover, recently growing evidence suggests that dietary factors play an important role in modulating endothelial function. Higher dietary glycemic loads have been associated with increased plasma concentrations of inflammatory cytokines and endothelial adhesion molecules, both of which are considered markers of endothelial dysfunction [9]. These findings provide additional biological mechanisms through which dietary factors, such as higher dietary glycemic load, influence the risk of cardiovascular disease. In addition, several epidemiologic and interventional studies have examined the relationship between overall dietary patterns and endothelial dysfunction. In general, a “prudent diet” (high intake of vegetables, legumes, fish and whole grains) is associated with a beneficial effect on the endothelium.
Thus, under physiological conditions, endothelial cells (EC) lining the lumen of all vasculature, act as an interface between circulating blood and vascular tissue and they can be considered the first vascular cells to sensor humoral changes. Therefore, due to their position, EC facilitate a complex array of functions in intimate interaction with vSMC, as well as cells within the blood compartment such as monocytes or platelets. Indeed, it has been established that the endothelium plays a critical role in overall homeostasis of the vessels. Their functions are integrated by a complex system of chemical mediators including endothelin-1 (ET-1), the vasoactive peptide angiotensin II (Ang II), leukocyte adhesion molecules, the anti-fibrinolytic factor plasminogen activator inhibitor-1 (PAI-1), reactive oxygen species (ROS), bradykinin and reactive nitrogen species such as nitric oxide (NO) [10–13]. The “endothelial system” exerts actions on the surrounding vSMC and cells in the blood that lead to the following biological effects: (1) vasodilation (bradykinin and NO) or vasoconstriction (ET-1, Ang II, ROS) of vSMC; (2) stimulation of growth and change in phenotypic characteristics of vSMC (Ang II, ROS) or inhibition of vSMC proliferation (NO); (3) maintenance of blood fluidity and normal coagulation (PAI-1) [10–13]. Therefore, the cellular effects of EC maintain a dynamic balance of opposing physiological and molecular effects with the ultimate result of allowing a proper blood supply to tissues and regulating the potential proinflammatory actions of adhesion molecules and coagulating factors [11, 12].
Nitric oxide and endothelial function
The key factor in preserving physiological endothelial functions is NO, which is prevalently generated via the constitutive endothelial nitric oxide synthase (eNOS). Endogenous NO is synthesized via the conversion of the amino acid l-arginine in to l-citrulline by the enzyme nitric oxide synthase, of which several isoforms have been isolated, purified, and cloned. Three distinct isoforms of NOS have been identified as products of different genes, with different localization, catalytic properties and inhibitor sensitivities [11, 14, 15]. Neuronal NOS (nNOS, also known as type I NOS) predominates in neuronal tissue; it is functionally relevant in the central control of vascular homeostasis [11, 15, 16]. Inducible NOS (iNOS, also known as type II NOS), is found in a wide range of tissues and cells. Its expression is only promoted under pathophysiological situations, including endothelial dysfunction, in which macrophages exert cytotoxic effects in response to cytokines [11, 15–17]. Endothelial NOS (eNOS, also known as type III NOS) is the isoform first found in vascular EC, it is constitutively expressed and is essential for the control of vascular tone in response to several stimuli, including mechanical (i.e. shear stress), receptor dependent (i.e. acetylcholine) and receptor independent (i.e. calcium ionophore) effects.
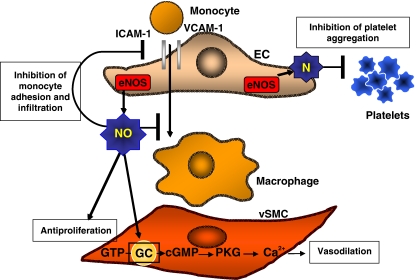
As schematically represented in Fig. 1, NO produced by eNOS in the endothelium diffuses to the vSMC where it activates the enzyme guanylate cyclase (GC). The concomitant increase in cyclic GMP (cGMP) then induces relaxation of the vascular smooth muscle [11, 15, 16]. Thus, the net effect of NO is an increase in vasodilation. The continual vasodilation produced by basal NO generation has a role in regulating blood pressure as well. Moreover, physiological NO acts as a pleiotropic molecule and capable of preserving vascular wall homeostasis [18, 19] also contributing to the prevention of platelet aggregation and adhesion to the vascular wall [11]; it also controls the expression of vascular cell adhesion molecules and finally, besides its effect on relaxation of vSMC, it also inhibits vSMC proliferation and migration [18, 19].
Fig. 1.
Schematic and simplified representation of endothelial function. Please see text for details. EC indicates endothelial cells; vSMC vascular smooth muscle cells; eNOS endothelial nitric oxide synthase, NO nitric oxide; VCAM-1 vascular cell adhesion molecule-1; ICAM-1 intercellular adhesion molecule-1; GTP guanosine 5′-triphosphate; GC guanylate cyclase; cGMP cyclic guanosine monophosphate; PKG protein kinase G
Thus, impaired NO synthesis and/or availability may result in endothelial and vascular wall dysfunction. Diminished NO bioavailability [4, 20, 21] has been demonstrated experimentally when vascular cells are exposed either in vitro or in vivo to a diabetic environment. The endothelium can be viewed as a target of the diabetic milieu and endothelial dysfunction is thought to play an important role in the vasculopathy of this disease state. A large body of evidence in humans indicates that endothelial dysfunction is closely associated with alteration of large vessels and atherosclerosis in type 2 diabetes [11, 22–25].
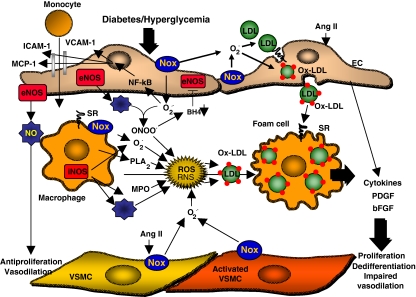
As mentioned above, under physiological conditions endothelium transmits the information about humoral changes to the other vascular cell types, in particular to vSMC, by changing their gene expression profile and coordinate the production of growth factors, cytokines, adhesion molecules and other bioactive molecules. It is known that chronic hyperglycemia, likely via increased oxidative stress, plays a critical role in endothelial dysfunction and in the etiology of atherosclerosis in diabetes [4]. Different mechanisms have been described to account for increased oxidative stress in hyperglycemic conditions: activation of Nox-based NAD(P)H oxidases [26–28], xanthine oxidase [29], or uncoupling eNOS [29, 30]. Such evidence is discussed in this review and is shown in schematic form in Fig. 2. In particular, uncoupling of eNOS (a situation in which eNOS synthesizes superoxide rather than NO), may be explained by several mechanisms. First, it can occur when reactive species such as peroxynitrates (ONOO−) oxidize the essential NOS cofactor, tetrahydrobiopterin (BH4), yielding to the formation of O2·− and H2O2 instead of NO [30–32]. Second, ONOO− may also release Zn2+ from the complex Zn2+-thiolate complex resulting in disruption of eNOS dimer and enzyme uncoupling [33]. Third, protein kinase C (PKC) may cause phosphorylation of eNOS in Thr495 leading to the uncoupling of electron flux in eNOS to NO production [34]. These events may lead, in turn, to redox-dependent nuclear factor-kB (NF-kB)-mediated expression of adhesion molecules and recruitment of monocytes, which become activated macrophages and secrete myeloperoxidase (MPO) [26, 35]. Dedifferentiated smooth muscle cells are activated by Ang II or cytokines, such as tumor necrosis factor-α (TNF-α), and also ROS via NAD(P)H oxidase [10, 26]. Increased ROS as well as the reactive species resulting from their reaction with NO will provoke oxidation of low density lipoproteins (LDL).
Fig. 2.
Schematic and simplified representation of endothelial dysfunction. Please see text for details. EC indicates endothelial cells; vSMC vascular smooth muscle cells; eNOS endothelial nitric oxide synthase; iNOS inducible nitric oxide synthase; NO nitric oxide; VCAM-1 vascular cell adhesion molecule-1; ICAM-1 intercellular adhesion molecule-1; MCP-1 monocyte chemoattractant protein-1; NF-kB nuclear factor-kB; Nox NADPH-oxidase; ONOO−, peroxynitrates; BH4 tetrahydrobiopterin; PLA2 phospholipase A2; MPO myeloperoxidase; SR scavenger receptor; AngII angiotensin II; PDGF platelet-derived growth factor; bFGF, basic fibroblast growth factor; ROS reactive oxygen species; RNS reactive nitrogen species; ox-LDL oxidized low density lipoproteins
Oxidized LDL (OxLDL) induces atherosclerosis by (1) stimulating monocyte infiltration, (2) stimulating vSMC migration and proliferation, (3) contributing to impairment of the vasodilator function of arteries, and (4) participating in atherothrombosis.
Adhesion and infiltration of macrophages contributes to fatty streak formation. OxLDL induces endothelium to express adhesion molecules for monocytes, intercellular adhesion molecule-1 (ICAM-1) and vascular adhesion molecule-1 (VCAM-1), E-selectin, and fibronectin. OxLDL induces expression of secretory phospholipase A2 (sPLA2) and MPO in macrophages [35–37]. sPLA2 liberates polyunsaturated fatty acid from LDL and increases the formation of oxidized phospholipids. sPLA2 also enhances the accumulation of cholesterol ester hydroperoxides induced by lipoxygenase and thereby oxidize LDL [35, 38, 39]. OxLDL stimulates the endothelium to secrete the chemokine, monocyte chemotactic protein-1 (MCP-1), which induces macrophage infiltration into the endothelial space [35, 40, 41]. On the other hand, the H2O2–MPO system further oxidizes LDL and converts them into a form recognized by the scavenger receptor (SR, LOX-1) [35, 37, 42]. Interaction of OxLDL with SR induces unregulated uptake of modified LDL into macrophages leading to massive cholesterol accumulation and formation of foam cells [35, 42].
The OxLDL also induces migration of vSMC by increasing the expression of platelet-derived growth factor (PDGF) by EC, vSMC, and macrophages [43–46]. OxLDL stimulates vSMC proliferation by inducing expression of basic fibroblast growth factor (bFGF) by ECs and smooth muscle cells [47–51]. OxLDL and thromboxan A2 (TxA2) released by aggregating platelets have a synergistic interaction on vSMC proliferation [35, 52].
Intimal thickening in arteries is caused by accumulation of foam cells and by vSMC migration and proliferation. It results in reduction of the arterial lumen, which is exacerbated by impairment of vasodilation capacity of the artery. It has been proposed that this modulation of the vasomotion occurs by interaction of ROS with endogenous vasoactive mediators secreted by EC. For instance, O2·− reacts with endothelium-derived NO rapidly and inactivate its biological effects [30, 35, 53, 54]. Moreover, it has been recently demonstrated that in various disease conditions all three types of NOS (neuronal, inducible, and endothelial) may generate oxidants through not fully known mechanisms [32]. In particular, iNOS is expressed in atherosclerotic plaques and local release of large amount of NO and O2·− has been linked to the production of harmful oxidative products such as peroxynitrite [17]. Importantly, it has also been shown that diabetes/hyperglycemia increases the concentration of asymmetric dimethylarginine (ADMA), an endogenous inhibitor of NOS, via a redox-dependent mechanism [55]. Moreover, OxLDL also induce vasoconstriction through inhibition of NO production and induction of expression of ET-1 [35]. OxLDL inactivates NO and inhibits the expression and the enzymatic activity of NOS [30, 35, 56–58] as well as GC, the target enzyme of NO in smooth muscle cells [30].
Endothelial dysfunction is associated with enhanced platelet adhesion, increased procoagulant activity, and impaired fibrinolysis. OxLDL stimulates platelet adhesion and aggregation by decreasing endothelial production of NO, increasing prostacyclin production, and stimulating the synthesis of prostaglandins and prostaglandin precursors. OxLDL enhances the procoagulant activity of endothelium by inducing release of tissue factor (TF) by EC and smooth muscle cells [35, 59, 60]. TF is a cofactor of factor VIIa that activates factors IX and X, resulting in thrombin formation. OxLDL reduces the fibrinolytic activity of endothelium by decreasing secretion of tissue-type plasminogen activator (tPA) and increasing the release of PAI-1 [35, 61, 62].
Actually, although our understanding of endothelial dysfunction in diabetes has lately greatly improved (as schematically summarized in Fig. 2), researchers have yet to understand the precise mechanisms that leads to diabetic vascular disease. As previously mentioned, hyperglycemia has been identified as independent risk factor for micro- and macrovascular complications. Although elevated levels of glucose, likely mediated by PKC activation [34, 63, 64], induce expression of procoagulant and extracellular matrix proteins, inhibit fibrinolysis, decrease endothelium proliferation and increase apoptosis of endothelial cells [4, 65–69], most of the current knowledge about the molecular mechanism used by glucose to regulate gene expression is based on studies that employed cells with insulin-dependent glucose transport, such as adipocytes and hepatocytes [70, 71].
Regulation of vascular genes by glucose
Due to a central importance of glucose as both a fuel for energy and a substrate for the biosynthesis of cell components, all cell types have evolved mechanisms to sense glucose levels in their environment and to adapt the expression of their genes to glucose availability. In case of chronic hyperglycemia, the effect of glucose in cells that are not dependent on insulin for glucose transport, such as vascular cells, results in rapid and uncontrollable transport glucose into the cell, whereby normal metabolic pathways become rapidly overloaded. Although elevated levels of glucose can cause most vascular diabetic complications directly [72–75], very little is known about mechanism of gene regulation by glucose in vascular cells. One crucial issue stands out in understanding pathways of glucose regulated vascular gene transcription. How does intracellular high glucose level transduced transcriptional activities of the cell? Most of our knowledge of transcriptional and translational mechanisms activated by glucose has come from studies on hepatocytes, adipocytes and pancreatic β-islet cells [70, 71]. As mentioned above, these cells are very different from vascular cells both in glucose uptake mechanism and response to hyperglycemia.
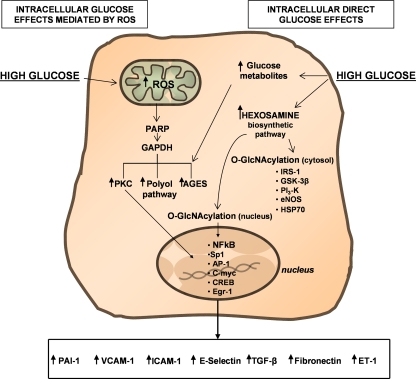
Recently, excellent reviews have detailed the vascular mechanisms of activation of main metabolic pathways by glucose [4] suggesting that the generation of ROS in response to high glucose levels in vascular cells is recognized as a mechanism responsible for the majority of the effects of glucose [76], as shown in schematic form on the left of Fig. 3. Nishikawa et al.[77] suggested that glucose increased production of intracellular ROS, likely through the inhibition of GAPDH activity by poly(ADP)-ribosylation, augmented diacylglycerol (DAG) levels leading to PKC activation, advanced glycation product (AGEs) formation, and the activation of polyol pathway. Inhibition of mitochondrial ROS production suppresses the high glucose-induced effects on PKC, AGEs, sorbitol formation and NF-kB activation. Although down-regulating ROS production by antioxidant treatments has been effective in vitro and can show benefits in animal models, antioxidants have failed to demonstrate any beneficial effects in terms of prevention of coronary heart disease and death [78] or on the development of vascular diabetic complication in large scale clinical trials [1, 79].
Fig. 3.
Schematic overview of high glucose intracellular effects. Please see text for details. ROS indicates reactive oxygen species; PARP Poly ADP-Ribose Polymerase; GAPDH glyceraldehyde-3-phosphate dehydrogenase; PKC protein kinase C; AGEs advanced glycation product; NF-kB nuclear factor-kB; IRS-1 insulin receptor substrate-1; GSK-3β glycogen synthase kinase-3beta kinase; PI3-K phosphatidylinositol 3-kinase; eNOS endothelial nitric oxide synthase; HSP70 heat shock protein 70; VCAM-1 vascular cell adhesion molecule-1; ICAM-1 intercellular adhesion molecule-1; CREB CRE-binding protein; Egr-1 early growth response gene-1; PAI-1 plasminogen activator inhibitor-1; TGF-β transforming growth factor- β; ET-1 endothelin-1
In cultured cells stimulated with high glucose, experimental data suggest that other events can account for the initiation of signaling event leading to vascular complications. Based on these studies, it has been hypothesized that glucose metabolites can directly initiate formation of intracellular AGEs [80] and/or, by activation of hexosamine pathway, glucose might induce direct glycation of cytosolic and membrane proteins, receptors and their ligands, and that alterations in lipid patterns can change the normal signaling events [81, 82]. Interestingly, as shown in Fig. 3, modification of transcription factors and signaling messengers by glucose metabolites may also result in activation of gene transcription in the nucleus [71]. Among the mechanisms by which glucose activates any specific gene, several studies clearly indicate a role of enzymatic intracellular O-glycosylation (O-GlcN-acylation) in mediating cellular events associated with hyperglycemia-induced complications [83]. High glucose levels increase O-GlcN-acylation in a variety of cell types [84–86] and this reaction is concentrated in the nucleus, where it has been identified on transcription factors, such as Sp1, c-myc, YY1, and CRE-binding protein (CREB), as well as other nuclear proteins [83, 87]. As schematically shown in Fig. 3, certain cytosolic proteins have also been reported to be subject to O-GlcN-acylation, including eNOS [87], insulin receptor substrate-1 (IRS-1) [88, 89], glycogen synthase [89], heat shock protein 70 (HSP70) and α-tubulin [83, 90]. For example, very recently [91] it has been demonstrated in glomerular mesangial cells that O-GlcN-acylation is indispensable for high-glucose induced PAI-1 gene expression, PAI-1 promoter activation, and Sp1 transcription activation. As recently reviewed by Zachara and Hart [92], O-GlcN-Ac has also been implicated in mediating many of the cytoplasmic molecular alterations induced by hyperglycemia. Infact, high glucose may also inhibit eNOS activity [93–95] by O-GlcN-Ac modification of Ser-1177, which apparently blocks insulin induced Akt phosphorylation of this aminoacid, thereby preventing enzymatic activation [93–95]. O-GlcNAc might also alter the function of vascular tissue by modulating the levels of proteins such as PAI-1 and TGF-α/β [96–101]. Thus, these data suggest a more complicated model for the glucose induced changes in intracellular pathways, where O-GlcN-acylation level may have not only a traditional role in energy metabolism but also serve as a downstream effector produced by glucose directly involved in regulating nuclear and cytoplasmic molecular activities.
In conclusion, although elevated levels of glucose may cause most vascular diabetic complication directly, our current knowledge about the molecular mechanisms of gene regulation by glucose in vascular cells is incomplete and controversial. As our understanding of the mechanisms of vascular complications associated with type II diabetes, it is becoming clear that rather than merely scavenging reactive radicals, a more comprehensive approach aimed at preventing both the generation of these reactive species and/or inhibition of enzymatic intracellular O-GlcN-acylation mechanisms may prove more beneficial.
Regulation of vascular NOS levels and activity by glucose
Although it has been demonstrated that diabetes is associated with endothelial dysfunction and it is known that endothelial-dependent vasodilation is significantly impaired in diabetic patients [102–104], whether and in which way elevated glucose levels might affect NO synthesis and bioavailability is still a matter of controversy [6, 104, 105]. The impaired endothelium-mediated vasodilation observed in diabetic patients might suggest a deficit in NO synthesis in these subjects. Federici et al. [94] observed a blunted increase in eNOS activity in response to insulin in human artery endothelial cells cultured in elevated glucose concentrations and Du et al. [93] have reported that elevated glucose concentrations induce a decrease in eNOS activity in bovine aortic endothelial cells. However, these observations are at odds with the results of several studies demonstrating that elevated glucose concentrations in vitro are capable of up-regulating eNOS gene and protein expression to stimulate NO production in both animal [103, 106] and human [107, 108] cells. It should be pointed out, however, that increased NO synthesis does not necessarily correspond to increased NO availability. For example, there is substantial evidence that under several conditions associated with accelerated atherosclerosis, such as hypertension, hypercholesterolemia, smoking, etc., NO production is not impaired, but NO bioavailability is reduced because of oxidative inactivation by excessive production of the superoxide anion in the vascular wall [109]. In the presence of high glucose concentrations, superoxide anion generation is increased and this leads to a pro-oxidant environment. Thus, it is conceivable that in the presence of a pro-oxidant environment brought about by hyperglycemia, NO and O2− react very rapidly to form peroxynitrite (ONOO−): this might also be consistent with the impaired endothelial function observed in diabetes [107, 109]. Moreover, synthesis of ONOO− has been considered as a channeling mechanism that diverts NO from physiological target to pathophysiological targets. Indeed, it has been reported that NO inhibits NF-kB activation in vascular cells, including human endothelial cells [110] and rodent vSMC [111]. Paradoxically, NO has also been reported to trigger NF-kB activation in various situations [112]. There are several explanations for this apparent conflicting action of NO, as noted above, co-synthesis of O2− in the inflammatory environment effectively channels NO to production of ONOO−, potentially leading to NF-kB activation in vascular cells. Moreover, Du et al. [113] suggests that peroxynitrite is a mediator by NF-kB activation of cytotoxic effects of high glucose on endothelial cells.
Our recent data obtained in HUVEC cultured from umbilical cords from gestational diabetic (GD) women [20], and thus exposed to chronic hyperglycemia in vivo, lend further support to this hypothesis: indeed, in this cellular model, Sobrevia et al. showed an increased basal NO synthesis which was maintained in cells cultured for up to five passages in vitro [114, 115] most likely via increased eNOS expression [116].
Furthermore, although endothelial cells are by far the main site of vascular NO synthesis, vSMC do express NOS isoforms and NO synthesis in vSMC might be important in vessel’s function [15, 117, 118]. However, though proliferative modification of vSMC and impaired bioavailability of NO have both been proposed among the mechanisms linking diabetes and atherosclerosis, diabetes induced modifications in phenotype and eNOS expression and activity in vSMC have not been fully characterized [21, 103, 119, 120].
The loss of vascular homeostasis is also characterized by migration and proliferation of vascular smooth muscle cells (vSMC) and, in the arterial intima, they are key events in the development of atherosclerosis [121]. Thus, alterations in vSMC behaviour and characteristics might contribute to vascular pathology in diabetes. At least two distinct vSMC phenotypes are present in the vessel wall [122–124], the so called contractile or differentiated vSMC phenotype, which is more abundant in healthy blood vessels, and a non-contractile or “synthetic” phenotype, particularly represented in areas of intense vascular remodeling such as the initial intimal atherosclerotic lesions [125–127]. In vSMC with the latter phenotype, the so called dedifferentiated vSMC, expression of proteins regulating contractile function (e.g., smooth muscle-specific isoform of myosin, alpha-actin and calponin), is decreased, while the capacity of generating extracellular matrix is increased [128, 129]. The process by which vSMC undergo a change from a quiescent state to an activated proliferative and migratory phenotype [128] is termed phenotypic modulation; it is the hallmark of atherosclerosis [121, 130] and might be enhanced in diabetes [131–133]. Indeed Suzuki et al. [134] have shown increased vSMC accumulation in atherosclerotic lesions of diabetic pigs fed a cholesterol-rich diet and abnormal cell morphology has been observed in culture of vSMC obtained from vessels of diabetic patients with severe atherosclerosis [135].
In vSMC, altered phenotypic modulation might also involve alterations in the nitrergic systems [136, 137]. As a matter of fact, several studies have reported impaired NO synthesis and/or action in diabetes and endothelial dysfunction is often thought to be present in this disease [6, 138, 139]. In in vitro systems mimicking diabetic milieu, decreased NO availability has been consistently observed in endothelial cell cultures [108, 140–143], although several conflicting mechanisms have been proposed to explain this reduced NO availability [102, 144].
For these reasons, we recently compared phenotypic characteristics, eNOS expression and activity and NO bioavailability in aortic smooth muscle cells from diabetic and non-diabetic rats. The results showed that diabetes is associated with modifications in vSMC phenotype and that these changes are associated with both increased eNOS expression/activity and greater basal superoxide anion release. On the other hand, NO bioavailability, as indirectly shown by lower intracellular cGMP levels, was reduced in cells from diabetic animals [21].
It is interesting to note that, in agreement with the observations by Etienne et al. [145], in vSMC obtained from diabetic rats (which were 90% pancreatectomized), we observed a phenotypic modulation process characterized by a decreased smooth muscle alpha-actin content: this indicates that vSMC from diabetic animals tend to switch from a contractile to a synthetic phenotype. These findings are consistent with those reported by Faries et al. [135], who, in vSMC cultures from diabetic patients vessels, observed a significant increase in cellular adhesion, migration and proliferation rate and an atypical culture morphology, suggestive of altered contact inhibition mechanisms. Furthermore, it appears that, in cultures from diabetic animals, proliferative potential is significantly increased.
The possible mechanism(s) responsible for the phenotypic modifications and increased eNOS expression observed in vSMC from diabetic animals were not addressed by our study. However, Watson et al. [133] have recently reported that, in rat aortic SMC, high glucose levels can reduce cAMP response element-binding protein (CREB) content. This seems to be mediated by glucose-induced reactive oxygen species [146], and it might be relevant for phenotype modulation, since cyclic nucleotides have an important role in maintaining the mature contractile phenotype in vSMC [124]. Indeed, it appears that, in vSMC, a close relationship exists between phenotype and redox state. Thus, on one side, it has been suggested that dedifferentiated SMC have a greater capacity to produce O2− by NAD(P)H oxidase [136] and, on the other side, there is evidence that NAD(P)H oxidase activity causes cellular transformation toward a more proliferative and migratory phenotype [137, 147].
Intracellular redox state is likely to be involved in the regulation of many cellular functions in vSMC, and it has been proposed that redox state might also affect NO synthesis and bioavailability [148]. vSMC are capable of NO synthesis and it is known that vSMC express iNOS, the NOS isoform inducible by inflammatory and atherogenic stimuli [117]. However, although diabetes mellitus represents a pro-atherogenic condition and it is associated with increased levels of inflammation markers [149], in vivo or in vitro evidence supporting increased vSMC iNOS expression in diabetes is so far very scant [150, 151]. On the other hand, our data show that rat vSMC do express the eNOS constitutively and that the enzyme is active in these cells. These data are in keeping with those obtained by Boulanger et al., who showed expression of both endothelial and neuronal NOS in human vSMC [152].
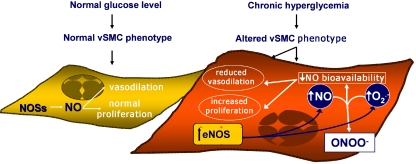
Our study also demonstrates that diabetes can affect eNOS expression and activity in rat vSMC. As a matter of fact, we observed increased eNOS mRNA levels and increased total NOS activity in vSMC from diabetic rats. As far as increased eNOS expression is concerned, our data are consistent with those reported by several groups, showing increased eNOS protein content in the aortic wall of diabetic rats [15, 103, 119]. In particular, Cai et al. [154] have recently demonstrated that, although total eNOS levels were increased, in diabetic mouse aortas NO levels were significantly decreased. Since the authors also demonstrated increased superoxide production in association with reductions in tetrahydrobiopterin, all together these data suggest that exposure to a diabetic milieu in vivo may results in greater vascular wall eNOS expression and reduced NO bioavailability (Fig. 4).
Fig. 4.
Schematic and simplified representation of glucose modified nitric oxide bioavailability and vascular smooth muscle cells behaviour. Please see text for details. ENOS indicates endothelial nitric oxide synthase; NO nitric oxide; ONOO− peroxynitrates; O2− superoxide anion
Consistent with this idea, when we measured intracellular cGMP levels in our system, we found them decreased in cells from diabetic animals. This suggests that, in spite of increased NO synthesis, NO availability was not increased in cells from diabetic animals.
Increased oxidative stress may play a role in reduced NO availability in diabetic rats (DR) vSMC: therefore, we evaluated O2− production by both cell strains. As schematically represented in Fig. 4, we observed that basal O2− release was greater in vSMC from diabetic as compared to control rats. It is, therefore, likely that in vSMC from diabetic animals a greater amount of NO was readily converted into peroxynitrite (ONOO−). Indeed, it has been suggested [109] that, in pro-oxidant conditions, a great amount of NO is rapidly converted into peroxynitrite and thus it is not available anymore to be released into the peri-cellular environment. In our experiments, vascular SMC from diabetic rats, as compared to vSMC from control rats, exhibited a less differentiated phenotype which has been associated with alteration of the redox state [136]. It is therefore conceivable that, in vSMC from diabetic rats, increased eNOS expression and activity indeed resulted in greater NO production, but that, due to a pro-oxidant intra-cellular status, a large portion of this NO was converted into peroxynitrite and was therefore unavailable for conversion into nitrate/nitrite and for guanylyl cyclase stimulation. Indeed, the probable increased generation of ONOO− in vSMC from diabetic rats could not compensate for the reduced NO bioavailability, since ONOO− is a much less potent activator of guanylyl cyclase than NO [155]. Our data are in keeping with those obtained by Etienne et al. [156], who showed a lower basal cGMP content in diabetic rats than in control rats vSMC [156]. If this were to be the case, the conversion of NO into peroxynitrite could be even greater in vivo, where the presence of hyperglycemia would further increase the generation of ROS in the intra- and extra-cellular environment.
In summary, it is possible hypothesized that in cells directly involved in atherosclerotic plaques formation and development like aortic SMC, chronic hyperglycemia might induce cellular dedifferentiation which persists in culture and which is associated with increased eNOS expression and activity. Since, however, chronic hyperglycemia was not associated with increased NO bioavailabilty, it is tempting to speculate that the observed dedifferentiation is also associated with a redox imbalance responsible for NO quenching and/or trapping. In conclusion, since the demonstration of an association between cellular dedifferentiation and altered NO synthetic pathways in cell strains explanted from diabetic animals may provides new insight on the mechanisms responsible for accelerated atherosclerosis in diabetes, the information about vascular NOSs localization, expression and dysfunction in diabetes and high glucose conditions is contradictory and more studies are clearly needed.
Conclusions
In conclusion, a large body of literature supports that in vivo, chronic hyperglicemia, via several glucose impaired intracellular pathways, may lead to endothelial dysfunction and accelerated atherosclerosis in diabetes. This lends support to the concept that glycemic control remains the major intervention to prevent vascular complication in diabetes.
Interestingly, recent evidences indicate the role of dietary factors (in particular of high dietary glycemic load) in the development of endothelial dysfunction, characterized by increased levels of plasma concentration of inflammatory cytokines and endothelial adhesion molecules [9]. Since endothelial dysfunction is an early step in the development of atherosclerosis, the recent study by Lopez-Garcia et al. suggests a role of dietary patterns in the pathogenesis of cardiovascular disease. Furthermore, insulin resistance syndrome and obesity are closely linked to atherosclerosis and may enhance the inflammatory process present in all stages of atherosclerosis [157]. Hyperglycemia associated with insulin resistance can lead to modification of macromolecules as advanced glycation end products (AGEs) that bind surface receptors, which, in turn, may augment the production of proinflammatory cytokines in vascular endothelial cells. Within the vessel wall, collagen-linked AGEs may “trap” plasma proteins, quench NO activity and interact with specific receptors to modulate a large number of cellular properties [158].
Actually, little data are available to evaluate the dietary predictors for the markers of systemic inflammation and endothelial dysfunction in patients with type 2 diabetes.
In addition, although dietary fibre intake was recently associated with serum inflammatory markers in the general population [159, 160], little is known about the effect of fibre intake on inflammatory markers among diabetic patients [161]. Because of the few number and the observational nature of these studies, further clinical trials are needed to test the potential benefits of these dietary factors among patients with type 2 diabetes.
Acknowledgments
We thank Sara Di Silvestre, Pamela Di Tomo and Annalisa Giardinelli for technical and editorial support. The authors were partially financed by Italian Government Grants (MIPAF 2000-200P to A.P.).
References
- 1.Johansen JS, Harris AK, Rychly DJ, Ergul A (2005) Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol 4:5 [DOI] [PMC free article] [PubMed]
- 2.Laakso M (1999) Hyperglycaemia and cardiovascular disease in type 2 diabetes. Diabetes 48:937–942 [DOI] [PubMed]
- 3.Kannel WB, McGee DL (1979) Diabetes and cardiovascular disease. JAMA 241:2035–2058 [DOI] [PubMed]
- 4.Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625 [DOI] [PubMed]
- 5.Balletshofer BM, Rittig K, Enderle MD, Volk A, Maerker E, Jacob S, Matthaei S, Rett K, Haring HU (2000) Endothelial dysfunction is detectable in young normotensive first-degree relatives of subjects with type 2 diabetes in association with insulin resistance. Circulation 101:1780–1784 [DOI] [PubMed]
- 6.Pieper GM, Dembny K, Siebeneich W (1998) Long-term treatment in vivo with NOX-101, a scavenger of nitric oxide, prevents diabetes-induced endothelial dysfunction. Diabetologia 41:1220–1226 [DOI] [PubMed]
- 7.Pricci F, Leto G, Amadio L, Iacobini C, Cordone S, Catalano S, Zicari A, Sorcini M, Di Mario U, Pugliese G (2003) Oxidative stress in diabetes-induced endothelial dysfunction. Involvement of nitric oxide and protein Kinase C. Free Radic Biol Med 35:683–694 [DOI] [PubMed]
- 8.UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood- glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853 [PubMed]
- 9.Lopez-Garcia E, Hu FB (2004) Nutrition and the endothelium. Curr Diabetes Rep 4:253–259 [DOI] [PubMed]
- 10.Brasier AR, Recinos A, Eledrisi MS (2002) Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol 22:1257–1266 [DOI] [PubMed]
- 11.Calles-Escandon J, Cipolla M (2001) Diabetes and endothelial dysfunction: a clinical perspective. Endocr Rev 22:36–52 [DOI] [PubMed]
- 12.De Meyer GR, Herman AG (1997) Vascular endothelial dysfunction. Prog Cardiovasc Dis 39:325–342 [DOI] [PubMed]
- 13.Plante GE (2002) Vascular response to stress in health and disease. Metabolism 51:25–30 [DOI] [PubMed]
- 14.Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357:593–615 [DOI] [PMC free article] [PubMed]
- 15.Papapetropoulos A, Rudic RD, Sessa WC (1999) Molecular control of nitric oxide synthases in the cardiovascular system. Cardiovasc Res 43:509–520 [DOI] [PubMed]
- 16.Li H, Forstermann U (2000) Nitric oxide in the pathogenesis of vascular disease. J Pathol 190:244–254 [DOI] [PubMed]
- 17.Kibbe M, Billiar T, Tzeng E (1999) Inducible nitric oxide synthase and vascular injury. Cardiovasc Res 43:650–657 [DOI] [PubMed]
- 18.Andrew P, Mayer B (1999) Enzymatic function of nitric oxide synthases. Cardiovasc Res 43:521–531 [DOI] [PubMed]
- 19.Harrison DG (1997) Nitric oxide and nitric oxide synthases—cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest 100(9):2153–2157 [DOI] [PMC free article] [PubMed]
- 20.Di Fulvio P, Formoso G, Di Silvestre S, Di Tomo P, Giardinelli A, La Sorda R, Di Pietro N, Piantelli M, Consoli A, Pandolfi A (2006) Increased vascular wall endothelial nitric oxide synthase (eNOS) levels in umbilical cords from gestational diabetic women. Atherosclerosis 14(W2):5 (Abstract)
- 21.Pandolfi A, Grilli A, Cilli C, Patruno A, Giaccari A, Di Silvestre S, De Lutiis MA, Pellegrini G, Capani F, Consoli A, Felaco M (2003) Phenotype modulation in cultures of vascular smooth muscle cells from diabetic rats: association with increased nitric oxide synthase expression and superoxide anion generation. J Cell Physiol 196:378–385 [DOI] [PubMed]
- 22.Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, King GL, LoGerfo FW, Horton ES, Veves A (1999) Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes 48:1856–1862 [DOI] [PubMed]
- 23.Jaap AJ, Shore AC, Tooke JE (1997) Relationship of insulin resistance to microvascular dysfunction in subjects with fasting hyperglycaemia. Diabetologia 40:238–243 [DOI] [PubMed]
- 24.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD (1996) Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 97:2601–2610 [DOI] [PMC free article] [PubMed]
- 25.Vehkavaara S, Seppala-Lindroos A, Westerbacka J, Groop PH, Yki-Jarvinen H (1999) In vivo endothelial dysfunction characterizes patients with impaired fasting glucose. Diabetes Care 22:2055–2060 [DOI] [PubMed]
- 26.Sorescu D, Szocs K, Griendling KK (2001) NAD(P)H oxidases and their relevance to atherosclerosis. Trends Cardiovasc Med 11:124–131 [DOI] [PubMed]
- 27.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK (2002) Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105:1429–1435 [DOI] [PubMed]
- 28.Warnholtz A, Nickenig G, Schultz E, Macharzina R, Bransen JH, Skatchkov M, Heitzer T, Stasch JP, Griendling KK, Harrison DG, Bohm M, Meinertz T, Munzel T (1999) Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation 99:2027–2033 [DOI] [PubMed]
- 29.Cai H, Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87:840–844 [DOI] [PubMed]
- 30.Matsuoka H (2001) Endothelial dysfunction associated with oxidative stress in human. Diabetes Res Clin Pract 54:S65–S72 [DOI] [PubMed]
- 31.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG (2001) Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103:1282–1288 [DOI] [PubMed]
- 32.Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA (2002) Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem 277:35552–35557 [DOI] [PubMed]
- 33.Zou MH, Shi C, Cohen RA (2002) Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest 109:817–826 [DOI] [PMC free article] [PubMed]
- 34.Rask-Madsen C, King GL (2005) Proatherosclerotic mechanism involving protein kinase C in diabetes and insulin resistance. Arterioscler Thromb Vasc Biol 24:1542–1548 [DOI] [PubMed]
- 35.Mertens A, Holvoet P (2001) Oxidized LDL and HDL: antagonists in atherothrombosis. FASEB J 15:2073–2084 [DOI] [PubMed]
- 36.Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R (1996) Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest 97:1535–1544 [DOI] [PMC free article] [PubMed]
- 37.Podrez EA, Schmitt D, Hoff HF, Hazen SL (1999) Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest 103:1547–1560 [DOI] [PMC free article] [PubMed]
- 38.Carpenter KL, Dennis IF, Challis IR, Osborn DP, Macphee CH, Leake DS, Arends MJ, Mitchinson MJ (2001) Inhibition of lipoprotein-associated phospholipase A2 diminishes the death-inducing effects of oxidised LDL on human monocyte-macrophages. FEBS Lett 505:357–363 [DOI] [PubMed]
- 39.Hanasaki K, Yamada K, Yamamoto S, Ishimoto Y, Saiga A, Ono T, Ikeda M, Notoya M, Kamitani S, Arita H (2002) Potent modification of low density lipoprotein by group X secretory phospholipase A2 is linked to macrophage foam cell formation. Biol Chem 277:29116–29124 [DOI] [PubMed]
- 40.Reddy S, Hama S, Grijalva V, Hassan K, Mottahedeh R, Hough G, Wadleigh DJ, Navab M, Fogelman AM (2001) Mitogen-activated protein kinase phosphatase 1 activity is necessary for oxidized phospholipids to induce monocyte chemotactic activity in human aortic endothelial cells. J Biol Chem 276:17030–17035 [DOI] [PubMed]
- 41.Sonoki K, Yoshinari M, Iwase M, Ino K, Ichikawa K, Ohdo S, Higuchi S, Iida M (2002) Glycoxidized low-density lipoprotein enhances monocyte chemoattractant protein-1 mRNA expression in human umbilical vein endothelial cells: relation to lysophosphatidylcholine contents and inhibition by nitric oxide donor. Metabolism 51:1135–1142 [DOI] [PubMed]
- 42.Kita T, Kume N, Yokode M, Ishii K, Arai H, Horiuchi H, Moriwaki H, Minami M, Kataoka H, Wakatsuki Y (2000) Oxidized-LDL and atherosclerosis. Role of LOX-1. Ann N Y Acad Sci 902:95–100 [DOI] [PubMed]
- 43.Malden LT, Chait A, Raines EW, Ross R (1991) The influence of oxidatively modified low density lipoproteins on expression of platelet-derived growth factor by human monocyte-derived macrophages. J Biol Chem 266:13901–13907 [PubMed]
- 44.Stiko-Rahm A, Hultgard-Nilsson A, strom J, Hamsten A, Nilsson J (1992) Native and oxidized LDL enhances production of PDGF AA and the surface expression of PDGF receptors in cultured human smooth muscle cells. ArteriosclerThromb Vasc Biol 12:1099–1109 [DOI] [PubMed]
- 45.Zhao SP, Xu DY (2000) Oxidized lipoprotein(a) enhanced the expression of P-selectin in cultured human umbilical vein endothelial cells. Thromb Res 100:501–510 [DOI] [PubMed]
- 46.Zwijsen RM, Japenga SC, Heijen AM, van den Bros RC, Koeman JH (1992) Induction of platelet-derived growth factor chain A gene expression in human smooth muscle cells by oxidized low density lipoproteins. Biochem and Biophys Res Commun 186:1410–1416 [DOI] [PubMed]
- 47.Ananyeva NM, Tjurmin AV, Berliner JA, Chisolm GM, Liau G, Winkles JA, Haudenschild CC (1997) Oxidized LDL mediates the release of fibroblast growth factor-1. Arterioscler Thromb Vasc Biol 17:445–453 [DOI] [PubMed]
- 48.Chai YC, Binion DG, Chisolm GM (2000) Relationship of molecular structure to the mechanism of lysophospholipid-induced smooth muscle cell proliferation. Am J Physiol Heart Circ Physiol 279:H1830–H1838 [DOI] [PubMed]
- 49.Chai YC, Binion DG, Macklis R, Chilsom GM (2002) Smooth muscle cell proliferation induced by oxidized LDL-borne lysophosphatidylcholine. Evidence for FGF-2 release from cells not extracellular matrix. Vasc Pharmacol 38:229–237 [DOI] [PubMed]
- 50.Chai YC, Howe PH, Di Corleto PE, Chisolm GM (1996) Oxidized low density lipoprotein and lysophosphatidylcholine stimulate cell cycle entry in vascular smooth muscle cells. Evidence for release of fibroblast growth factor-2. J Biol Chem 271:17791–17797 [DOI] [PubMed]
- 51.Chisolm GM, Chai Y (2000) Regulation of cell growth by oxidized LDL. Free Radic Biol Med 28:1697–1707 [DOI] [PubMed]
- 52.Koba S, Pakala R, Watanabe T, Katagiri T, Benedict CR (2000) Synergistic interaction between thromboxane A2 and mildly oxidized low density lipoproteins on vascular smooth muscle cell proliferation. Prostaglandins Leukot Essent Fatty Acids 63:329–335 [DOI] [PubMed]
- 53.Price DT, Vita JA, Keaney JF Jr (2000) Redox control of vascular nitric oxide bioavailability. Antioxid Redox Signal 2:919–935 [DOI] [PubMed]
- 54.Vanhoutte PM (2001) Endothelium-derived free radicals: for worse and for better. J Clin Invest 107:23–25 [DOI] [PMC free article] [PubMed]
- 55.Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, Kimoto M, Tsuji H, Reaven GM, Cooke JP (2002) Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 106:987–992 [DOI] [PubMed]
- 56.Liao JK, Shin WS, Lee WY, Clark SL (1995) Oxidized low-density lipoprotein decreases the expression of endothelial nitric oxide synthase. J Biol Chem 270:319–324 [DOI] [PubMed]
- 57.Nuszkowski A, Grabner R, Marsche G, Unbehaun A, Malle E, Heller R (2001) Hypochlorite-modified low density lipoprotein inhibits nitric oxide synthesis in endothelial cells via an intracellular dislocalization of endothelial nitric-oxide synthase. J Biol Chem 276:14212–14221 [DOI] [PubMed]
- 58.Vergnani L, Hatrik S, Ricci F, Passaro A, Manzoli N, Zuliani G, Brovkovynch V, Fellin R, Malinski T (2000) Effect of native and oxidized low-density lipoprotein on endothelial nitric oxide and superoxide production: key role of l-arginine availability. Circulation 101:1261–1266 [DOI] [PubMed]
- 59.Cui MZ, Penn MS, Chisolm GM (1999) Native and oxidized low density lipoprotein induction of tissue factor gene expression in smooth muscle cells is mediated by both Egr-1 and Sp1. J Biol Chem 274:32795–32802 [DOI] [PubMed]
- 60.Fei H, Berliner JA, Parhami F, Drake TA (1993) Regulation of endothelial cell tissue factor expression by minimally oxidized LDL and lipopolysaccharide. Arterioscler Thromb J Vasc Biol 13:1711–1717 [DOI] [PubMed]
- 61.Dichtl W, Stiko A, Eriksson P, Goncalves I, Calara F, Banfi C, Ares MP, Hamsten A, Nilsson J (1999) Oxidized LDL and lysophosphatidylcholine stimulate plasminogen activator inhibitor-1 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 19:3025–3032 [DOI] [PubMed]
- 62.Ren S, Man RY, Angel A, Shen GX (1997) Oxidative modification enhances lipoprotein(a)-induced overproduction of plasminogen activator inhibitor-1 in cultured vascular endothelial cells. Atherosclerosis 128:1–10 [DOI] [PubMed]
- 63.Koya D, King GL (1998) Proteinkinase C activation and development of diabetic complications. Diabetes 47:859–866 [DOI] [PubMed]
- 64.Xia P, Inoguchi T, Kern TS, Engerman RL, Oates PJ, King GL (1994) Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes 43:1122–1129 [DOI] [PubMed]
- 65.Feener EP, Xia P, Inoguchi T, Shiba T, Kunisaki M, King GL (1996) Role of protein kinase C in glucose- and angiotensin II-induced plasminogen activator inhibitor expression. Contrib Nephrol 118:180–187 [DOI] [PubMed]
- 66.Kuboki K, Jiang ZJ, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL (2000) Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation 101:676–681 [DOI] [PubMed]
- 67.Pandolfi A, Iacovello L, Capani F, Vitacolonna E, Donati MB, Consoli A (1996) Glucose and insulin independently reduce the fibrinolytic potential of human vascular smooth muscle cells in culture. Diabetologia 39:1425–1431 [DOI] [PubMed]
- 68.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A (2003) Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NADPH-oxidase activation. Diabetes 52:2795–2804 [DOI] [PubMed]
- 69.Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, Zuodar G, Ceriello A (2005) Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectine expression in human umbilical vein endothelial cells in culture: the distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis 183:259–267 [DOI] [PubMed]
- 70.Ferre P (1999) Regulation of gene expression by glucose. The Proceedings of the nutrition Society 58:621–623 [DOI] [PubMed]
- 71.Stenina OI (2005) Regulation of vascular gene by glucose. Curr Pharm Des 11:2367–2381 [DOI] [PubMed]
- 72.Ginsberg HN (2000) Insulin resistance and cardiovascular disease. J Clin Invest 106:453–58 [DOI] [PMC free article] [PubMed]
- 73.The Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long- term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986 [DOI] [PubMed]
- 74.UK Prospective Diabetes Study (UKPDS) Group (1998) Effect of intensive blood- glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352:854–865 [PubMed]
- 75.Wei M, Gaskill SP, Haffner SM, Stern MP (1998) Effect of diabetes and level of glycemia on all-cause and cardiovascular mortality. The san Antonio Heart Study. Diabetes Care 21:1167–1172 [DOI] [PubMed]
- 76.Ceriello A (2006) Oxidative stress and diabetes-associated complications. Endocr Pract 12:60–62 [DOI] [PubMed]
- 77.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404:787–790 [DOI] [PubMed]
- 78.Ueda S, Yasunari K (2006) What we learnt from randomized clinical trials and cohort studies of antioxidant vitamin?: focus on vitamin E and cardiovascular disease. Curr Pharm Biotechnol 7:69–72 [DOI] [PubMed]
- 79.Stevens MJ (2005) Oxidative-nitrosative stress as a contributing factor to cardiovascular disease in subjects with diabetes. Curr Vasc Pharmacol 3:253–266 [DOI] [PubMed]
- 80.Wautier JL, Schmidt AM (2004) Protein glycation: a firm link to endothelial cell dysfunction. Circ Res 95:233–238 [DOI] [PubMed]
- 81.Duraisamy Y, Slevin M, Smith N, Bailey J, Zweit J, Smith C, Ahmed N, Gaffney J (2001) Effect of glycation on basic fibroblast growth factor induced angiogenesis and activation of associated signal transduction pathways in vascular endothelial cells: possible relevance to wound healing in diabetes. Angiogenesis 4:277–288 [DOI] [PubMed]
- 82.Watala C, Gwozdzinski K, Pluskota E, Pietrucha T, Walkowiak B, Trojanowski Z, Cierniewski CS (1996) Diabetes mellitus alters the effect of peptide and protein ligands on membrane fluidity of blood platelets. Thromb Haemost 75:147–153 [PubMed]
- 83.Zachara NE, Hart GW (2004) O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in responce to nutrition and stress. Biochim Biophys Acta 1673:13–28 [DOI] [PubMed]
- 84.Burt DJ, Gruden G, Thomas SM, Tutt P, Dell’Anna C, Viberti GC, Gnudi L (2003) P38 mitogen-activated protein kinase mediates hexosamine-induced TGFβ1 mRNA expression in human mesangial cells. Diabetologia 46:531–537 [DOI] [PubMed]
- 85.Han I, Oh ES, Kudlow JE (2000) Responsiveness to the state of O-linked N-acetylglucosamine modification of nuclear pore protein p62 to the extracellular glucose concentration. Biochem J 350:109–114 [PMC free article] [PubMed]
- 86.Sayeski PP, Kudlow JE (1996) Glucose metabolism to glucosamine is necessary for glucose stimulation of trasforming growth factor-β gene transcription. J Biol Chem 271:15237–15243 [DOI] [PubMed]
- 87.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldeberg H, Ziyadeh F, Wu J, Brownlee M (2000) Hyperglycemia-induced mithocondrial superoxide overproduction activates the exosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA 97:12222–12226 [DOI] [PMC free article] [PubMed]
- 88.McClain DA, Lubas WA, Cooksy RC, Hazel M, Parker GJ, Love DC, Hanover JA (2002) Altereted glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci USA 99:10695–10699 [DOI] [PMC free article] [PubMed]
- 89.Patti ME, Virkamaki A, Landaker EJ, Kanh CR, Yki-Jarvinen H (1999) Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance of early postreceptor insulin signaling event in skeletal muscle. Diabetes 48: 1562–1571 [DOI] [PubMed]
- 90.Walgren JL, Vincent TS, Schey KL, Buse MG (2003) High glucose and insulin promote O-GlcNac modification of proteins, including α-tubulin. Am J Physiol Endocr Metab 284:E424–E434 [DOI] [PubMed]
- 91.Goldeberg HJ, Whiteside CI, Hart GH, Fantus IG (2006) Posttranslational, reversible O-glycosylation is stimulated by high glucose and mediates plasminogen Activator Inhibitor-1 gene expression and Sp1 transcriptional activity in glomerular mesangial cells. Endocrinology 147:222–231 [DOI] [PubMed]
- 92.Zachara NE, Hart GW (2006) Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta 1761:599–617 [DOI] [PubMed]
- 93.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M (2001) Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest 108:1341–1348 [DOI] [PMC free article] [PubMed]
- 94.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R (2002) Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 106:466–472 [DOI] [PubMed]
- 95.Musicki B, Kramer MF, Beker RE, Burnett AL (2005) Inactivation of phosphorylated endothelail nitric oxide synthase (Ser-1177) by O-GlcNAc in 37 diabetes- associated erectile dysfunction. Proc Natl Acad Sci USA 102:11870–11875 [DOI] [PMC free article] [PubMed]
- 96.Daniels MC, Kansal P, Smith TM, Paterson AJ, Kudlow JE, McClain DA (1993) Glucose regulation of transforming growth factor-alpha expression is mediated by products of the exosamine biosynthesis pathway. Mol Endocrinol 7:1041–1048 [DOI] [PubMed]
- 97.Gabriely I, Yang XM, Cases JA, Ma XH, Rossetti L, Barzilai N (2002) Hyper glycemia induces PAI-1 gene expression in adipose tissue by activation of the hexosamine biosynthetic pathway. Atherosclerosis 160:115–122 [DOI] [PubMed]
- 98.Goldeberg HJ, Scholey J, Fantus IG (2000) Glucosamine activates the plasminogen activator inhibitor -1 gene promoter throught Sp1 DNA binding sites in glomerular mesangial cells. Diabetes 49:863–871 [DOI] [PubMed]
- 99.Goldeberg HJ, Whiteside CI, Fantus IG (2002) The exosamine pathway regulates the plasminogen activator inhibitor-1 gene promoter and Sp1 transcriptional activation throught protein kinase C-beta I and delta. J Biol Chem 277:33833–33841 [DOI] [PubMed]
- 100.Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED (1998) High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J Clin Invest 101:160–169 [DOI] [PMC free article] [PubMed]
- 101.Weigert C, Brodbeck K, Staiger H, Kausch C, Machicao F, Häring H, Schleicher ED (2004) Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappaB. J Biol Chem 279:23942–23952 [DOI] [PubMed]
- 102.Graier WF, Wascher TC, Lackner L, Toplak H, Krejs GJ, Kukovetz WR (1993) Exposure to elevated d-glucose concentrations modulates vascular endothelial cell vasodilatatory response. Diabetes 42:1497–1505 [DOI] [PubMed]
- 103.Oyadomari S, Gotoh T, Aoyagi K, Araki E, Shichiri M, Mori M (2001) Coinduction of endothelial nitric oxide synthase and arginine recycling enzymes in aorta of diabetic rats. Nitric Oxide Biol Chem 5:252–260 [DOI] [PubMed]
- 104.Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, Creager MA (1998) Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation 97:1695–1701 [DOI] [PubMed]
- 105.Brodsky SV, Morrishow AM, Dharia N, Gross SS, Goligorsky MS (2001) Glucose scavenging of nitric oxide. Am J Physiol Renal Physiol 280:F480–F486 [DOI] [PubMed]
- 106.Graier WF, Posch K, Fleischhacker E, Wascher TC, Kostner GM (1999) Increased superoxide anion formation in endothelial cells during hyperglycemia: an adaptive response or initial step of vascular dysfunction? Diabetes Res Clin Pract 45:153–160 [DOI] [PubMed]
- 107.Cosentino F, Eto M, De Polis P, van der Loo B, Bachschmid M, Ullrich V, Kouroedov A, Delli Gatti C, Joch H, Volpe M, Lüscher TF (2003) High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells. Role of protein kinase C and reactive oxygen species. Circulation 107:1017–1023 [DOI] [PubMed]
- 108.Cosentino F, Hishikawa K, Katusic Z, Luscher TF (1997) High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 96:25–28 [DOI] [PubMed]
- 109.Kojda G, Harrison D (1999) Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 43:652–571 [DOI] [PubMed]
- 110.Peng HB, Libby P, Liao JK (1995) Induction and stabilization of I kappa B alpha by nitric oxide mediates inhibition of NF-kappa B. J Biol Chem 270:14214–14219 [DOI] [PubMed]
- 111.Katsuyama K, Shichiri M, Marumo F, Hirata Y (1998) NO inhibits cytpkine-induced iNOS expression and NF-kappaB activation by interfering with phpsphorylation and degradation of IkappaB-alpha. Arterioscler Thromb Vasc Biol 18:1796–1802 [DOI] [PubMed]
- 112.Lander HM, Ogiste JS, Teng KK, Novogrodsky A (1995) p21 ras as a common signaling target of reactive free radicals and cellular redox stress. J Biol Chem 270:21195–21198 [DOI] [PubMed]
- 113.Du XL, Stocklauser-Farber K, Rosen P (1999) Generation of reactive oxygen intermediates, activation of NF-kappaB, and induction of apoptosis in human endothelial cells by glucose: role on nitric oxide synthase? Free Radic Biol Med 27:752–763 [DOI] [PubMed]
- 114.Sobrevia L, Mann GE (1997) Dysfunction of the endothelial nitric oxide signaling pathway in diabetes and hyperglycemia. Exp Physiol 82:423–452 [DOI] [PubMed]
- 115.Sobrevia L, Yudilevich DL, Mann GE (1998) Elevated d-glucose induces insulin insensitivity in human umbilical endothelial cells isolates from gestational diabetic pregnancies. J Physiol 506:219–230 [DOI] [PMC free article] [PubMed]
- 116.Vasquez G, Sanhueza F, Vasquez R, Gonzalez M, SanMartýn R, Casanello P, Sobrevia L (2004) Role of adenosine transport in gestational diabetes-induced l-arginine transport and nitric oxide synthesis in human umbilical vein endothelium. J Physiol 560:111–122 [DOI] [PMC free article] [PubMed]
- 117.Luoma JS, Stralin P, Marklund SL, Hiltunen TP, Sarkioja T, Yla-Herttuala S (1998) Expression of extracellular SOD and iNOS in macrophages and smooth muscle cells in human and rabbit atherosclerotic lesion. Arterioscler Thromb Vasc Biol 18:157–167 [DOI] [PubMed]
- 118.Trovati M, Massucco P, Mattiello L, Costamagna C, Aldieri E, Cavalot F, Anfossi G, Bosia A, Ghigo D (1999) Human vascular smooth muscle cells express a costitutive nitric oxide synthase that insulin rapidly activates, thus increasing guanosine-3′,5′-cyclic monophosphate and adenosine-3′,5′-cyclic monophosphate concentrations. Diabetologia 42:831–839 [DOI] [PubMed]
- 119.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T (2001) Mechanisms underlaying endothelial dysfunction in diabetes mellitus. Circ Res 88:14–e22 [DOI] [PubMed]
- 120.Sugimoto H, Shikata K, Matsuda M, Kushiro M, Hayashi Y, Hiragushi K, Wada J, Makino H (1998) Increased expression of endothelial nitric oxide sinthase (ecNOS) in afferent and glomerular endothelial cells is involved in glomerular hyperfiltration of diabetic retinopathy. Diabetologia 41:1426–1434 [DOI] [PubMed]
- 121.Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809 [DOI] [PubMed]
- 122.Bochaton-Piallat ML, Ropraz P, Gabbiani F, Gabbiani G (1996) Phenotypic heterogeneity in rat smooth muscle cell clones. Arterioscler Thromb Vasc Biol 16:815–820 [DOI] [PubMed]
- 123.Frid MG, Moiseeva EP, Stenmark KR (1994) Multiple phenotypically distinct smooth muscle cell population exist in the adult and developing bovine pulmonary arterial media in vivo. Circ Res 75:669–681 [DOI] [PubMed]
- 124.Klemm DJ, Watson PA, Frid MG et al (2001) cAMP response element-binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. J Biol Chem 276:46132–46141 [DOI] [PubMed]
- 125.Kuro-o M, Nagai R, Nakahara K, Katoh H, Tsai RC, Tsuchimochi H, Yazaki Y, Ohkubo A, Takaku F (1991) cDNA cloning of a myosin heavy chain isoform in embrionic smooth muscle and its expression during vascular development and arterioscerosis. J Biol Chem 266:3768–3773 [PubMed]
- 126.Mosse PR, Campbell GR, Wang ZL, Capbell YH (1985) Smooth Muscle phenotypic expression in human carotid arteries. I. Comparison of cells with diffuse intimal thicknings adjacent to atheromatous plaques with those of the media. Lab Invest 53:556–562 [PubMed]
- 127.Rocnik E, Saward L, Pickering JG (2001) HSP47 expression by smooth muscle cells increased during arterial development and lesion formation is inhibited by fibrillar collagen. ArteriosclerThromb Vasc Biol 21:40–46 [DOI] [PubMed]
- 128.Owens GK (1995) Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75:487–517 [DOI] [PubMed]
- 129.Regan CP, Adam PJ, Madsen CS, Owens GK (2000) Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest 106:1139–1147 [DOI] [PMC free article] [PubMed]
- 130.Schwartz CJ, Valente AJ, Sprague EA, Kelley JL, Cayatte AJ, Rozek MM (1992) Pathogenesis of the atherosclerotic lesion. Implications for diabetes mellitus. Diabetes Care 15:1156–1167 [DOI] [PubMed]
- 131.Kansaki T, Shinomiya M, Ueda S, Morisaki N, Saito Y, Yoshida S (1994) Enhanced arterial intimal thickening after balloon catheter injury in diabetic animals accompanied by PDGF alfa- receptor overexpression of aortic media. Eur J Clin Invest 24:377–381 [DOI] [PubMed]
- 132.Moreno PR, Fallon JT, Murcia AM, Leon MN, Simosa H, Fuster V, Palacios IF (1999) Tissue characteristics of restenosis after percutaneus transluminal coronary angioplasty in diabetic patiens. J Am Coll Cardiol 34:1045–1049 [DOI] [PubMed]
- 133.Watson PA, Nesterova A, Burant CF, Klemm J, Reush JE-B (2001) Diabetes- related changes in cAMP response element- binding protein content enhance smooth muscle cell proliferation and migration. J Biol Chem 276:46142–46150 [DOI] [PubMed]
- 134.Suzuki LA, Poot M, Gerrity RG, Bornfeldt KE (2001) Diabetes accelerates smooth muscle accumulation in lesions of atherosclerosis. Diabetes 50:851–860 [DOI] [PubMed]
- 135.Faries Pl, Rohan DI, Takahara H, Wyers MC, Contreras MA, Quist WC, King GL, Logerfo FW (2001) Human vascular smooth muscle cells of diabetic origin exhibit increased proliferation, adhesion, and migration. J Vasc Surg 33:601–607 [DOI] [PubMed]
- 136.Pagano PJ (2001) NAD(P)H oxidase. Marker of the differentiated neointimal smooth muscle cell? Arterioscler Thromb Vasc Biol 21:175–177 [DOI] [PubMed]
- 137.West NEJ, Guzik J, Black E, Channon KM (2001) Enhanced superoxide production in experimental venous bypass graft intimal hyperplasia. Role of NAD(P)H oxidase. ArteriosclerThromb Vasc Biol 21:189–194 [DOI] [PubMed]
- 138.Cosentino F, Luscher T (1998) Endothelial dysfunction in diabetes mellitus. J Cardiovasc Pharmacol 32:S54–S61 [PubMed]
- 139.Felaco M, Grilli A, De Lutiis MA, Patruno A, Libertini N, Taccardi AA, Di Napoli P, Di Giulio C, Barbacane R, Conti P (2001) Endothelial nitric oxide synthase (eNOS) expression and localization in healthy and diabetic rat hearts. Ann Clin Lab Sci 31:179–186 [PubMed]
- 140.Graier WF, Simecek S, Kukovetz WR, Kostner GM (1996) High d-glucose-induced changes in endothelial Ca2+/EDRF signaling are due to generation of superoxide anions. Diabetes 45:1386–1395 [DOI] [PubMed]
- 141.Okuda Y, Kawashima K, Sawada T, Tsurumaru K, Asano M, Suzuki S, Soma M, Nakajima T, Yamashita K (1997) Eicosapentaenoic acid enhanced nitric oxide production by cultured human endothelial cells. Biochem Biophys Res Commun 232:487–491 [DOI] [PubMed]
- 142.Pieper GM, Dondlinger L (1997) Glucose elevations alter bradykinin- stimulated intracellular calcium accumulation in cultured endothelial cells. Cardiovasc Res 34:169–178 [DOI] [PubMed]
- 143.Sobrevia L, Nadal A, Yudilevich DL, Mann GE (1996) Activation of l- arginine transport system (y+) and nitric oxide synthase by elevated glucose and insulin in human endothelial cells. J Physiol 490:775–781 [DOI] [PMC free article] [PubMed]
- 144.Chakravarthy U, Hayes RG, Stitt AW, McAuley E, Archer DB (1998) Constitutive Nitric Oxide Synthase expression in retinal vascular endothelial cells is suppressed by high glucose and advanced glycation end products. Diabetes 47:945–942 [DOI] [PubMed]
- 145.Etienne P, Pares-Herbute N, Mani-Ponset L et al (1998) Phenotype modulation in primary cultures of aortic smooth muscle cells from streptozotocin-diabetic rats. Differentiation 63:225–236 [DOI] [PubMed]
- 146.Sharpe PC, Liu WH, Yue KK, McMaster D, Catherwood MA, McGinty AM, Trimble ER (1998) Glucose- induced oxidative stress in vascular contractile cells. Comparison of aortic smooth muscle cells and retinal pericytes. Diabetes 47:801–809 [DOI] [PubMed]
- 147.Pagano PJ, Haurani MJ (2006) Vascular cell locomotion: osteopontin, NADPH Oxidase, and matrix metalloproteinase-9. Circ Res 98:1453–1455 [DOI] [PubMed]
- 148.Thomas SR, Chen K, Keaney JF (2002) Hydrogen peroxide activates endothelial nitric oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-Kinase- dependent signaling pathway. J Biol Chem 277:6017–6024 [DOI] [PubMed]
- 149.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286:327–334 [DOI] [PubMed]
- 150.Consoli A, Di Tomo P, Giardinelli A, Di Silvestre S, Patrono A, Grilli A, Capani F, Felaco M, Pandolfi A (2004) Inducile Nitric Oxide Synthase (iNOS) activity is increased in vascular smooth muscle cells (vSMC) from thoracic oarta of diabetic rats. Diabetologia 47:A87 (Abstract)
- 151.Nagareddy PR, Xia Z, McNeill JH, MacLeod KM (2005) Increased expression of iNOS is associated with endothelial dysfunction and impaired pressor responsiveness in streptozotocin-induced diabetes. Am J Physiol Heart Circ Physiol 289:H2144–H2152 [DOI] [PubMed]
- 152.Boulanger CM, Heymes C, Benessiano J, Geske RS, Lévy BI, Vanhoutte PM (1998) Neuronal nitric oxide synthase is expressed in rat vascular smooth muscle cells. Activation by Angiotensin II in hypertension. Circ Res 83:1271–1278 [DOI] [PubMed]
- 153.Bitar MS, Wahid S, Mustafa S, Al-Saleh E, Dhaunsi GS, Al-Mulla F (2005) Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur J Pharmacol 511:53–64 [DOI] [PubMed]
- 154.Cai S, Khoo J, Mussa S, Alp NJ, Channon KM (2005) Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia 48:1933–1940 [DOI] [PubMed]
- 155.Wolin MS, Davidson CA, Kaminski PM, Fayngersh RP, Mohazzab-H KM (1998) Oxidant-nitric oxide signaling mechanisms in vasculr tissue. Biochemistry (Mosc) 63:810–816 (Review) [PubMed]
- 156.Etienne P, Pares-Herbutè N, Monnier L (1996) Enhanced antiproliferative effect of nitric oxide in cultured smooth muscle cells from diabetic rats. J Cardiovasc Pharmacol 27:140–146 [DOI] [PubMed]
- 157.Libby P, Ridker PM, Maseri A (2002) Inflammation and atherosclerosis. Circulation 105:1135–1143 [DOI] [PubMed]
- 158.Basta G, Schmidt AM, De Caterina R (2004) Advanced glycation end products and vascular inflammation: implication for accetarated atherosclerosis in diabetes. Cardiovasc Res 63:582–592 [DOI] [PubMed]
- 159.Ajani UA, Ford ES, Mokdad AH (2004) Dietary fiber and C-reactive protein: findings from national healt and nutrition examination survey data. J Nutr 134:1181–1185 [DOI] [PubMed]
- 160.King DE, Egan BM, Geesey ME (2003) Relation of dietary fat and fier to elevation of C-reactive protein. Am J Cardiol 92:1335–1339 [DOI] [PubMed]
- 161.Qi L, van Dam RM, Liu S, Franz M, Mantzoros C, Hu FB (2006) Whole- grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care 29:207–211 [DOI] [PubMed]