Abstract
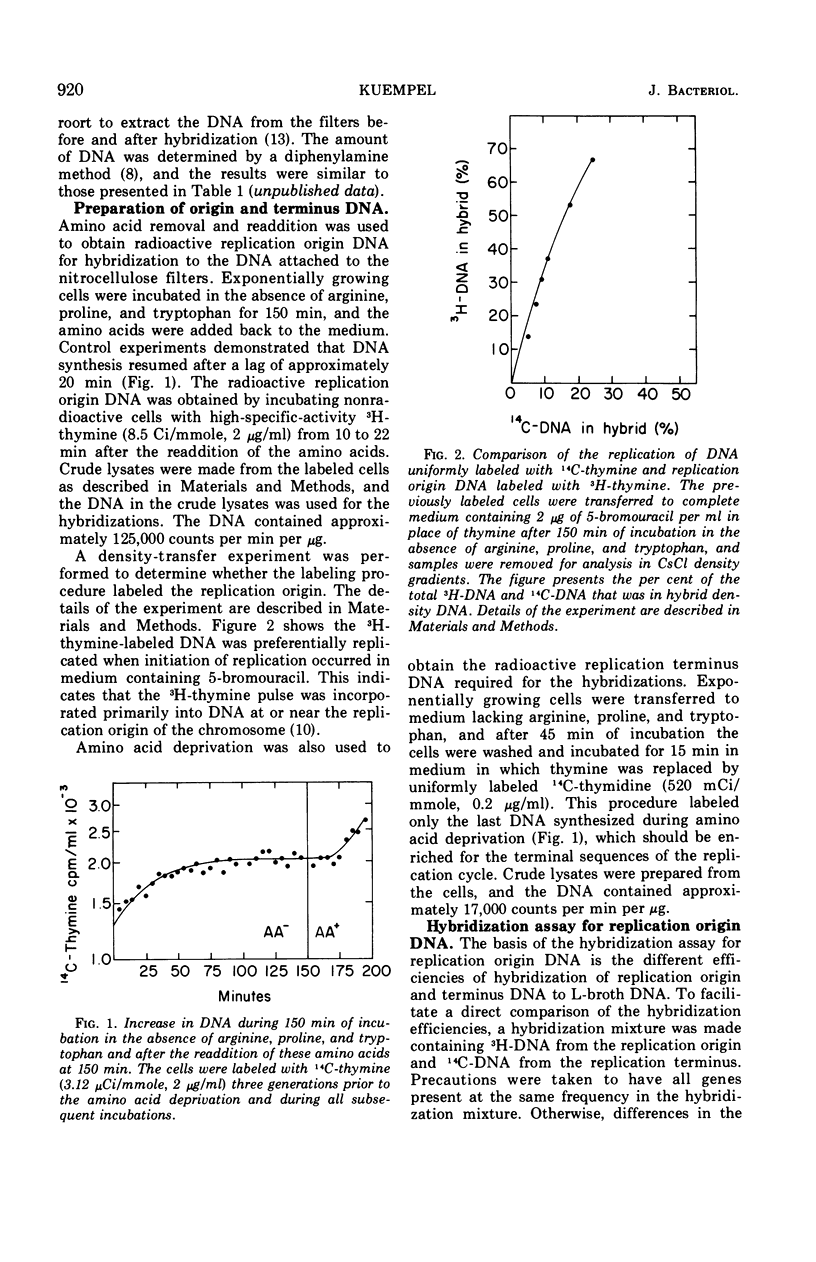
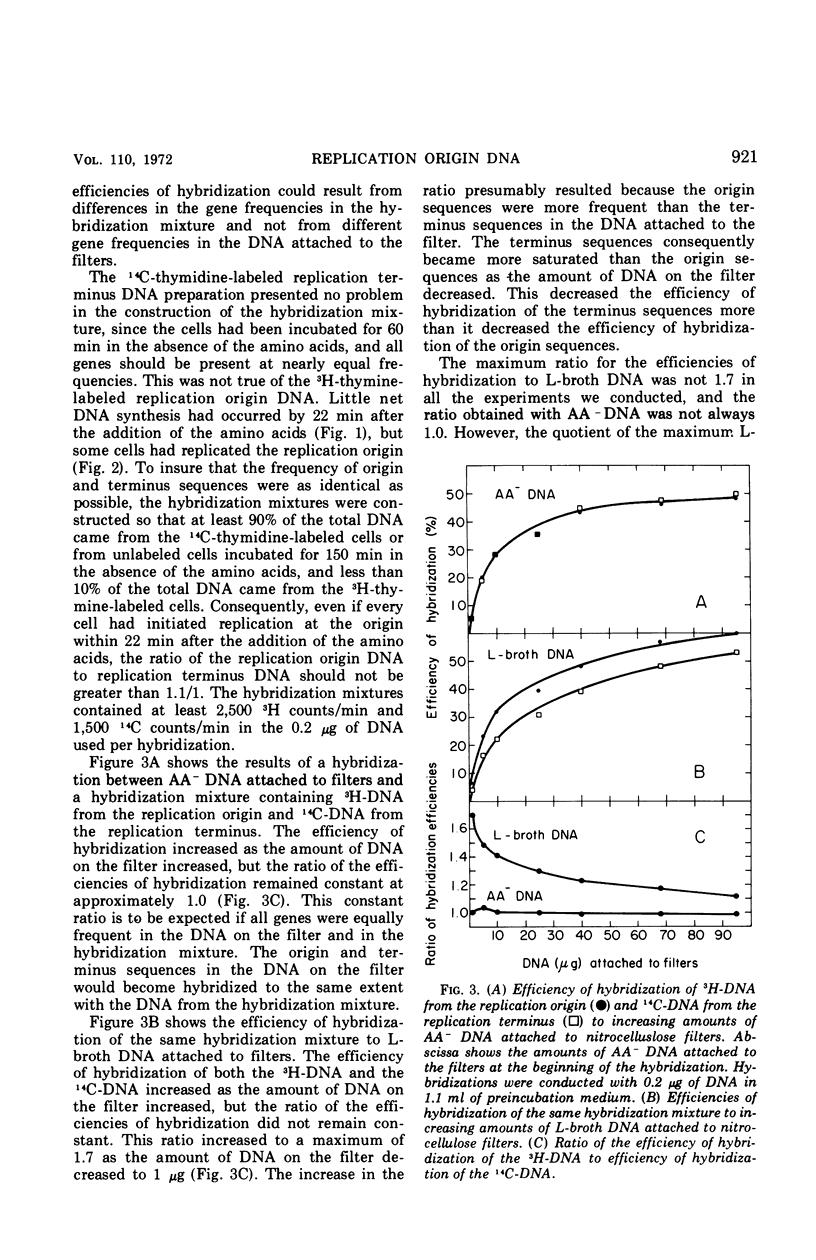
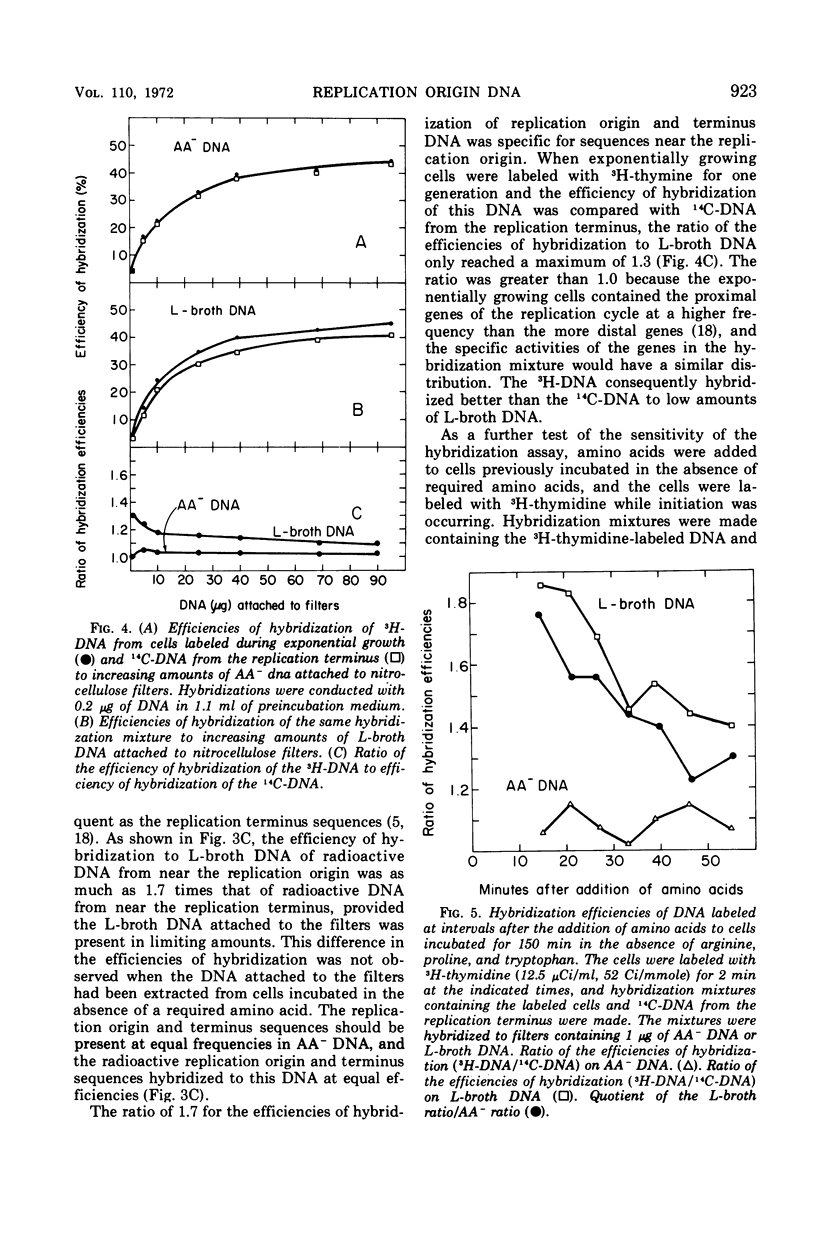
Deoxyribonucleic acid (DNA)-DNA hybridization on nitrocellulose filters can be used to assay for replication origin DNA from Escherichia coli if the DNA attached to the filters is enriched for the replication origin sequences. Such DNA can be readily isolated from very rapidly growing cells. When low amounts of this DNA were attached to filters, radioactively labeled DNA from the replication origin hybridized 1.7 times as well as radioactive replication terminus DNA. Under identical conditions, radioactively labeled DNA from exponentially growing cells hybridized only 1.3 times as well as radioactive replication terminus DNA. The replication origin, replication terminus, and randomly labeled DNA hybridized with similar efficiencies to filters containing DNA isolated from cells incubated in the absence of required amino acids. This DNA appeared to have all sequences present at equal frequencies. The hybridization assay was used to demonstrate that the DNA synthesized shortly after the addition of amino acids to cells previously deprived of required amino acids was primarily from the replication origin and then rapidly became similar to DNA synthesized by exponentially growing cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard R. W., Palleroni N. J., Doudoroff M., Stanier R. Y., Mandel M. Taxonomy of the aerobic pseudomonads: Pseudomonas cepacia, P. marginata, P. alliicola and P. caryophylli. J Gen Microbiol. 1970 Feb;60(2):199–214. doi: 10.1099/00221287-60-2-199. [DOI] [PubMed] [Google Scholar]
- Bodmer W. F. Integration of deoxyribonuclease-treated DNA in bacillus subtilis transformation. J Gen Physiol. 1966 Jul;49(6):233–258. doi: 10.1085/jgp.49.6.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk C. F., Leick V. Rapid equilibrium isopycnic CsC1 gradients. Biochim Biophys Acta. 1969 Mar 18;179(1):136–144. doi: 10.1016/0005-2787(69)90129-4. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Hall B. D. Transforming activity in single-stranded DNA from Bacillus subtilis. J Mol Biol. 1968 Jun 28;34(3):439–451. doi: 10.1016/0022-2836(68)90171-x. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Kuempel P. L. Temperature-sensitive initiation of chromosome replication in a mutant of Escherichia coli. J Bacteriol. 1969 Dec;100(3):1302–1310. doi: 10.1128/jb.100.3.1302-1310.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark K. G. Regulation of chromosome replication and segregation in bacteria. Bacteriol Rev. 1966 Mar;30(1):3–32. doi: 10.1128/br.30.1.3-32.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Masters M., Broda P. Evidence for the bidirectional replications of the Escherichia coli chromosome. Nat New Biol. 1971 Aug 4;232(31):137–140. doi: 10.1038/newbio232137a0. [DOI] [PubMed] [Google Scholar]
- Meijs W. H., Schilperoort R. A. Determination of the amount of DNA on nitrocellulose mebrane filters. FEBS Lett. 1971 Jan 12;12(3):166–168. doi: 10.1016/0014-5793(71)80059-5. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A., Sueoka N. Sequential replication of the Bacillus subtilis chromosome. IV. Genetic mapping by density transfer experiment. J Mol Biol. 1967 Jul 28;27(2):349–368. doi: 10.1016/0022-2836(67)90025-3. [DOI] [PubMed] [Google Scholar]
- Quinn W. G., Sueoka N. Symmetric replication of the Bacillus subtilis chromosome. Proc Natl Acad Sci U S A. 1970 Oct;67(2):717–723. doi: 10.1073/pnas.67.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Worcel A. Reinitiation of chromosome replication in a thermosensitive DNA mutant of Escherichia coli. II. Synchronization of chromosome replication after temperature shifts. J Mol Biol. 1971 Oct 28;61(2):329–342. doi: 10.1016/0022-2836(71)90383-4. [DOI] [PubMed] [Google Scholar]
- Seidler R. J., Mandel M. Quantitative aspects of deoxyribonucleic acid renaturation: base composition, state of chromosome replication, and polynucleotide homologies. J Bacteriol. 1971 May;106(2):608–614. doi: 10.1128/jb.106.2.608-614.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N., Yoshikawa H. The chromosome of Bacillus subtilis. I. Theory of marker frequency analysis. Genetics. 1965 Oct;52(4):747–757. doi: 10.1093/genetics/52.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa T. Replication of phage lambda DNA. Cold Spring Harb Symp Quant Biol. 1968;33:533–551. doi: 10.1101/sqb.1968.033.01.061. [DOI] [PubMed] [Google Scholar]
- Warmaar S. O., Cohen J. A. A quantitative assay for DNA-DNA hybrids using membrane filters. Biochem Biophys Res Commun. 1966 Aug 23;24(4):554–558. doi: 10.1016/0006-291x(66)90356-1. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H., Haas M. On the regulation of the initiation of DNA replication in bacteria. Cold Spring Harb Symp Quant Biol. 1968;33:843–855. doi: 10.1101/sqb.1968.033.01.096. [DOI] [PubMed] [Google Scholar]


