Abstract
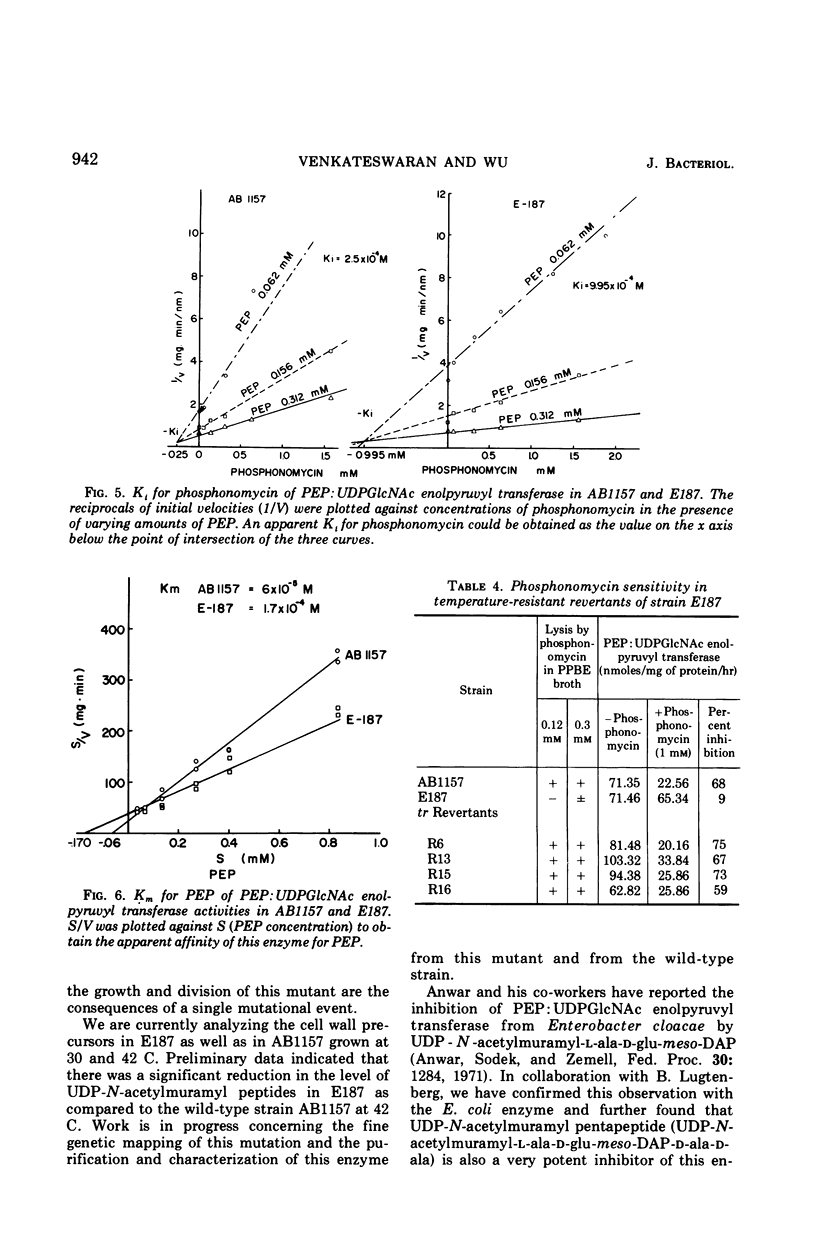
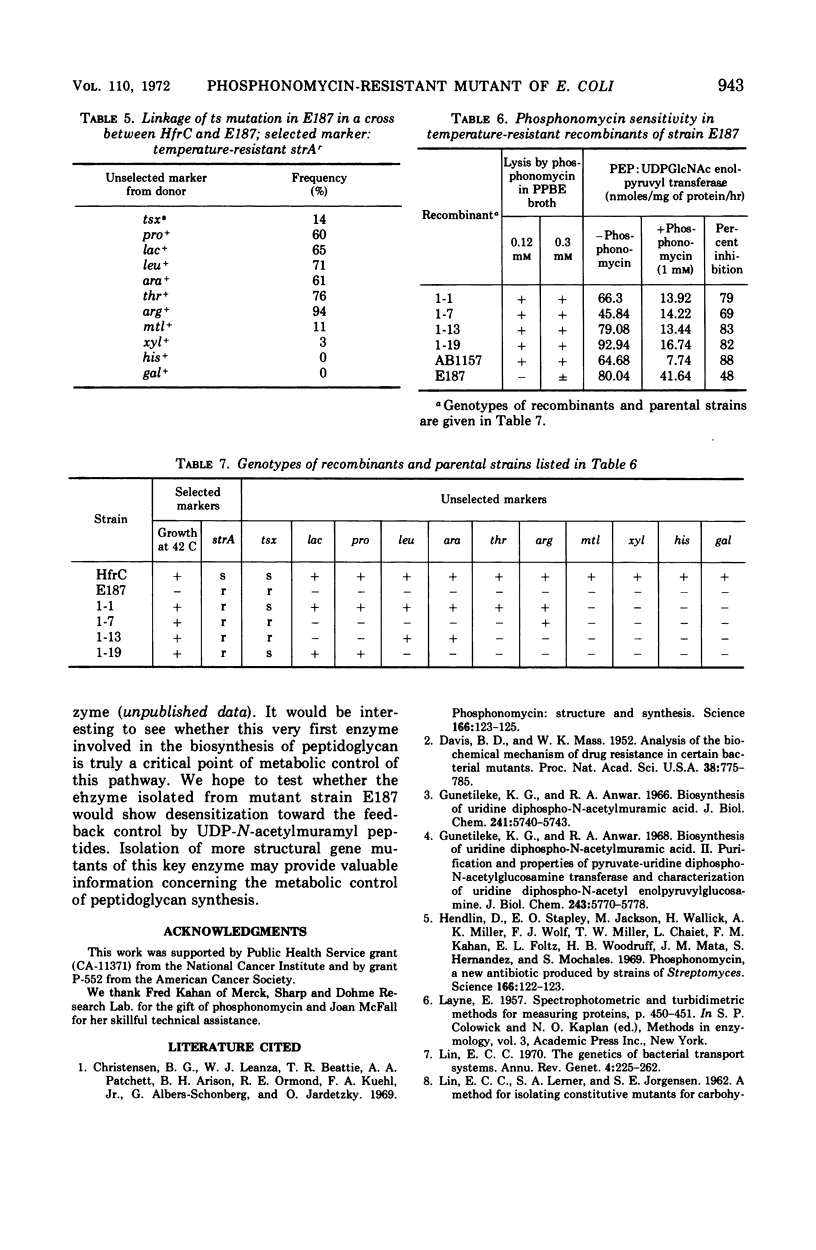
A mutant was isolated from Escherichia coli K-12 which showed increased resistance towards phosphonomycin, a new bactericidal antibiotic recently isolated from strains of Streptomyces. Evidence is presented which suggests that this mutant is resistant to lysis by phosphonomycin because of a lower affinity of phosphoenolpyruvate: uridine diphospho-N-acetylglucosamine enolpyruvyl transferase for this antibiotic. This mutant was also found to be temperature-sensitive in growth. At 42 C mutant cells grew poorly, and the rate of incorporation of 3H-diaminopimelic acid into trichloroacetic acid-insoluble material was also greatly reduced. Genetic studies indicate that the increased resistance toward phosphonomycin and temperature sensitivity in growth of this mutant are probably the consequences of a single mutation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen B. G., Leanza W. J., Beattie T. R., Patchett A. A., Arison B. H., Ormond R. E., Kuehl F. A., Jr, Albers-Schonberg G., Jardetzky O. Phosphonomycin: structure and synthesis. Science. 1969 Oct 3;166(3901):123–125. doi: 10.1126/science.166.3901.123. [DOI] [PubMed] [Google Scholar]
- Davis B. D., Maas W. K. Analysis of the Biochemical Mechanism of Drug Resistance in Certain Bacterial Mutants. Proc Natl Acad Sci U S A. 1952 Sep;38(9):775–785. doi: 10.1073/pnas.38.9.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunetileke K. G., Anwar R. A. Biosynthesis of uridine diphospho-N-acetyl muramic acid. J Biol Chem. 1966 Dec 10;241(23):5740–5743. [PubMed] [Google Scholar]
- Gunetileke K. G., Anwar R. A. Biosynthesis of uridine diphospho-N-acetylmuramic acid. II. Purification and properties of pyruvate-uridine diphospho-N-acetylglucosamine transferase and characterization of uridine diphospho-N-acetylenopyruvylglucosamine. J Biol Chem. 1968 Nov 10;243(21):5770–5778. [PubMed] [Google Scholar]
- Hendlin D., Stapley E. O., Jackson M., Wallick H., Miller A. K., Wolf F. J., Miller T. W., Chaiet L., Kahan F. M., Foltz E. L. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science. 1969 Oct 3;166(3901):122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin E. C. The genetics of bacterial transport systems. Annu Rev Genet. 1970;4:225–262. doi: 10.1146/annurev.ge.04.120170.001301. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Reitz R. H., Slade H. D., Neuhaus F. C. The biochemical mechanisms of resistance by streptococci to the antibiotics D-cycloserine and O-carbamyl-D-serine. Biochemistry. 1967 Aug;6(8):2561–2570. doi: 10.1021/bi00860a038. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L. Enzymic transfer of pyruvate to uridine diphosphoacetylglucosamine. Biochim Biophys Acta. 1958 Dec;30(3):645–646. doi: 10.1016/0006-3002(58)90119-7. [DOI] [PubMed] [Google Scholar]
- Taku A., Gunetileke K. G., Anwar R. A. Biosynthesis of uridine diphospho-N-acetylmuramic acid. 3. Purification and properties of uridine diphospho-N-acetylenolpyruvyl-glucosamine reductase. J Biol Chem. 1970 Oct 10;245(19):5012–5016. [PubMed] [Google Scholar]
- Wu H. C., Wu T. C. Isolation and characterization of a glucosamine-requiring mutant of Escherichia coli K-12 defective in glucosamine-6-phosphate synthetase. J Bacteriol. 1971 Feb;105(2):455–466. doi: 10.1128/jb.105.2.455-466.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



