Abstract
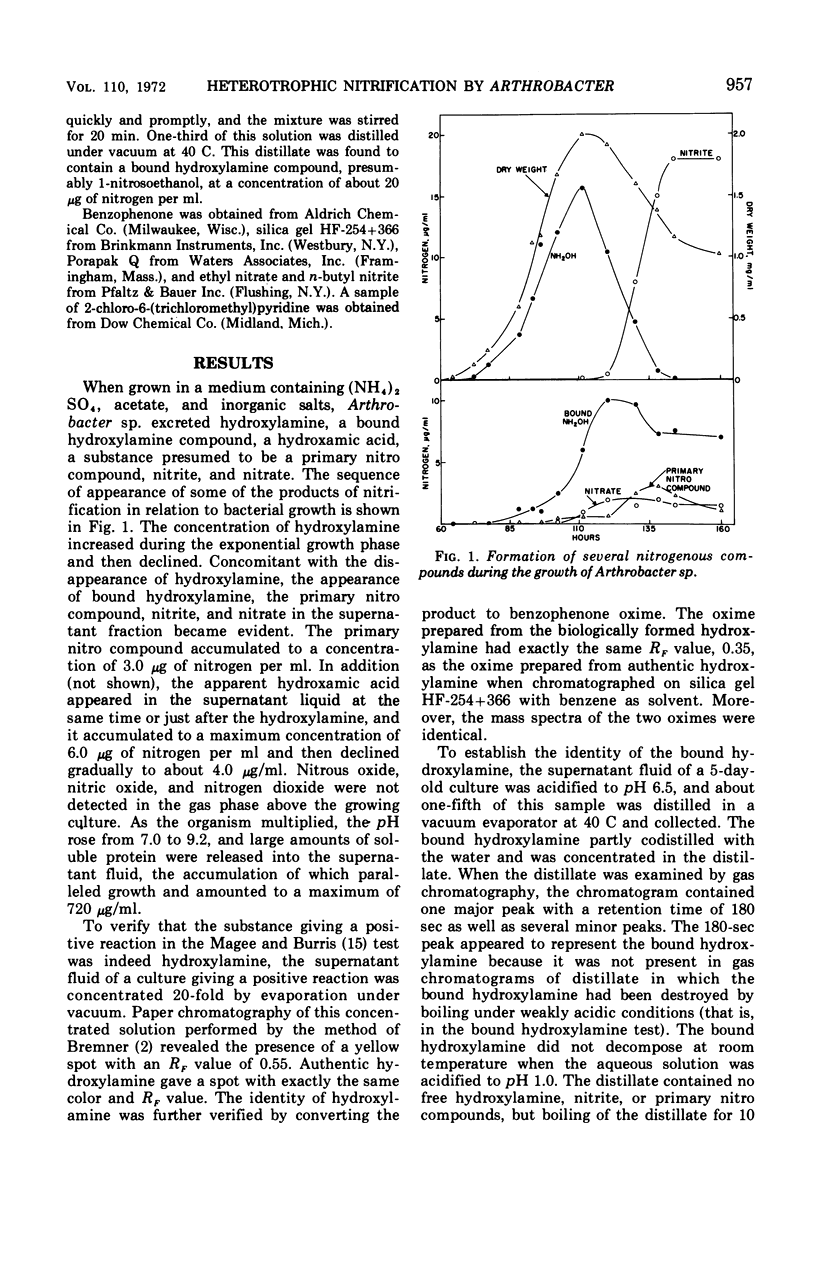
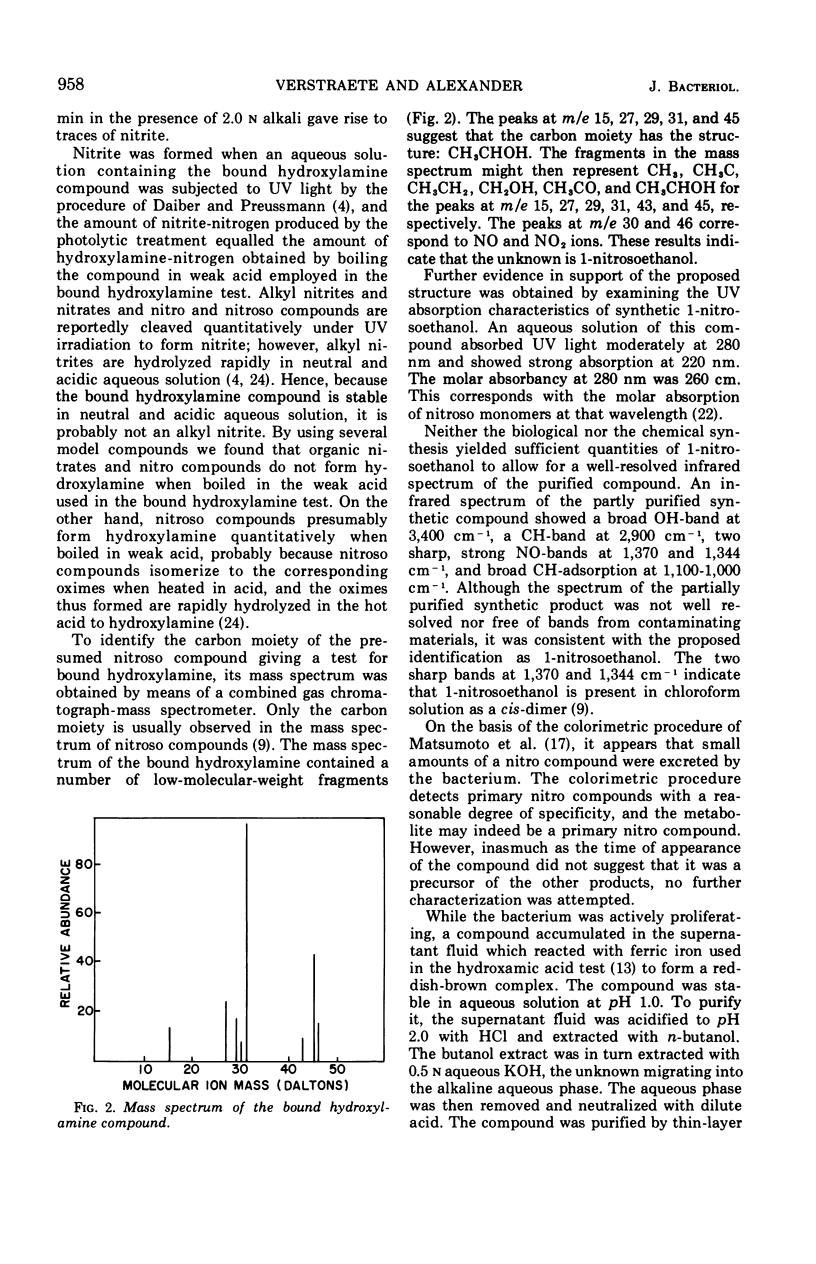
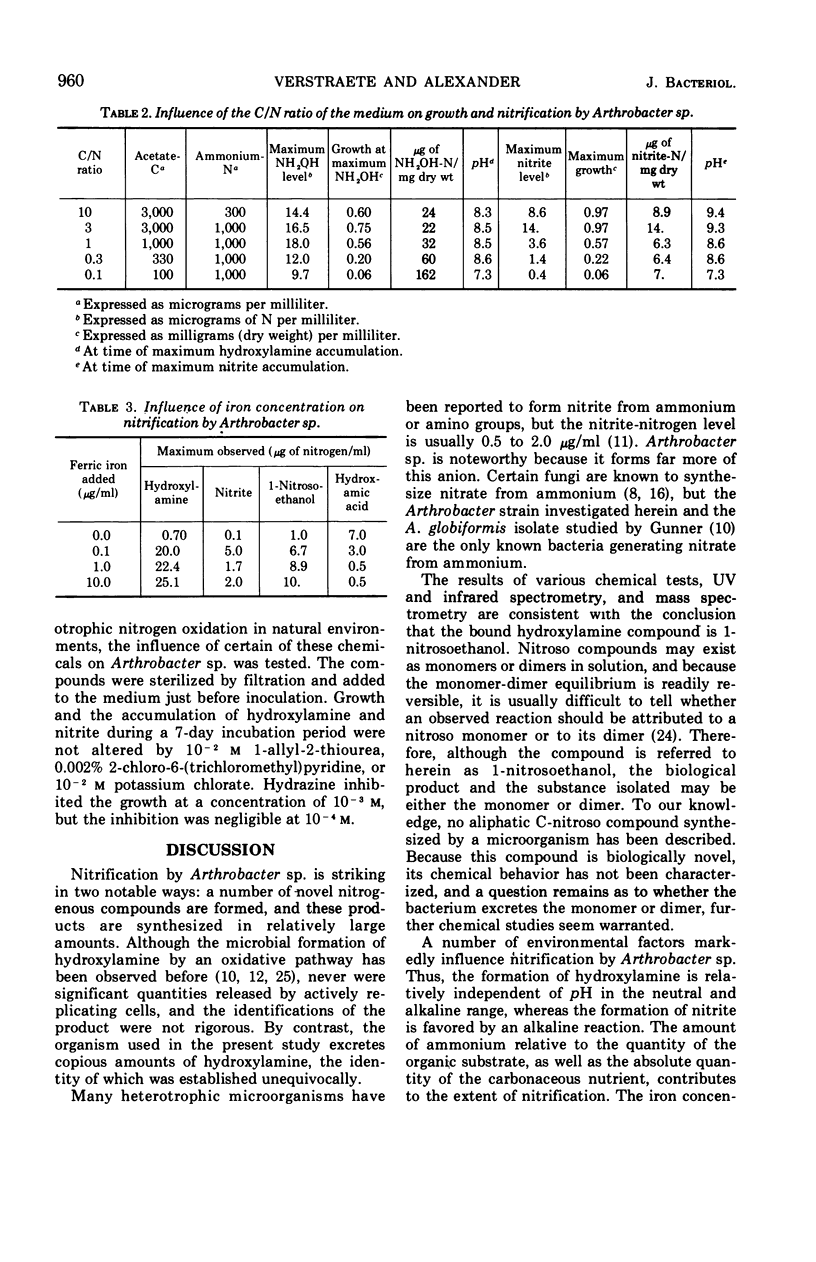
Arthrobacter sp. isolated from sewage oxidized ammonium to hydroxylamine, a bound hydroxylamine compound, a hydroxamic acid, a substance presumed to be a primary nitro compound, nitrite, and nitrate. The concentration of free hydroxylamine-nitrogen reached 15 μg/ml. The identification of hydroxylamine was verified by mass spectrometric analysis of its benzophenone oxime derivative. The bound hydroxylamine was tentatively identified as 1-nitrosoethanol on the basis of its mass spectrum, chemical reactions, and infrared and ultraviolet spectra. Hydroxylamine formation by growing cells was relatively independent of pH, but the accumulation of nitrite was strongly favored in alkaline solutions. The formation of hydroxylamine but not nitrite was regulated by the carbon to nitrogen ratio of the medium. The hydroxamic acid was the dominant product of nitrification in iron-deficient media, but hydroxylamine, nitrite, and 1-nitrosoethanol formation was favored in iron-rich solutions. Heterotrophic nitrification by Arthrobacter sp. was not inhibited by several compounds at concentrations which totally inhibited autotrophic nitrification.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CORNFORTH J. W., JAMES A. T. Structure of a naturally occurring antagonist of dihydrostreptomycin. Biochem J. 1956 May;63(1):124–130. doi: 10.1042/bj0630124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYLAR O. R., Jr, SCHMIDT E. L. A survey of heterotrophic micro-organisms from soil for ability to form nitrite and nitrate. J Gen Microbiol. 1959 Jun;20(3):473–481. doi: 10.1099/00221287-20-3-473. [DOI] [PubMed] [Google Scholar]
- HIRSCH P., OVERREIN L., ALEXANDER M. Formation of nitrite and nitrate by actinomycetes and fungi. J Bacteriol. 1961 Sep;82:442–448. doi: 10.1128/jb.82.3.442-448.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARSHALL K. C., ALEXANDER M. Nitrification by Aspergillus flavus. J Bacteriol. 1962 Mar;83:572–578. doi: 10.1128/jb.83.3.572-578.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy Y. K., Thiemann J. E., Coronelli C., Sensi P. Alanosine, a new antiviral and antitumour agent isolated from a Streptomyces. Nature. 1966 Sep 10;211(5054):1198–1199. doi: 10.1038/2111198a0. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Hydroxamic acids in nature. Science. 1967 Jun 16;156(3781):1443–1447. doi: 10.1126/science.156.3781.1443. [DOI] [PubMed] [Google Scholar]
- WILEY P. F., HERR R. R., MACKELLAR F. A., ARGOUDELIS A. D. THREE CHEMICALLY RELATED METABOLITES OF STREPTOMYCES. II. STRUCTURAL STUDIES. J Org Chem. 1965 Jul;30:2330–2334. doi: 10.1021/jo01018a051. [DOI] [PubMed] [Google Scholar]


